Abstract
We evaluated species richness, abundance, alpha diversity, and true diversity of Phlebotominae sand flies temporal changes in domiciles within the northern Argentina city of Corrientes. A total of 16 sampling nights were conducted seasonally throughout the years 2012-2014 through light traps supplemented with CO2. Meteorological and remote sensing environmental factors were used to assessed for vectors implications in disease transmission through Generalized Mixt Models. Lutzomyia longipalpis was the most abundant and common species, followed by Nyssomyia neivai and Migonemyia migonei. Lutzomyia longipalpis was more abundant in urban areas, Ny. neivai was associated with vegetation in periurban areas, both were found all sampling years with higher abundance during the rainy season. Positive association of Lu. longipalpis with precipitation and relative humidity and negative association with temperature were observed. Models showed humidity and vegetation as making effects on Lu. longipalpis abundance. Precipitation was significant for Mg. migonei models, with higher abundance in periurban and periurban-rural environments. For Ny. neivai models, relative humidity was the most important variable, followed by precipitation frequency. Our findings led to identify high risk areas and develop predictive models. These are useful for public health stakeholders giving tolls to optimized resources aim to prevent leshmaniasis transmission on the area.
Key words
Species abundance; species richness; modeling; public health entomology; remote sensing
INTRODUCTION
Argentina has reported leishmaniasis cases from the two clinical forms, the visceral (VL) form which is the more serious form, and the tegumentary (TL) form which is the most common and presents manifestations in the skin and mucous (Salomón et al. 2008aSALOMÓN OD, QUINTANA MG & ROSA JR. 2008a. Ecoepidemiología de la Leishmaniasis cutánea en Argentina. Salud i Ciencia 16: 514-520., Locatelli et al. 2014LOCATELLI FM ET AL. 2014. The isolation and molecular characterization of Leishmania spp. from patients with American tegumentary leishmaniasis in northwest Argentina. Act Trop 131: 16-21.). VL is caused by Leishmania (Leishmania) infantun Nicolle and TL is caused by Le. (Viannia) braziliensis Vianna. The latter is the most frequent species of Leishmania isolated in Argentina (Salomón et al. 2006bSALOMÓN OD, SOSA-ESTANI S, RAMOS K, ORELLANO PW, SANGUESA G, FERNÁNDEZ G, SINAGRA A & RAPASCIOLLI G. 2006b. Tegumentary leishmaniasis outbreak in Bella Vista City, Corrientes, Argentina during 2003. Mem Inst Oswaldo Cruz 101(7): 767-774.). Other detected Leishmania species that cause tegumentary manifestations are Le. (V.) guyanensis, Le. (L.) amazonensis, and Le. (V.) panamensis (Marco et al. 2005MARCO JD ET AL. 2005. Species assignation of Leishmania from human and canine American tegumentary leishmaniasis cases by multilocus enzyme electrophoresis in North Argentina. Am J Trop Med Hyg 72(5): 606-611., 2006MARCO JD, UEZATO H, MIMORI T, BARROSO PA, KORENAGA M, NONAKA S, BASOMBRÍO MA, TARANTO NJ & HASHIGUCHI Y. 2006. Are cytochrome B gene sequencing and polymorphism-specific polymerase chain reaction as reliable as multilocus enzyme electrophoresis for identifying Leishmania spp. from Argentina?. Am J Trop Med Hyg 75(2): 256-260., 2012MARCO JD ET AL. 2012. Polymorphism-specific PCR enhances the diagnostic performance of American tegumentary leishmaniasis and allows the rapid identification of Leishmania species from Argentina. BMC Infect Dis 15: 12:191., Salomón et al. 2012aSALOMÓN OD, MASTRANGELO AV, SANTINI MS, RUVINSKY S, ORDUNA T, SINAGRA A, LUNA C, RIARTE A, CASAS N & AMIOTTI P. 2012a. Leishmaniasis visceral: senderos que confluyen, se bifurcan. Salud Colectiva 8(Supl 1): S49-S63., bSALOMÓN OD, QUINTANA MG, MASTRANGELO AV & FERNÁNDEZ MS. 2012b. Leishmaniasis and climate change—case study: Argentina. J Trop Med 2012: 601242.).
In Argentina, different species of phlebotominae sand flies were found to be involved in the cycle of transmission of leishmaniasis. Nyssomyia neivai (Pinto), Micropygomyia quinquefer (Dyar) and cortelezzii complex have been found infected with Leishmania sp. by “Leishmania DNA detection” (Córdoba Lanús et al. 2006CÓRDOBA LANÚS E, LIZARRALDE DE GROSSO M, PIÑERO JE, VALLADARES B & SALOMÓN OD. 2006. Natural infection of Lutzomyia neivai with Leishmania spp. in northwestern argentina. Act Trop 98(1): 1-5., Rosa et al. 2012ROSA JR, PEREIRA DP, BRAZIL RP, FILHO JD, SALOMÓN OD & SZELAG E. 2012. Natural infection of cortelezzii complex (Diptera: Psychodidae: Phlebotominae) with Leishmania braziliensis in Chaco, Argentina. Act Trop 123(2): 128-131.). Lutzomyia longipalpis (Lutz and Neiva), Migonemyia migonei (França) and Ny. whitmani (Antunes & Coutinho) have been found infected with Le. infantum by “Leishmania DNA detection” (Acardi et al. 2010ACARDI S, LIOTTA DJ, SANTINI MS, ROMAGOSA CM & SALOMÓN OD. 2010. Detection of Leishmania infantum in naturally infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis familiaris in Misiones, Argentina: First report of PCR-RFLP and a sequencing confirmation assay. Mem Inst Oswaldo Cruz 105(6): 796-799., de Carvalho et al. 2010DE CARVALHO MR, VALENÇA HF, DA SILVA FJ, DE PITA-PEREIRA D, DE ARAÚJO PEREIRA T, BRITTO C, BRAZIL RP & FILHO SP. 2010. Natural Leishmania infantum infection in Migonemyia migonei (França, 1920) (Diptera:Psychodidae:Phleboto minae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Act Trop 116: 108-110., Moya et al. 2015MOYA SL, GIULIANI MG, MANTECA ACOSTA M, SALOMÓN OD & LIOTTA DJ. 2015. First description of Migonemyia migonei (França) and Nyssomyia whitmani (Antunes & Coutinho) (Psychodidae: Phlebotominae) natural infected by Leishmania infantum in Argentina. Act Trop 152: 181-184.). Likewise Evandromyia cortelezzii (Brethes) has been found infected with Le. infantum DNA in Brazil (Saraiva et al. 2010SARAIVA L, FILHO JDA, DE OLIVEIRA SILVA S, DE ANDRADE ASR & MELO MN. 2010. The molecular detection of different Leishmania species within sand flies from a cutaneous and visceral leishmaniasis sympatric area in Southeastern Brazil. Mem Inst Oswaldo Cruz 105: 1033-1039.). Lutzomyia longipalpis was detected in 2004 for the first time in Clorinda city, Formosa province, northern Argentina (Salomón & Orellano 2005SALOMÓN OD & ORELLANO PW. 2005. Lutzomyia longipalpis in Clorinda, Formosa province, an area of potential visceral leishmaniasis transmission in Argentina. Mem Inst Oswaldo Cruz 100: 475-476.), and the first VL human case as well as canine VL was reported by 2006 in Posada city (Salomón et al. 2008bSALOMÓN OD, QUINTANA MG & ZAIDENBERG M. 2008c. Urban distribution of Phlebotominae in a cutaneous leishmaniasis focus, Argentina. Mem Inst Oswaldo Cruz 103(3): 282-287.). In 2008, Lu. longipalpis was first reported in Corrientes city (Salomón et al. 2009aSALOMÓN OD, QUINTANA MG, BRUNO MR, QUIRICONI RV & CABRAL V. 2009a. Visceral leishmaniasis in border areas: clustered distribution of phlebotomine sand flies in Clorinda, Argentina. Mem Inst Oswaldo Cruz 104(5): 801-804., bSALOMÓN OD, RAMOS LK, QUINTANA MG, ACARDI SA, SANTINI MS & SCHNEIDER A. 2009b. Distribución de vectores de Leishmaniasis visceral en la provincia de Corrientes, 2008. Medicina (Buenos Aires). 2009(69): 625-630.).
