Abstract
The objective was to apply a simplex lattice design to determine the properties of polyvinyl alcohol-graft-lactic acid (PVA-g-LA) with different values for two independent variables: curing time (X1) and LA ratio (X2). Each independent variable was varied among three levels: –1, 0, and +1. Three coded levels were 120 min, 150 min, and 180 min for X1 and 2.5 g, 5 g, and 7.5 g for X2. Dependent variables of swelling behavior in various swelling media and thermal analysis parameters were monitored. The optimal formulation was selected based on the desirability value. The prediction was accurate, showing a low value of percent error. The morphology of the selected formulation with the highest desirability value showed a compact and dense film. Propranolol hydrochloride used as a model drug, was loaded into PVA-g-LA film. The propranolol hydrochloride content was 4.19 ± 1.05 mg/cm2. The cumulative release and permeation of drug were 61.94 ± 8.03% and 59.96 ± 6.61%, respectively. Thus, response surface methodology can be used as a tool to predict or optimize the process parameters for PVA-g-LA transdermal films in an accurate manner. PVA-g-LA could control the release and permeation of drug from the film layer.
Key words
Film; optimization; transdermal film; polyvinyl alcohol-graft-lactic acid
INTRODUCTION
Hydrogels are hydrophilic polymeric cross-linked network structures that can absorb and retain a significant amount of water or biological fluid, usually to equilibrium. The hydrogel structure is created by hydrophilic groups or domains in a polymeric cross-linked network upon hydration in an aqueous environment. Hydrogels possess a degree of flexibility similar to that of natural tissue due to their significant water content. They can be broadly classified into two categories. (I) Permanent or chemical hydrogels are covalently cross-linked networks that replaced the hydrogen bond, whose equilibrium swelling state depends on the polymer and water interaction and the cross-linking density. (II) Reversible or physical hydrogel, physically cross-linked hydrogels are prevented by physical interactions which exist between different more than two polymer chains by molecular entanglements with secondary forces including ionic, hydrogen bonding, or hydrophobic interactions. These interactions are reversible and can be disrupted by changes in physical and environmental conditions (Gulrez & Al-Assaf 2011GULREZ SKH & AL-ASSAF S. 2011. Hydrogels: Methods of Preparation, Characterisation and Applications. In: Progress in Molecular and Environmental Bioengineering - From Analysis and Modeling to Technology Applications. Croatia: InTech., Hennink & van Nostrum 2002HENNINK WE & VAN NOSTRUM CF. 2002. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 54: 13-36., Rosiak & Yoshii 1999ROSIAK JM & YOSHII F. 1999. Hydrogels and their medical applications. Nucl Instrum Meth B 151: 56-64.). Because hydrogels have high water absorption capacity and biocompatibility, they have been produced and applied in various fields such as tissue engineering (Zheng Shu et al. 2004ZHENG SHU X, LIU Y, PALUMBO FS, LUO Y & PRESTWICH GD. 2004. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 25: 1339-1348.), pharmaceutical (Gwon et al. 2010GWON H-J, LIM Y-M, CHANG H-N & NHO Y-C. 2010. Reduction of postsurgical adhesion formation with CM-chitosan hydrogel barriers prepared by using γ-irradiation. J Appl Polym Sci 116: 3682-3687., Kumar et al. 2008KUMAR S, SASMAL D & PAL S. 2008. Rheological characterization and drug release studies of gum exudates of Terminalia catappa Linn. AAPS PharmSciTech 9: 885-890.), and biomedical (Hoffman 2002HOFFMAN AS. 2002. Hydrogels for biomedical applications. Adv Drug Deliv Rev 54: 3-12.), including use in drug delivery (Zhou et al. 2008ZHOU HY, CHEN XG, KONG M, LIU CS, CHA DS & KENNEDY JF. 2008. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr Polym 73: 265-273.), transdermal systems (An et al. 2003AN N-M, KIM D-D, SHIN Y-H & LEE C-H. 2003. Development of a novel soft hydrogel for the transdermal delivery of testosterone. Drug Dev Ind Pharm 29: 99-105., Fang et al. 2009FANG J-Y, HUNG C-F, CHI C-H & CHEN C-C. 2009. Transdermal permeation of selegiline from hydrogel-membrane drug delivery systems. Int J Pharm 380: 33-39.), and dental applications (Cavalcanti et al. 2013CAVALCANTI BN, ZEITLIN BD & NÖR JE. 2013. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater 29: 97-102.).
Among hydrogels, polyvinylalcohol (PVA) is a water-soluble synthetic polyhydroxy polymer that is hydrolyzed from polyvinyl acetate. PVA has low toxicity and is biodegradable (Alexy et al. 2003ALEXY P, BAKOŠ D, CRKOŇOVÁ G, KRAMÁROVÁ Z, HOFFMANN J, JULINOVÁ M, CHIELLINI E & CINELLI P. 2003. Poly(vinyl alcohol)–collagen hydrolysate thermoplastic blends: II. Water penetration and biodegradability of melt extruded films. Polym Test 22: 811-818.). PVA can be prepared as a hydrogel for controlled release systems. For example, PVA hydrogels prepared by a low-temperature crystallization method containing bunitrolol-HCl can be used as transdermal delivery systems. The active drug is released from the PVA hydrogel following Fickian diffusion or the Higuchi model; the drug release, profile versus square root of release (Morimoto et al. 1990MORIMOTO K, NAGAYASU A, FUKANOKI S, MORISAKS K, HYON S-H & IKADA Y. 1990. Evaluation of polyvinyl alcohol hydrogel as sustained-release vehicle for transdermal sytem of bunitrolol-HCl. Drug Dev Ind Pharm 16: 13-29.). PVA can be cross-linked with succinyl, adipoyl, or sebacoyl chloride to obtain hydrogel-forming polymers and used in colon-specific drug-delivery systems for diclofenac sodium, propranolol hydrochloride, and vitamin B6 hydrochloride as hydrophilic model drugs (Orienti et al. 2001ORIENTI I, TRERÉ R & ZECCHI V. 2001. Hydrogels formed by cross-linked polyvinylalcohol as colon-specific drug delivery systems. Drug Dev Ind Pharm 27: 877-884.). PVA hydrogels with different degrees of cross-linking can also be used topically for transdermal delivery of propranolol hydrochloride (Orienti et al. 2000ORIENTI I, DI PIETRA A, LUPPI B & ZECCHI V. 2000. Crosslinked polyvinylalcohol hydrogels as vehicles for hydrophilic drugs. Archiv der Pharmazie 333: 421-424.). In addition, PVA hydrogels in which cross-linking is induced by light allow in situ polymerization for minimally invasive implantation methods (Schmedlen et al. 2002SCHMEDLEN RH, MASTERS KS & WEST JL. 2002. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 23: 4325-4332.). Therefore, PVA is favored as a hydrogel component for controlled-release drug-delivery systems.
Propranolol hydrochloride has a low molecular weight of 295.81 g/mol. It is a nonselective β-adrenergic blocking agent widely used for treatment of hypertension, angina pectoris, and many other cardiovascular disorders. Although it is well absorbed in the gastrointestinal tract, its bioavailability is low (15–23%) as a result of extensive first-pass metabolism (Cid et al. 1986CID E, MELLA F, LUCCHINI L, CÁRCAMO M & MONASTERIO J. 1986. Plasma concentrations and bioavailability of propranolol by oral, rectal and intravenous administration in man. Biopharm Drug Dispos 7: 559-566., Patel et al. 2007PATEL V, PRAJAPATI B & PATEL M. 2007. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS Pharm Sci Tech 8: E147-E154.). Administration via the buccal route can bypass the hepatic first-pass effect and improve the bioavailability of propranolol hydrochloride, such that the dose of propranolol hydrochloride can be reduced (Amores et al. 2014AMORES S, DOMENECH J, COLOM H, CALPENA AC, CLARES B, GIMENO Á & LAUROBA J. 2014. An improved cryopreservation method for porcine buccal mucosa in ex vivo drug permeation studies using Franz diffusion cells. Eur J Pharm Sci 60: 49-54., Patel et al. 2007PATEL V, PRAJAPATI B & PATEL M. 2007. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS Pharm Sci Tech 8: E147-E154.). Propranolol hydrochloride prepared as a hydrogel is non-toxic and allows for high levels of drug loading. It has attracted considerable attention as a medium for controlled release of active drug and has been employed extensively in hydrophilic matrix hydrogels (Cerchiara et al. 2002CERCHIARA T, LUPPI B, BIGUCCI F, ORIENTI I & ZECCHI V. 2002. Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drugs. J Pharm Pharmacol 54: 1453-1459., Ganga et al. 1992GANGA S, SINGH PN & SINGH J. 1992. Formulation, in-vitro release and therapeutic effect of hydrogels based controlled release tablets of propranolol hydrochloride. Drug Dev Ind Pharm 18: 2049-2066., Gil et al. 2006GIL EC, COLARTE AI, BATAILLE B, PEDRAZ JL, RODRÍGUEZ F & HEINÄMÄKI J. 2006. Development and optimization of a novel sustained-release dextran tablet formulation for propranolol hydrochloride. Int J Pharm 317: 32-39., Inoue et al. 1997INOUE T, CHEN G, NAKAMAE K & HOFFMAN AS. 1997. A hydrophobically-modified bioadhesive polyelectrolyte hydrogel for drug delivery. J Controlled Release 49: 167-176.).