Vector-borne diseases are influenced, along with the insects’ population dynamics, by environmental factors that could be characterized and studied by meteorological and remote sensing (RS) data (Estallo et al. 2015ESTALLO EL, LUDUEÑA-ALMEIDA FF, INTROINI MV, ZAIDENBERG M & ALMIRÓN WR. 2015. Weather Variability Associated with Aedes (Stegomyia) aegypti (Dengue Vector) Oviposition Dynamics in Northwestern Argentina. PLoS ONE 10(5): e0127820., 2016ESTALLO EL, BENÍTEZ EM, LANFRI MA, SCAVUZZO CM & ALMIRÓN WR. 2016. MODIS environmental data to assess chikungunya, dengue, and zika diseases through Aedes (Stegomyia) aegypti oviposition activity estimation. IEEE J. SelecTop. Appl Earth Observ Remote Sens 9(12): 5461-5466.). In the last decade, the use of RS data for vector-borne diseases has increased as a tool to estimate the environmental conditions where vectors could breed and develop (Estallo et al. 2016ESTALLO EL, BENÍTEZ EM, LANFRI MA, SCAVUZZO CM & ALMIRÓN WR. 2016. MODIS environmental data to assess chikungunya, dengue, and zika diseases through Aedes (Stegomyia) aegypti oviposition activity estimation. IEEE J. SelecTop. Appl Earth Observ Remote Sens 9(12): 5461-5466., 2018ESTALLO EL, SANGERMANO F, GRECH M, LUDUEÑA-ALMEIDA FF, FRÍAS-CÉSPEDES M, AINETE M, ALMIRÓN WR & LIVDAHL T. 2018. Modelling the distribution of the vector Aedes aegypti in a central Argentine city. Med Vet Entomol 32: 451-461.). Remote sensing allows environmental factor characterization in different ways such as vegetation quantification through vegetation indices like the Normalized Difference Vegetation Index (NDVI) and the Enhanced Vegetation Index (EVI) or through land surface temperatures (LST) (Estallo et al. 2016ESTALLO EL, BENÍTEZ EM, LANFRI MA, SCAVUZZO CM & ALMIRÓN WR. 2016. MODIS environmental data to assess chikungunya, dengue, and zika diseases through Aedes (Stegomyia) aegypti oviposition activity estimation. IEEE J. SelecTop. Appl Earth Observ Remote Sens 9(12): 5461-5466., Artun & Kavur 2017ARTUN O & KAVUR H. 2017. Investigation of the spatial distribution of sandfly species and cutaneous leishmaniasis risk factors by using geographical information system technologies in Karaisali district of Adana province, Turkey J Vector Borne Dis 54: 233-239.).
There have been studies on several species of phlebotomine sand flies aimed at investigating environmental factors and urban areas associated with VL (Santini et al. 2012SANTINI MS, FERNÁNDEZ MS, PÉREZ AA, SANDOVAL AE & SALOMÓN OD. 2012. Lutzomyia longipalpis abundance in the city of Posadas, northeastern Argentina: variations at different spatial scales. Mem Inst Oswaldo Cruz 107(6): 767-771.). Likewise, different studies have been carried out to know the influence of modified environments and urbanized areas in the domestic and peridomestic TL transmission (Salomón et al. 2006a, b, 2008a). Many of these previous studies had focused primarily on Misiones province as a risk area for Argentina. Relevant studies of phlebotomine sand flies communities in new risk areas of VL of the country such as Corrientes city are lacking however as only a few studies have begun analyzing macro and microenvironmental factors mainly on a spatial scale (Berrozpe et al. 2017BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, UTGES ME & SALOMÓN OD. 2017. Environmental suitability for Lutzomyia longipalpis in a subtropical city with a recently established visceral leishmaniasis transmission cycle, Argentina. Mem Inst Oswaldo Cruz 112(10): 674-680., 2018). Herein, we aim to evaluate the species richness and abundance of phlebotomine sand flies (Diptera: Psychodidae) in Corrientes city, temporal changes assessed by 16 sampling events over two years of sampling, and the relationship between meteorological factors and remote sensing environmental factors for vector implications in disease transmission.
MATERIALS AND METHODS
Study area
The study area was in Corrientes province, located in the Neotropical Region of northeastern Argentina. The soil permeability is moderately slow, which allows the formation of flooded areas (Bruniard 1978BRUNIARD ED. 1978. El Gran Chaco Argentino. Ensayo de interpretación geográfica, GEOGRÁFICA 4. Facultad de Humanidades, UNNE, Resistencia, Chaco, Argentina.). Corrientes city (27º28’08” S, 58º49’50” W) is located in the biogeographic Chacoan province, the floodplain area of the Paraná and Paraguay rivers (Secretaría de Desarrollo Sustentable y Política Ambiental 1999SECRETARÍA DE DESARROLLO SUSTENTABLE Y POLÍTICA AMBIENTAL. 1999. Estudio integral de la región del parque chaqueño. Segunda edición. Ministerio de Desarrollo Social y Medio Ambiente, Buenos Aires, Argentina.). The mean annual temperature is 23ºC and the mean annual rainfall is 1280 mm. In the summer, the mean temperature is 27ºC, with temperatures as high as 43ºC recorded. The mean temperature in winter is 15ºC, with -1.1 ºC as the lower recorded. The rainy season is between November and April, with peaks of rainfall in spring (September-October) and autumn (March- April) (Bruniard 1978BRUNIARD ED. 1978. El Gran Chaco Argentino. Ensayo de interpretación geográfica, GEOGRÁFICA 4. Facultad de Humanidades, UNNE, Resistencia, Chaco, Argentina.).
Sampling sites
The study was conducted seasonally for two years, from March 2012 to May 2014, attaining a total of 16 nights of collection, simultaneously on each sampling site. Within the urban area of the city of Corrientes, 8 sites were selected for phlebotomine sand flies sampling, six of them (Figure 1: sites 1, 2, 3, 4, 6, 8) corresponded to houses that fulfilled the criterion of worst epidemiological scenario (Table I). A house fulfilling such criterion is an inhabited dwelling with favorable conditions for vector development, shady trees, hens or poultry in the peridomicile, and forest patches not less than 100m from the dwelling (Salomón et al. 2009a). One of the sampling sites met the conditions but was without a history of VL (Figure 1: site 5), and one site did not meet this criterion and had a high concentration of people throughout the day due to the Argentine Army Regiment (Figure 1: site 7). Sites 1, 2, 6, 7 and 8 were located in the urban area, which higher human density, while sites 3, 4 and 5 they were in the suburban area with less human density (Figure 1). The urban sites have higher population density and well –developed infrastructure, and periurban sites are within an area with houses located in a green matrix (Berrozpe et al. 2018BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98.). Each sampling site was geo-referenced using a GPS (Global Positioning System) (Garmin GPSMAP ® 60GSx).