In a previous report, we presented the preparation and characterization of PVA-graft-lactic acid (PVA-g-LA). We investigated the influence of different ratios of PVA and LA (1:0.5, 1:1, 1:1.5, and 1:2), different curing times (120 min, 150 min, and 180 min) and different curing temperatures (100°C and 120°C) (Suksaeree & Chuchote 2018SUKSAEREE J & CHUCHOTE C. 2018. Swelling properties of propranolol HCl-loaded polyvinyl alcohol-graft-lactic acid hydrogel films. MATEC Web Conf 237: 02004., Suksaeree et al. 2016SUKSAEREE J, LUPRASONG C & MONTON C. 2016. Swelling behavior of polyvinyl alcohol and lactic acid hydrogel films. Asian J Pharm Sci 11: 102-103., 2015). In the present work, the swelling behavior and thermal analysis parameters of PVA-g-LA were summarized, predicted, and optimized by response surface methodology using Design-Expert® version 11 (Stat-Ease, Inc, USA). Thus, the aim of this work was a preparation of PVA-g-LA using two independent variables: curing time (X1) and LA ratio (X2). Seven dependent variables of swelling and three dependent variables of thermal analysis parameters were monitored. The best formula resulting from optimization of the process parameters was studied for possible use as a transdermal film, mixed with propranolol hydrochloride as a model drug. The in vitro release and permeation of the drug from the film were studied, and the kinetics including Zero-order, First-order, and Higuchi models were calculated.
MATERIALS AND METHODS
Materials
PVA (Mw = ˜195,000 g/mol) and 80% L-(+)-lactic acid (LA) were purchased from Sigma-Aldrich, USA. Propranolol hydrochloride (BP grade) was purchased from Chang Zhou Yabang Pharmaceutical Co., Ltd., China. Ultrapure water was produced by Puris, Expe-UP water system (model: Expe-UP series, Korea) to stringent specifications of 18.2 MΩ∙cm at 25°C of resistivity, 5–10 ppb of total organic carbon (TOC), <0.05 ppb of inorganics, and <1 CFU/mL of bacteria.
PVA-g-LA film preparation
PVA pellets (5 g) were dissolved in ultrapure water by heating in a water bath at 70 ± 2°C with continuous stirring. The clear PVA solution was cooled at ambient temperature and adjusted to 100 g with ultrapure water. LA solution was slowly added into the PVA solution with the help of a mechanical stirrer for 60 min. Thirty grams of the mixture solution was weighed into a petri dish and cured in an oven for different curing times (120 min, 150 min, and 180 min) at 120°C.