Study site. a: Map of Argentina showing the Corrientes province (northeast) and city of Corrientes. b: Map of the study locality: city of Corrientes indicating where the sampling point were located.
Entomological sampling
Sampling was done 16 months at each sampling site, during the sampling period (from March 2012 to May 2014) at list ones in each climatic season. Phlebotomine sand flies were captured using 1 CDC-like light trap per site, supplemented with CO2, and located in peridomestic environments. The traps were set at a height of 1 to 1.5 m above the ground and were active for one night from 3 p.m. to 10 a.m. during each sampling date of the month.
All phlebotomine sand flies were euthanized via freezing (-20°C) and preserved in 70% alcohol solution prior to processing. The specimens were cleared in lactophenol and identified according to Galati (2003)GALATI E. 2003. Classificação de Phlebotominae, p.23-51. In: Rangel E and Lainson R (Eds). Flebotomíneos do Brasil. Fiocruz, Rio do Janeiro..
Females of Evandromyia cortelezzii and Ev. sallesi cannot be distinguished by morphological characteristics, so female specimens were included within the Cortelezzii complex (Szelag et al. 2018SZELAG EA, ROSA JR, GALATI EAB, ANDRADE FILHO JD & SALOMÓN OD. 2018. Considerations on the Species Complex of the Cortelezzii series (Diptera: Psychodidae) and Description of Evandromyia chacuensis sp. nov., a New Phlebotomine Species of the Chaco Region, Argentina. J Med Entomolartun 55(4): 902-909.).
Environmental factors
Meteorological data were provided by the National Meteorological Service weather station from the city of Corrientes Aero station (27º27´ S 58º 50´O). Mean temperature (°C), relative humidity (%), accumulated precipitation (mm) and precipitation frequency (days) from 2012 to 2014 were obtained. The environmental characterization through remote sensing (RS) data was registered by the Moderate-resolution imaging spectroradiometer (MODIS) satellite products that are useful for vector-borne disease studies in Argentina (Estallo et al. 2016ESTALLO EL, BENÍTEZ EM, LANFRI MA, SCAVUZZO CM & ALMIRÓN WR. 2016. MODIS environmental data to assess chikungunya, dengue, and zika diseases through Aedes (Stegomyia) aegypti oviposition activity estimation. IEEE J. SelecTop. Appl Earth Observ Remote Sens 9(12): 5461-5466.). MODIS products: MOD13Q1 (NDVI and EVI) vegetation satellite products at 250 meters and 16-day compositing periods and LST MOD11A2 satellite product at 8-day compositing period and 1km spatial resolution were used. The NDVI saturates in high biomass regions which provides continuity with NOAA’s AVHRR NDVI time series record for historical and climate applications, while EVI remained sensitive to canopy variations because it minimizes canopy-soil variations and improves sensitivity over dense vegetation conditions (Huete et al. 2002HUETE A, DIDAN K, MIURA T, RODRIGUEZ EP, GAO X & FERREIRA LG. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens Environ 83: 195-213.).
Statistical analysis to evaluate the phlebotomine sand fly community structure
To compare the species abundance in the 8 sampling sites, the Index of species abundance (ISA) was calculated for individual species according to Roberts & Hsi (1979): (ISA = a + Rj/K).
The nonparametric richness estimator ICE (Abundance-based coverage estimator, Chao & Lee 1992CHAO A & LEE S. 1992. Estimating the Number of Classes via Sample Coverage. J Amer Statist Assoc 87(417): 210-217.) was calculated to measure the completeness of the phlebotominae sand flies inventory (Colwell & Coddington 1994COLWELL RK & CODDINGTON JA. 1994. Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc Lond B Biol Sci 345(1311): 101-118.). This analysis was performed using the free Estimate Sv9.1.0 software (http://purl.oclc.org/estimates; Colwell 2013COLWELL RK. 2013. EstimateS, Version 9.1: Statistical Estimation of Species Richness and Shared Species from Samples.). Alpha diversity was estimated by specific richness (S) and the method proposed by Jost (2006)JOST L. 2006. Entropy and diversity. Oikos 113(2): 363-375..
Statistical analysis of meteorological and remote sensing environmental factors for vector implications in disease transmission
We selected the three most abundant phlebotomine sand flies species (Lu. longipalpis, Mg. migonei, and Ny. neivai), with response variables to develop the models to investigate how the environment affects relative abundance of the species sampled. First, correlations were obtained between each species abundance and meteorological (mean temperature, relative humidity, accumulated precipitation) and remote sensing environmental factors (EVI and LST) with and without time lags (1- and 2-months’ time lags). This way, the best-correlated lag explanatory variable was selected for each species to model the species abundance. We used precipitation frequency (FrecPrec) without time lags.
In repeated measures data, individuals typically display a high degree of similarity in responses over time. Therefore, we used generalized linear mixed models (GLMM) known as multilevel models to evaluate the relationship between the observed species of Phlebotominae sand fly abundance (Lu. longipalpis, Mg. migonei and Ny. neivai) and environmental factors (meteorological and remote sensing variables).
Phlebotominae sand fly abundance (Lu. longipalpis, Mg. migonei and Ny. neivai) and environmental factors (meteorological and remote sensing variables)
We use a negative binomial distribution instead of Poisson because the exploratory analysis revealed that variance was much higher than the mean. To select the best model we tested two families of negative binomial distributions: family=nbinom1, where the variance linearly increases with the mean: ⌠2= µ (1+<), with < > 0; and family=nbinom2, where the variance is a quadratic function of the mean as ⌠2= µ (1 + µ /⎝), with ⎝ > 0.
These kinds of models are adequate for counting with overdispersion (where variance is higher than medium values) because of the number of null frequencies. Exploratory analysis showed that abundance depends on the year, therefore in every case the years were used as random factors. At the second step, three different models were compared for each species:
1-Negative binomial distribution (for family=nbinom1 and family=nbinom2) without considering the variable that determines overdispersion;
2-Negative binomial distribution (for family=nbinom1 and family=nbinom2) considering months as overdispersion variable;
3-Negative binomial distribution (for family=nbinom1 and family=nbinom2) zero-inflated model, considering month as zi (the variable where the presence of zeros could depend).
A total of 6 models (the above describe models for two different families each one) were developed for each species abundance in order to see which environmental variables affect the species abundance, and selection of the best model was based on the Akaike´s information criterion (AIC- Akaike 1974AKAIKE H. 1974, A new look at the statistical model identification. IEEE Transactions on Automatic Control 19(6): 716-723.). The model with the lowest AIC was selected as the best one of the 6 compared models. The selected model was implemented and developed in each case for each species, in order to evaluate the effects of the explanatory variables.
For each species, at third step, the selected model was implemented with groups of explanatory variables to avoid collinearity. Therefore for each species model, explanatory variables were grouped as:
A-mean EVI (EVIm), precipitation frequency (FrecPrec) and medium relative humidity (HRm);
B- mean land surface temperatures (LSTm), FrecPrec, HRm and precipitation (prec).
We used Generalized Linear Mixed Model throughout the GLMMTMB package on R software 3.5.0 (04-23-2018).
RESULTS
In all trapping periods, 1310 phlebotomine sand flies were captured belonging to 7 species from 5 genera. Lutzomyia longipalpis was the most abundant (n = 1,121) and most common species from the study area (ISAs=0.925), followed by Ny. neivai (ISAs=0.625) and Mg. migonei (ISAs=0.525). Site 1 showed the highest abundance of sand flies over the capture period. Cortelezzii complex females were scarce although they were captured in 4/8 sites (ISAs = 0.225). The rest of the species showed ISAs=0.05 (Table II).