Prediction of swelling behavior and thermal analysis parameters by response surface methodology
Response surface methodology was applied to predict swelling behavior in various swelling media as well as thermal analysis parameters. Two independent variables were investigated: curing time (X1) and LA ratio (X2). Three levels were investigated for each independent variable: –1, 0, and +1. The three levels were 120 min, 150 min, and 180 min for X1 and 2.5 g, 5 g, and 7.5 g for X2 (Table I). Seven dependent variables of swelling and three dependent variables of thermal analysis parameters were monitored. The coded dependent variables of swelling (Y1 to Y7) were moisture content; swelling behavior at pH 2, pH 4, pH 7, and pH 10; and swelling behavior in NaCl and KCl solutions, respectively. The coded dependent variables of thermal analysis parameters (Y8 to Y10) were glass transition temperature (Tg), melting temperature (Tm), and decomposition temperature (Td), respectively. The prediction was performed using Design-Expert® version 11 (Stat-Ease, Inc, USA). Response surfaces for each model condition were produced to predict each dependent variable. The actual equations for the prediction of each dependent variable were also reported. The optimal formulation was selected based on the desirability value. The criteria for selecting the optimal formulation were based on the maximized moisture content and maximized swelling at pH 7. The three formulations with the highest desirability value were prepared and evaluated again. The data obtained from the experiments were compared to the predicted values and the percent errors of the prediction were calculated and reported.
Swelling study of the PVA-g-LA films
The swelling behavior of PVA-g-LA samples in solutions with different pH levels or chloride salts were determined. We used 1 mol/L sodium hydroxide and 1 mol/L hydrochloric acid aqueous solution in distilled water to adjust pH to 2, 4, 7, and 10. Saline solutions of 10 mmol/L NaCl and 10 mmol/L KCl were prepared. PVA-g-LA samples were prepared and immersed in swelling media for different time intervals. Once they reached swelling equilibrium, the PVA-g-LA samples were weighed and the difference between the final and initial weight was calculated (W2 – W1) and expressed as a percentage of the initial weight (W1).
Moisture content measurement
PVA-g-LA films (1.0–1.5 g) were placed on an aluminum pan and heated at 120°C. The percentage of moisture content was measured by a moisture analyzer (model: MAC 50/NH, Poland). The percentage of moisture content was calculated as the difference between the initial weight and the dry weight (Winitial – Wdry) compared to the initial weight (Winitial).
Differential scanning calorimetry (DSC) determination
A DSC instrument was used to determine the glass transition temperature (Tg), melting temperature (Tm), and degradation temperature (Td) of the PVA-g-LA samples. Each sample was weighed in a DSC pan (~5–10 mg) and then hermetically sealed. The sample was run from 25°C to 400°C under a liquid nitrogen atmosphere with a heating rate of 10°C/min.
Scanning electron microscopy (SEM) study
The surface morphology of the PVA-g-LA samples was examined by SEM (model: JSM-5800 LV, JEOL, Japan) with high vacuum and high voltage of 20 kV and using an Everhart-Thornley detector. Each sample was immersed in liquid nitrogen and then cut in half. The sample was placed onto a copper stub coated with gold in a sputter coater.
Loading of PVA-g-LA film with propranolol hydrochloride
PVA pellets (5 g) were dissolved in ultrapure water by heating in a water bath at 70±2°C with continuous stirring. The clear PVA solution was cooled at ambient temperature and adjusted to 100 g with ultrapure water. LA solution was slowly added to the PVA solution with the help of a mechanical stirrer for 60 min. Propranolol hydrochloride was subsequently mixed into the homogeneous dispersion by slow stirring with a magnetic stirrer. The mixture solution (about 30 g) was weighed into a petri dish and cured in a hot air oven.
Weight measurement of PVA-g-LA film loaded with propranolol hydrochloride
The 1 cm × 1 cm square size of the film sample was weighed by analytical balance. The results were recorded in five replicates with the obtained mean result.
Thickness measurement of PVA-g-LA film loaded with propranolol hydrochloride
The 1 cm × 1 cm square size of the film sample had measured the thickness using a micrometer. The results were recorded in five replicates with the obtained mean result.
Surface pH measurement of PVA-g-LA film loaded with propranolol hydrochloride
The 1 cm × 1 cm square size of the film sample was initially contacted with distilled water 1 mL in glass tubes. The excess distilled water was removed. The pH of the film at the surface area was determined by pH meter and maintained the electrode on the wetted surface of the film to equilibrate for 1 min. The results were recorded in five replicates with the obtained mean result.