Species collected during the trapping period with their abundance, the trapping site of the species and the Index of species abundance (ISA).
Diversity and richness
According to the richness estimator calculated, representation of the sampling was 71.57% (ICE mean= 9.78). While the greatest species richness was found in site 4 (S = 6), followed by sites 3 (S = 5), 2 (S = 4) and 8 (S = 4) (Table III), site 4 also had the least abundance of sandflies. The effective number of species was higher in environment 4 (1D = 5.29 effective species), followed by site 3 (1D = 3.97 effective species). On the contrary, site 1 presented a reduction of 76% in phlebotomine sand flies diversity compared to the most diverse site and the most abundant of sand flies with a high predominance of Lu. longipalpis. This was a large open area (1/4 ha approx.), with a house and a large open space with some scattered trees (Table I). Collections at sites 3 and 5 consisted largely of Ny. neivai.
Seasonal distribution of phlebotomine sand flies abundance
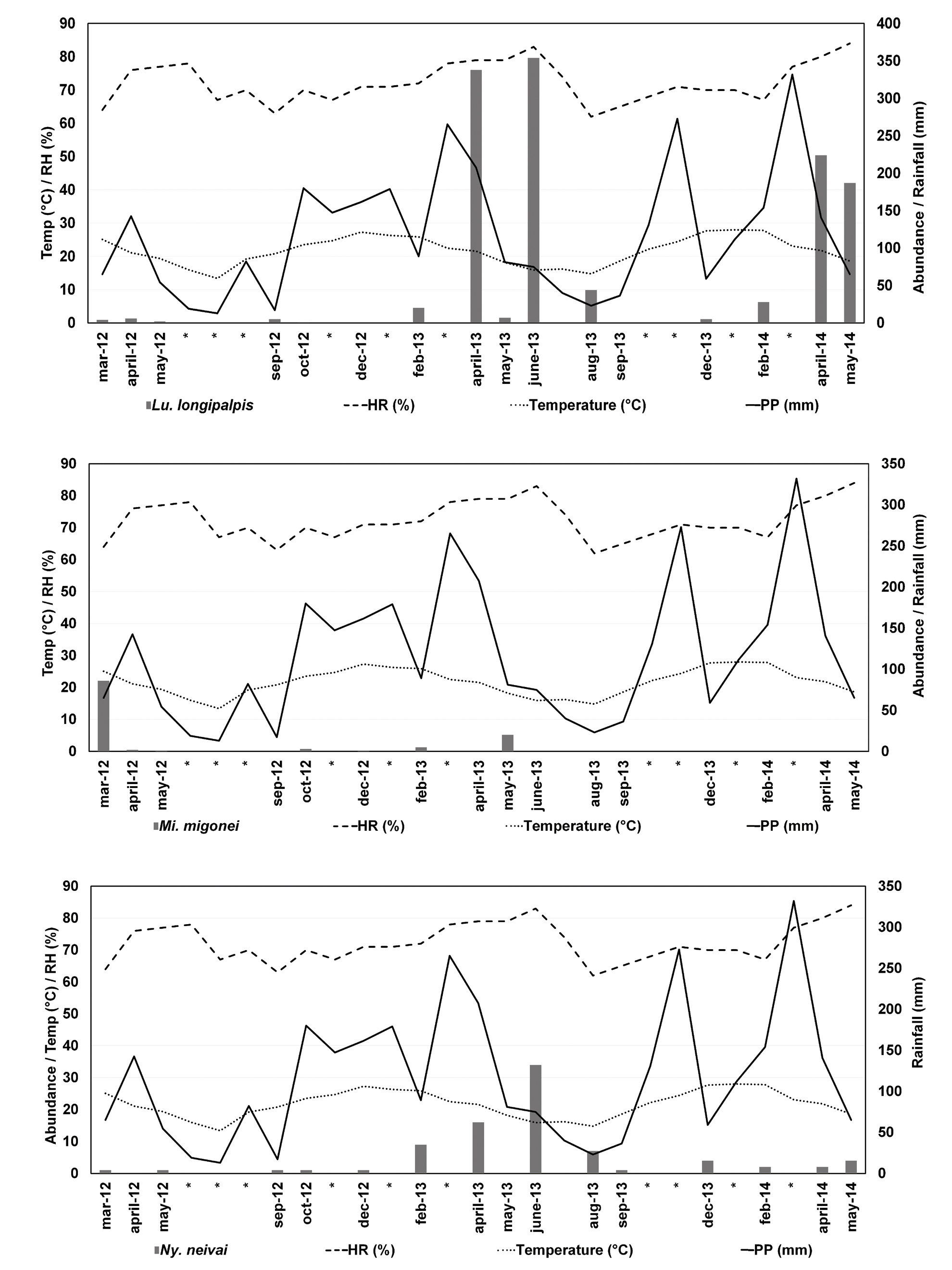
Lutzomyia longipalpis and Ny. neivai were collected during all sampling years, while Mg. migonei was collected from March 2012 to May 2013 (sampling was done during June-July-August-September 2012 that correspond to south hemisphere winter time) (Figure 2). Females of Ev. cortelezzi-sallesi were found only two times in autumn of 2012 and ones at summer of 2013. Nyssomyia whitmani was captured once in December 2012 and Psathyromyia (Psathyromyia) bigeniculata (Floch & Abonnenc) once in April of the same year.
The highest overall species richness was recorded twice, one in the beginning of autumn 2012 (March) and the highest at the end of 2013 summer season (February). During the winter of 2013 (June and August), only two species were recorded, Lu. longipalpis and Ny. neivai. The two species presented similar patterns of temporal distribution throughout the year, with greatest abundance in autumn and winter, followed by summer and then spring. Migonemyia migonei was most abundant during autumn.
Meteorological and remote sensing environmental factors for implications in vector-borne disease transmission
The GLMM considered the sampling sites as the random factor for each model developed. Explanatory variables were selected according to the best lag correlation with each phlebotomine sand flies species (Table IV), and the fluctuation for temperature, mean rainfall and relative humidity for Lutzomyia longipalpis, Ny. neivai and Mg. migonei are shown also in Figure 3.
Abundance of Lu. longipalpis, Mg. migonei and Ny. neivai, mean temperature, mean rainfall, and relative humidity data.
Spearman correlation coefficients between the abundance of Lu. longipalpis, Mi. migonei and Ny. neivai with the meteorological and satellite variables: with and without time lags. In the table is showed the week with the best correlation value selected for the models.
Lutzomyia longipalpis models
Explanatory variables EVIm2, FrecPrec, and HRm1
Comparing the goodness of fit (AIC) from the 6 models developed as a first step, we found the negative binomial distribution (family=nbinom2: where dispersion parameter as a quadratic function of the mean) zero-inflated model, as the best one.
Explanatory variables LSTmN2, FrecPrec, HRm1, and prec1
In this particular case, the negative binomial with the dispersion parameter as a linear function of the mean (family=nbinom1) without considering the variable that gives overdispersion was the best.
For both models developed for this species (Table Va, b), we found that relative humidity with 1-month time lag (HRm1) was the only significant variable. Also we saw that the intercept variance of the random factor for both models was very small, indicating that Lu. longipalpis abundance doesn’t change between years.
GLMM parameter estimates for group of selected explanatory variables for Lutzomia longipalpis model.
Migonemyia migonei models
Explanatory variables EVIm1, PrecFrec, and RHm1
Comparing the goodness of fit (AIC) from the 6 models developed as a first step, we found the negative binomial distribution (family=nbinom1) without dispersion parameter model as the best one to use.
We found no evidence of the existence of a significant effect of any of the variables considered on Mg. migonei abundance (Table Vc).
Explanatory variables LSTmN2 PrecFrec, RHm1, and prec2
The best model to apply was a mixed model without dispersion parameter using a negative binomial distribution as a quadratic of the mean (family=nbinom2).
The only variable that has a positive significant effect over the abundance of Mg. migonei, in this case, was the precipitation (p = 0.0152) (Table Vd).
Nyssomyia neivai models
Explanatory variables EVIm1, FrecPrec, and HRm1
We found the mixed model that considered zero-inflation with negative binomial distribution as a quadratic of the mean (family=nbinom2) as the best one to apply in this particular case (Table Ve). The relative humidity at 1 month time lag (z =4.52; p =0.00000618) and the precipitation frequency (without time lags) (z =2.65; p =0.00817) were both significant variables. There is no evidence that Ny. neivai abundance could be influenced by EVI because this variable showed not significance (z =-0.052; p =0.95867).
Explanatory variables LSTmN2, FrecPrec, HRm1, and prec2
We found as the best model for these variables the mixed model with zero-inflation and negative binomial distribution as a quadratic of the mean (family=nbinom2) and dispersion parameter (Table Vf).
From the GLMM developed here only the relative humidity at lag 1-month (RHm1), was significant (z =4.070; p =0.000047).
DISCUSSION
Previous studies on the distribution of Phlebotomine sand flies fauna in Argentina mentioned 12 species for the province of Corrientes (Salomón et al. 2008aSALOMÓN OD, QUINTANA MG & ROSA JR. 2008a. Ecoepidemiología de la Leishmaniasis cutánea en Argentina. Salud i Ciencia 16: 514-520., Quintana et al. 2012QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48.).
Psathyromyia (Psathyromyia) bigeniculata (Floch and Abonnenc) was previously considered a junior synonyms of Pa. shannoni. However, it taxonomic status was revalidated, and based on the morphological differences presented by Sábio et al. (2014)SÁBIO PB, ANDRADE AJ & GALATI EAB. 2014. Assessment of the Taxonomic Status of Some Species Included in the Shannoni Complex, With the Description of a New Species of Psathyromyia (Diptera: Psychodidae: Phlebotominae). J Med Entomol 51: 331-341. to distinguish Pa. bigeniculata from Pa. shannoni, the presence of Pa. bigeniculata in Corrientes city is confirmed for the present study. Rosa et al. (1999)ROSA JR, BORDA CE, REA MJF & MOSQUEDA LA. 1999. Cría de especies de Lutzomyia de la provincia de Corrientes, Argentina en condiciones experimentales. Medicina (Buenos Aires). 59(Supl. III): 49. recorded three Pa. bigeniculata (previously Lutzomyia shanonni) individuals in Corrientes city using Shannon traps. A few years later, Borda et al. (2002)BORDA CE, REA MJF & ROSA JR. 2002. Urbanization of the human leishmaniasis in the Northeast of the Argentina. Entomol Vect 9(1): 56. presented a review of phlebotomine sand flies in 11 departments of Corrientes province where this species is mentioned without indicating the geographic location of capture. Also Salomón et al. (2008a)SALOMÓN OD, QUINTANA MG & ROSA JR. 2008a. Ecoepidemiología de la Leishmaniasis cutánea en Argentina. Salud i Ciencia 16: 514-520. mention its presence, without mentioning the geographic location of capture in Corrientes province. Berrozpe et al. (2017, 2018) does not capture this species in the following years from these studies, therefore we considered that Pa. bigeniculata had not been registered in Corrientes city since the first detection by Rosa et al. (1999)ROSA JR, BORDA CE, REA MJF & MOSQUEDA LA. 1999. Cría de especies de Lutzomyia de la provincia de Corrientes, Argentina en condiciones experimentales. Medicina (Buenos Aires). 59(Supl. III): 49. during 1997 samplings. Sábio et al. (2014)SÁBIO PB, ANDRADE AJ & GALATI EAB. 2014. Assessment of the Taxonomic Status of Some Species Included in the Shannoni Complex, With the Description of a New Species of Psathyromyia (Diptera: Psychodidae: Phlebotominae). J Med Entomol 51: 331-341. mentioned the need for a review of the specimens identified as Pa. shannoni to determine the actual distribution of Pa. bigeniculata and the possible presence of other species of Shannoni complex in Argentina.
The present study contributes to the confirmation of 5 Phlebotomine sand flies species of medical importance in northeastern Argentina. Two of them, Lu. longipalpis and Ny. neivai, were collected throughout the sampling period with a wide spatial range in the city of Corrientes. Migonemyia migonei was the species in third highest abundance as was also registered by Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98.. These findings are in accordance with other studies in different places of northern Argentina like Santo Tomé (Corrientes), Puerto Iguazú (Misiones) and Corrientes city where Lu. longipalpis was the most abundant species (Santini et al. 2015SANTINI MS, UTGÉS ME, BERROZPE P, MANTECA ACOSTA M, CASAS N, HEUER P & SALOMÓN OD. 2015. Lutzomyia longipalpis Presence and Abundance Distribution at Different Micro-spatial Scales in an Urban Scenario. PLoS Negl Trop Diseases 9(8): e0003951., 2017SANTINI MS, FERNÁNDEZ MS, CAVIA R & SALOMÓN OD. 2017. Co-occurrence and seasonal and environmental distributions of the sandflies Lutzomyia longipalpis and Nyssomyia whitmani in the city of Puerto Iguazú, northeastern Argentina. Med Vet Entomol 32(2): 197-205., Berrozpe et al. 2017BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, UTGES ME & SALOMÓN OD. 2017. Environmental suitability for Lutzomyia longipalpis in a subtropical city with a recently established visceral leishmaniasis transmission cycle, Argentina. Mem Inst Oswaldo Cruz 112(10): 674-680., 2018). It is important to note that in the present study a higher richness of phlebotomine sand flies species was found for the city of Corrientes, compared to previous studies (Rosa et al. 1999ROSA JR, BORDA CE, REA MJF & MOSQUEDA LA. 1999. Cría de especies de Lutzomyia de la provincia de Corrientes, Argentina en condiciones experimentales. Medicina (Buenos Aires). 59(Supl. III): 49., Borda et al. 2002BORDA CE, REA MJF & ROSA JR. 2002. Urbanization of the human leishmaniasis in the Northeast of the Argentina. Entomol Vect 9(1): 56., Berrozpe et al. 2017BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, UTGES ME & SALOMÓN OD. 2017. Environmental suitability for Lutzomyia longipalpis in a subtropical city with a recently established visceral leishmaniasis transmission cycle, Argentina. Mem Inst Oswaldo Cruz 112(10): 674-680.). The sites of greatest species richness were found in the peri-urban city area, characterized by less constructions and lower human population density, with surrounding vegetation areas and presence of domestic animals, whereas Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. found lower phlebotomine sand flies richness in areas with low vegetation measure through NDVI values.