Drug determination
Squares measuring 1 cm x 1 cm were cut from five different sites of the prepared drug-containing film and dissolved in 20 mL isotonic phosphate buffer solution having pH 7.4 and containing 5% (v/v) methanol. The solution was vigorously shaken for 12 h, sonicated for 15 min, and centrifuged at 5000 rpm for 30 min. The solution was filtered through number 42 Whatman filter paper, and 1 ml of the filtrate was placed in a test tube and diluted with the same solvent. An ultraviolet (UV)–visible spectrophotometer was used to determine drug content at λmax 290 nm. The placebo film was taken as a blank solution.
In vitro release of propranolol hydrochloride from PVA-g-LA film
For the in vitro release study, we used a modified Franz-type diffusion cell apparatus. The effective diffusion area of the Franz diffusion cell was 1.77 cm2. The receptor was filled with 12 ml isotonic phosphate buffer solution (pH 7.4) as the diffusion medium. The Franz diffusion cell was thermoregulated with a water jacket at 37 ± 0.5°C and constantly stirred at 600 rpm with a magnetic stirrer. A cellulose membrane molecular cut-off of 3500 was used as a partition between the donor and receptor compartments. PVA-g-LA film loaded with propranolol hydrochloride were cut and placed directly on a cellulose membrane. At sampling time, one mL of receptor medium was withdrawn and immediately replaced with an equal volume of fresh receptor medium to maintain the sink condition. After suitable dilution, the samples were analyzed for drug content using a UV spectrophotometer at λmax 290 nm. The in vitro study was carried out for 24 hours. The in vitro experiments were performed in triplicate.
In vitro permeation of propranolol hydrochloride from PVA-g-LA film
For the in vitro permeation study, we used a modified Franz-type diffusion cell apparatus. The effective diffusion area of the Franz diffusion cell was 1.77 cm2. The receptor was filled with 12 ml isotonic phosphate buffer solution (pH 7.4) as the diffusion medium. The Franz diffusion cell was thermoregulated with a water jacket at 37 ± 0.5°C and constantly stirred at 600 rpm with a magnetic stirrer. The newborn pigs of 1.4 to 1.8 kg weight that had died by natural causes shortly after birth were freshly purchased from a local pig farm in Thailand. The full thickness of flank pig skin was excised, epidermal hairs were surgically removed, and subcutaneous fat and other extraneous tissues were trimmed with a scalpel, cleaned, dried by blotting, wrapped with aluminum foil and stored frozen. Before the skin permeation experiments, the isolated pig skins, which varied from 100 to 130 µm in thickness, were soaked overnight in receptor medium and mounted on the modified Franz-type diffusion cell with the epidermis facing upward on the donor compartment. PVA-g-LA film loaded with propranolol hydrochloride were cut and placed directly on a pig skin. At sampling time, one mL of receptor medium was withdrawn and immediately replaced with an equal volume of fresh receptor medium to maintain the sink condition. After suitable dilution, the samples were analyzed for drug content using a UV spectrophotometer at λmax 290 nm. The in vitro study was carried out for 24 hours. The in vitro experiments were performed in triplicate.