The sampling at site 1 made up 89.1% of the total collected Lu. longipalpis. This area is highly urbanized with a landscape appropriate for vector development represent by trees, bushes, and high grass as well as surrounding abandoned green spaces, similar characteristics of those suggested by Quintana et al. (2012)QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48., where they also associated high abundance of Lu. longipalpis at 100 meters around the sampling point with similar landscape. In Corrientes city urbanized area, the first VL human case was reported in March 2012 where our sampling site 1 was placed. This leads to thinking that this area could act as a hotspot for the disease, which is in accordance with several studies that showed the presence of Lu. longipalpis in areas with an urbanized structure (Quintana et al. 2012QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48., Gómez-Bravo et al. 2017GÓMEZ-BRAVO A, GERMAN A, ABRIL M, SCAVUZZO M & SALOMÓN OD. 2017. Spatial population dynamics and temporal analysis of the distribution of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in the city of Clorinda, Formosa, Argentina. Parasit Vectors 10: 352., Berrozpe et al. 2018BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98., Santini et al. 2015SANTINI MS, UTGÉS ME, BERROZPE P, MANTECA ACOSTA M, CASAS N, HEUER P & SALOMÓN OD. 2015. Lutzomyia longipalpis Presence and Abundance Distribution at Different Micro-spatial Scales in an Urban Scenario. PLoS Negl Trop Diseases 9(8): e0003951.). We found the highest abundance of Lu. longipalpis in urban areas (site 1) and less abundance in periurban areas as sites 3 and 5. Quintana et al. (2012)QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48. hypothesized that Lu. longipalpis populations of high and medium abundance in urban areas act like hotspots, as they are immersed in a matrix with low presence or absence of vector activity that act as sink populations. The results of the present study provide evidence that our site 1 could be acting as proposed by Quintana et al. (2012)QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48.. Lutzomyia longipalpis was not only the most abundant species, but also the most widely distributed in the study area. The latter point is important to consider because of the importance of multiple spots of transmission in the urban area. Nyssomyia neivai abundance has been associated with the presence of vegetation patches in a periurban area of the cities (Quintana et al. 2012QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48.) in accordance with places where we found the higher species abundance during the present study.
Even though sites 6 and 8, have the social conditions, domestic animals, chickens and pigs to support Phlebotomine sand flies development, the vector abundance was low throughout the sampling period. We do not found any explanation for this. We believe that other studies that include other environmental variables not considered in the present one, such as wind speed, should be taken into account.
The presence of avenue street lighting in sites 7 and 8 could also be a factor affecting the presence of Phlebotomine sand flies. As we see on the present study, the highest Phlebotomine species richness was observed where no water or even garbage service were available, considering also that this city sites have animal reservoirs presence (like pigs and chickens) with a high vegetation coverage, which is in accordance with Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98..
Lutzomyia longipalpis was found during the entire sampling period with high abundance during autumn and winter, which is in opposition with the findings of Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. who found high abundance during spring and summer seasons for the same city. One potential explanation for this discrepancy in findings is that our sampling was done monthly during the sampling period and Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. sampled one occasion during autumn because of operational reasons. The remainder of the seasons in Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. were sampled on two occasions during each season, which may be insufficient to represent the real seasonal variation of the city. Additionally, Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. begin their research immediately after the end of this study in Corrientes city, so the differences found could be due to changes in climatic conditions between seasons of the different years. In Brazil (Sobral, Ceará), Deane & Deane (1955)DEANE LM & DEANE MP. 1995. Leishmaniose visceral urbana (no cão e no homem) em Sobral, Ceará. O Hospital 47: 75-87. showed an association between sampling seasons and Lu. longipalpis, finding higher abundance during the rainy season. In our study area as we saw in results, two precipitation picks occurred, as we see one in autumn and the other in spring. Here we observed on this study the higher abundance of Lu. longipalpis in accordance with the autumn precipitation pick which could lead to vegetation growth, less insolation, higher soil humidity and thus better insect development.
Oliveira et al. (2008)OLIVEIRA AG, GALATI EA, FERNANDES CE, DORVAL ME & BRAZIL RP. 2008. Seasonal variation of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in endemic area of visceral leishmaniasis, Campo Grande, state of Mato Grosso do Sul, Brazil. Act Trop 105(1): 55-61. in Campo Grande, Mato Grosso do Sul, Brazil, found high Lu. longipalpis abundance during autumn and winter after a one or two-month lag in precipitation as we found in our study with best correlations at one-month time lag followed by two month time lags. Our study also provides evidence for a positive association not only of Lu. longipalpis with precipitation but also with relative humidity and a negative association with temperature. The models developed showed that humidity at a one-month time lag and EVI (vegetation) at a two month time lag had significant effects on Lu. longipalpis abundance. Satellite vegetation measured with Landsat 8 images by Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. found that extreme values of vegetation index correspond with lower vector abundance.
The negative association with temperature could be an indication of a maximum threshold tolerance of this vector and emphasizes the need for further studies on this point that could be very useful for decision makers. Berrozpe et al. (2018)BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98. found that extreme temperature values (measure as LST from Landsat 8 satellite) corresponded to lowest Lu. longipalpis abundance.
For our Mg. migonei models, only precipitation at a 2-month lag time was significant. In a temperate area of central Argentina, Ontivero et al. (2018)ONTIVERO IM, BERANEK MD, ROSA JR, LUDUEÑA-ALMEIDA FF & ALMIRÓN WR. 2018. Seasonal distribution of Phlebotominae sandfly in a vulnerable area for tegumentary leishmaniasis transmission in Córdoba, Argentina. Act Trop 178: 81-85. found a positive correlation of the abundance of this Phlebotomine sand flies with precipitation from 15 to 45-day lag time periods, but no correlation was found with mean temperature or relative humidity.
Migonemyia migonei abundance was higher in periurban and periurban-rural environments (Sites 2 and 3) with the presence of chickens and pigs. In Santiago del Estero, Mg. migonei was the only species found in all sampling sites with autochthonous VL cases reports and was the most abundant in households with a history of canine VL (Salomón et al. 2010SALOMÓN OD, QUINTANA MG, BEZZI G, MORÁN ML, BETBEDER E & VALDÉZ DV. 2010. Lutzomyia migonei as putative vector of visceral leishmaniasis in La Banda, Argentina. Act Trop 113: 84-87.), so these authors propose Mg. migonei as a putative vector in La Banda, Argentina. These findings are in accordance with the results of the present study, where the predominance of this species was in a household with 2 confirmed canine VL cases (personal communication, Ministerio de Salud de la Nación).
This species was also associated with periurban-rural transition environments and with domestic animals in Córdoba city, and was associated with the peri-domiciliary as well for Misiones (Ontivero et al. 2018ONTIVERO IM, BERANEK MD, ROSA JR, LUDUEÑA-ALMEIDA FF & ALMIRÓN WR. 2018. Seasonal distribution of Phlebotominae sandfly in a vulnerable area for tegumentary leishmaniasis transmission in Córdoba, Argentina. Act Trop 178: 81-85., Fernández 2012FERNÁNDEZ MS. 2012. Eco-epidemiología de vectores de Leishmania spp. en el noreste de la Argentina (Provincia de Misiones). Doctoral thesis, University of Buenos Aires, Argentina.). It is necessary to note that Mg. migonei have been found infected with with L. infantum in Brazil by “Leishmania DNA detection” (de Carvalho et al. 2010DE CARVALHO MR, VALENÇA HF, DA SILVA FJ, DE PITA-PEREIRA D, DE ARAÚJO PEREIRA T, BRITTO C, BRAZIL RP & FILHO SP. 2010. Natural Leishmania infantum infection in Migonemyia migonei (França, 1920) (Diptera:Psychodidae:Phleboto minae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Act Trop 116: 108-110.).
For Ny. neivai models relative humidity was the most important variable with one-month time lag, followed by precipitation frequency. This showed the effect of relative humidity during the previous month and precipitation frequency due to the importance of moisture for adult and immature survival. This could be related to soil moisture and the effects of environment for larval survival. These findings are in accordance with those of Salomón et al. (2008c).
Through the models developed here, we show the utility of knowing the relationship between meteorological and satellite environmental variables (with time lags) and the vector abundance and the importance of these relationships in anticipating and determining risk transition periods and the influence of the environment on those periods.
As final considerations, the study area showed a rich Phlebotomine sand flies of public health importance with a risk of pathogen transmission, suggesting the need for permanent surveillance studies on human and canine cases as well as additional entomological studies. Our findings emphasize the need to consider further studies on the effects of temperatures and maximum thresholds and the need of considering time lag effects in studies of these vectors. Such studies are important for developing predictive models for species distribution and abundance so that decision makers could use these findings to manage the vectors and prevent leishmaniasis transmission.
ACKNOWLEDGMENTS
This work was partially funded by Dirección Nacional de Control de Vectores, Ministerio de Salud de la Nación- Instituto de Medicina Regional. Universidad Nacional del Nordeste. We thank Ministerio de Salud de la Nación (National Health Ministry) nowadays Health Secretary for their technical support in the collecting specimens, especially to the technician Benitez César. ELE and MS are members of the Consejo de Investigaciones Científicas y Tecnológicas (CONICET) from Argentina. MEM is an assistant researcher at IIBYT, CONICET-Universidad Nacional de Córdoba (RHCD 288/2019). We thank Dr. Michael Robert from the department of mathematics and applied mathematics from Virginia Commonwealth University, Virginia, USA, for the editing and critical review of the manuscript.
REFERENCES
- ACARDI S, LIOTTA DJ, SANTINI MS, ROMAGOSA CM & SALOMÓN OD. 2010. Detection of Leishmania infantum in naturally infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis familiaris in Misiones, Argentina: First report of PCR-RFLP and a sequencing confirmation assay. Mem Inst Oswaldo Cruz 105(6): 796-799.
- AKAIKE H. 1974, A new look at the statistical model identification. IEEE Transactions on Automatic Control 19(6): 716-723.
- ARTUN O & KAVUR H. 2017. Investigation of the spatial distribution of sandfly species and cutaneous leishmaniasis risk factors by using geographical information system technologies in Karaisali district of Adana province, Turkey J Vector Borne Dis 54: 233-239.
- BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, TORRUSIO SE & SALOMÓN OD. 2018. Spatiotemporal dynamics of Lutzomyia longipalpis and macro-habitat characterization using satellite images in a leishmaniasis-endemic city in Argentina. Med Vet Entomol 33(1): 89-98.
- BERROZPE PE, LAMATTINA D, SANTINI MS, ARAUJO AV, UTGES ME & SALOMÓN OD. 2017. Environmental suitability for Lutzomyia longipalpis in a subtropical city with a recently established visceral leishmaniasis transmission cycle, Argentina. Mem Inst Oswaldo Cruz 112(10): 674-680.
- BORDA CE, REA MJF & ROSA JR. 2002. Urbanization of the human leishmaniasis in the Northeast of the Argentina. Entomol Vect 9(1): 56.
- BRUNIARD ED. 1978. El Gran Chaco Argentino. Ensayo de interpretación geográfica, GEOGRÁFICA 4. Facultad de Humanidades, UNNE, Resistencia, Chaco, Argentina.
- CHAO A & LEE S. 1992. Estimating the Number of Classes via Sample Coverage. J Amer Statist Assoc 87(417): 210-217.
- COLWELL RK. 2013. EstimateS, Version 9.1: Statistical Estimation of Species Richness and Shared Species from Samples.
- COLWELL RK & CODDINGTON JA. 1994. Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc Lond B Biol Sci 345(1311): 101-118.
- CÓRDOBA LANÚS E, LIZARRALDE DE GROSSO M, PIÑERO JE, VALLADARES B & SALOMÓN OD. 2006. Natural infection of Lutzomyia neivai with Leishmania spp. in northwestern argentina. Act Trop 98(1): 1-5.
- DE CARVALHO MR, VALENÇA HF, DA SILVA FJ, DE PITA-PEREIRA D, DE ARAÚJO PEREIRA T, BRITTO C, BRAZIL RP & FILHO SP. 2010. Natural Leishmania infantum infection in Migonemyia migonei (França, 1920) (Diptera:Psychodidae:Phleboto minae) the putative vector of visceral leishmaniasis in Pernambuco State, Brazil. Act Trop 116: 108-110.
- DEANE LM & DEANE MP. 1995. Leishmaniose visceral urbana (no cão e no homem) em Sobral, Ceará. O Hospital 47: 75-87.
- ESTALLO EL, BENÍTEZ EM, LANFRI MA, SCAVUZZO CM & ALMIRÓN WR. 2016. MODIS environmental data to assess chikungunya, dengue, and zika diseases through Aedes (Stegomyia) aegypti oviposition activity estimation. IEEE J. SelecTop. Appl Earth Observ Remote Sens 9(12): 5461-5466.
- ESTALLO EL, LUDUEÑA-ALMEIDA FF, INTROINI MV, ZAIDENBERG M & ALMIRÓN WR. 2015. Weather Variability Associated with Aedes (Stegomyia) aegypti (Dengue Vector) Oviposition Dynamics in Northwestern Argentina. PLoS ONE 10(5): e0127820.
- ESTALLO EL, SANGERMANO F, GRECH M, LUDUEÑA-ALMEIDA FF, FRÍAS-CÉSPEDES M, AINETE M, ALMIRÓN WR & LIVDAHL T. 2018. Modelling the distribution of the vector Aedes aegypti in a central Argentine city. Med Vet Entomol 32: 451-461.
- FERNÁNDEZ MS. 2012. Eco-epidemiología de vectores de Leishmania spp. en el noreste de la Argentina (Provincia de Misiones). Doctoral thesis, University of Buenos Aires, Argentina.
- GALATI E. 2003. Classificação de Phlebotominae, p.23-51. In: Rangel E and Lainson R (Eds). Flebotomíneos do Brasil. Fiocruz, Rio do Janeiro.
- GÓMEZ-BRAVO A, GERMAN A, ABRIL M, SCAVUZZO M & SALOMÓN OD. 2017. Spatial population dynamics and temporal analysis of the distribution of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in the city of Clorinda, Formosa, Argentina. Parasit Vectors 10: 352.
- HUETE A, DIDAN K, MIURA T, RODRIGUEZ EP, GAO X & FERREIRA LG. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens Environ 83: 195-213.
- JOST L. 2006. Entropy and diversity. Oikos 113(2): 363-375.
- LOCATELLI FM ET AL. 2014. The isolation and molecular characterization of Leishmania spp. from patients with American tegumentary leishmaniasis in northwest Argentina. Act Trop 131: 16-21.
- MARCO JD ET AL. 2005. Species assignation of Leishmania from human and canine American tegumentary leishmaniasis cases by multilocus enzyme electrophoresis in North Argentina. Am J Trop Med Hyg 72(5): 606-611.
- MARCO JD, UEZATO H, MIMORI T, BARROSO PA, KORENAGA M, NONAKA S, BASOMBRÍO MA, TARANTO NJ & HASHIGUCHI Y. 2006. Are cytochrome B gene sequencing and polymorphism-specific polymerase chain reaction as reliable as multilocus enzyme electrophoresis for identifying Leishmania spp. from Argentina?. Am J Trop Med Hyg 75(2): 256-260.
- MARCO JD ET AL. 2012. Polymorphism-specific PCR enhances the diagnostic performance of American tegumentary leishmaniasis and allows the rapid identification of Leishmania species from Argentina. BMC Infect Dis 15: 12:191.
- MOYA SL, GIULIANI MG, MANTECA ACOSTA M, SALOMÓN OD & LIOTTA DJ. 2015. First description of Migonemyia migonei (França) and Nyssomyia whitmani (Antunes & Coutinho) (Psychodidae: Phlebotominae) natural infected by Leishmania infantum in Argentina. Act Trop 152: 181-184.
- OLIVEIRA AG, GALATI EA, FERNANDES CE, DORVAL ME & BRAZIL RP. 2008. Seasonal variation of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in endemic area of visceral leishmaniasis, Campo Grande, state of Mato Grosso do Sul, Brazil. Act Trop 105(1): 55-61.
- ONTIVERO IM, BERANEK MD, ROSA JR, LUDUEÑA-ALMEIDA FF & ALMIRÓN WR. 2018. Seasonal distribution of Phlebotominae sandfly in a vulnerable area for tegumentary leishmaniasis transmission in Córdoba, Argentina. Act Trop 178: 81-85.
- QUINTANA M, SALOMÓN OD, GUERRA R, LIZARRALDE DE GROSSO M & FUENZALIDA A. 2012. Phlebotominae of epidemiological importance in cutaneous leishmaniasis in northwestern Argentina: risk maps and ecological niche models. Med Vet Entomol 27(1): 39-48.
- ROBERTS DR & HIS BP. 1979. An index of species abundance for use with mosquito surveillance data. Environ Entomol 8: 1007-1013.
- ROSA JR, BORDA CE, REA MJF & MOSQUEDA LA. 1999. Cría de especies de Lutzomyia de la provincia de Corrientes, Argentina en condiciones experimentales. Medicina (Buenos Aires). 59(Supl. III): 49.
- ROSA JR, PEREIRA DP, BRAZIL RP, FILHO JD, SALOMÓN OD & SZELAG E. 2012. Natural infection of cortelezzii complex (Diptera: Psychodidae: Phlebotominae) with Leishmania braziliensis in Chaco, Argentina. Act Trop 123(2): 128-131.
- SÁBIO PB, ANDRADE AJ & GALATI EAB. 2014. Assessment of the Taxonomic Status of Some Species Included in the Shannoni Complex, With the Description of a New Species of Psathyromyia (Diptera: Psychodidae: Phlebotominae). J Med Entomol 51: 331-341.
- SALOMÓN OD, MASTRANGELO AV, SANTINI MS, RUVINSKY S, ORDUNA T, SINAGRA A, LUNA C, RIARTE A, CASAS N & AMIOTTI P. 2012a. Leishmaniasis visceral: senderos que confluyen, se bifurcan. Salud Colectiva 8(Supl 1): S49-S63.
- SALOMÓN OD & ORELLANO PW. 2005. Lutzomyia longipalpis in Clorinda, Formosa province, an area of potential visceral leishmaniasis transmission in Argentina. Mem Inst Oswaldo Cruz 100: 475-476.
- SALOMÓN OD, ORELLANO PW, LAMFRI M, SCAVUZZO M, DRI L, FARACE MI & QUINTANA DO. 2006a. Phlebotominae spatial distribution asssociated with a focus of tegumentary leishmaniasis in Las Lomitas, Formosa, Argentina, 2002. Mem Inst Oswaldo Cruz 101(3): 295-299.
- SALOMÓN OD, QUINTANA MG, BEZZI G, MORÁN ML, BETBEDER E & VALDÉZ DV. 2010. Lutzomyia migonei as putative vector of visceral leishmaniasis in La Banda, Argentina. Act Trop 113: 84-87.
- SALOMÓN OD, QUINTANA MG, BRUNO MR, QUIRICONI RV & CABRAL V. 2009a. Visceral leishmaniasis in border areas: clustered distribution of phlebotomine sand flies in Clorinda, Argentina. Mem Inst Oswaldo Cruz 104(5): 801-804.
- SALOMÓN OD, QUINTANA MG, MASTRANGELO AV & FERNÁNDEZ MS. 2012b. Leishmaniasis and climate change—case study: Argentina. J Trop Med 2012: 601242.
- SALOMÓN OD, QUINTANA MG & ROSA JR. 2008a. Ecoepidemiología de la Leishmaniasis cutánea en Argentina. Salud i Ciencia 16: 514-520.
- SALOMÓN OD, QUINTANA MG & ZAIDENBERG M. 2008c. Urban distribution of Phlebotominae in a cutaneous leishmaniasis focus, Argentina. Mem Inst Oswaldo Cruz 103(3): 282-287.
- SALOMÓN OD, RAMOS LK, QUINTANA MG, ACARDI SA, SANTINI MS & SCHNEIDER A. 2009b. Distribución de vectores de Leishmaniasis visceral en la provincia de Corrientes, 2008. Medicina (Buenos Aires). 2009(69): 625-630.
- SALOMÓN OD, SINAGRA A, NEVOT MC, BARBERIAN G, PAULIN P, ESTEVEZ J, RIARTE A & ESTEVEZ J. 2008b. First visceral leishmaniasis focus in Argentina. Mem Inst Oswaldo Cruz 103: 109-111.
- SALOMÓN OD, SOSA-ESTANI S, RAMOS K, ORELLANO PW, SANGUESA G, FERNÁNDEZ G, SINAGRA A & RAPASCIOLLI G. 2006b. Tegumentary leishmaniasis outbreak in Bella Vista City, Corrientes, Argentina during 2003. Mem Inst Oswaldo Cruz 101(7): 767-774.
- SANTINI MS, FERNÁNDEZ MS, CAVIA R & SALOMÓN OD. 2017. Co-occurrence and seasonal and environmental distributions of the sandflies Lutzomyia longipalpis and Nyssomyia whitmani in the city of Puerto Iguazú, northeastern Argentina. Med Vet Entomol 32(2): 197-205.
- SANTINI MS, FERNÁNDEZ MS, PÉREZ AA, SANDOVAL AE & SALOMÓN OD. 2012. Lutzomyia longipalpis abundance in the city of Posadas, northeastern Argentina: variations at different spatial scales. Mem Inst Oswaldo Cruz 107(6): 767-771.
- SANTINI MS, UTGÉS ME, BERROZPE P, MANTECA ACOSTA M, CASAS N, HEUER P & SALOMÓN OD. 2015. Lutzomyia longipalpis Presence and Abundance Distribution at Different Micro-spatial Scales in an Urban Scenario. PLoS Negl Trop Diseases 9(8): e0003951.
- SARAIVA L, FILHO JDA, DE OLIVEIRA SILVA S, DE ANDRADE ASR & MELO MN. 2010. The molecular detection of different Leishmania species within sand flies from a cutaneous and visceral leishmaniasis sympatric area in Southeastern Brazil. Mem Inst Oswaldo Cruz 105: 1033-1039.
- SECRETARÍA DE DESARROLLO SUSTENTABLE Y POLÍTICA AMBIENTAL. 1999. Estudio integral de la región del parque chaqueño. Segunda edición. Ministerio de Desarrollo Social y Medio Ambiente, Buenos Aires, Argentina.
- SZELAG EA, ROSA JR, GALATI EAB, ANDRADE FILHO JD & SALOMÓN OD. 2018. Considerations on the Species Complex of the Cortelezzii series (Diptera: Psychodidae) and Description of Evandromyia chacuensis sp. nov., a New Phlebotomine Species of the Chaco Region, Argentina. J Med Entomolartun 55(4): 902-909.
Publication Dates
-
Publication in this collection
29 Oct 2021 -
Date of issue
2021
History
-
Received
21 Oct 2019 -
Accepted
31 Mar 2020