RESULTS AND DISCUSSION
Prediction of swelling behavior and thermal analysis parameters by response surface methodology
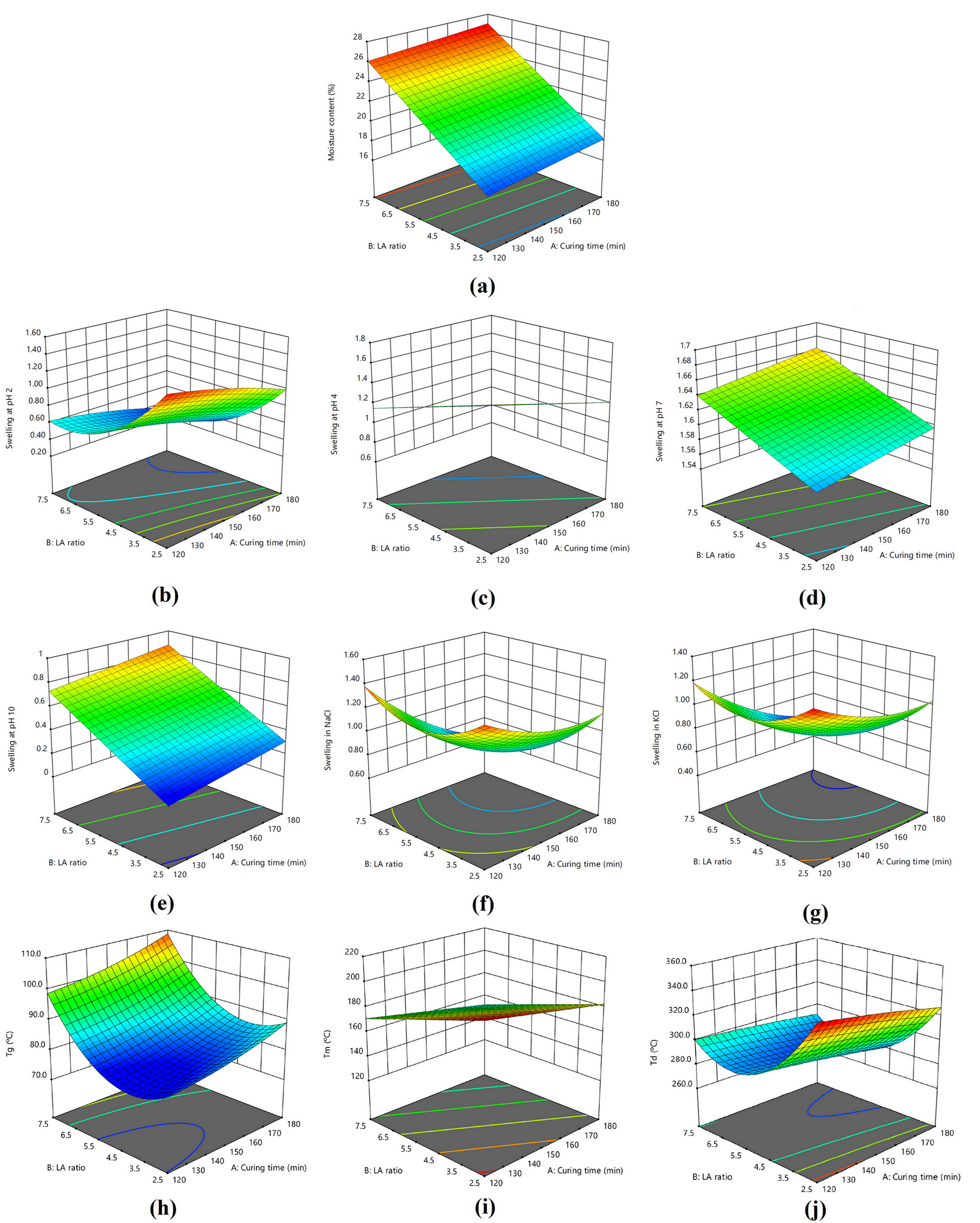
A curing time of 120 min with an LA ratio of 2.5 (Run 1) produced samples with the lowest values for moisture content (Y1) and swelling at pH 10 (Y5), while a curing time of 180 min with an LA ratio of 7.5 (Run 9) produced the highest values for these variables (Table I). Swelling at pH 2 (Y2) and in KCl (Y7) showed the opposite response to curing time, reaching their highest values in Run 1 and lowest values in Run 9. Swelling at pH 4 (Y3), at pH 7 (Y4), and in NaCl (Y6) reached their highest values in Run 1, Run 6, and Run 3, respectively, and lowest values in Run 6, Run 4, and Run 9, respectively.
Figure 1a shows the response surfaces to predict the moisture content. High LA ratio gave high values for moisture content but curing time did not affect moisture content. For swelling at pH 2 (Y2) (Figure 1b), in NaCl (Y6) (Figure 1f), and in KCl (Y7) (Figure 1g), the response surfaces were saddle-shaped: low values were found with long curing time and high LA ratio, while high values were found with short curing time and low LA ratio. Swelling at pH 4 was high with short curing time and low LA ratio but low with long curing time and high LA ratio (Figure 1c). Conversely, swelling at pH 7 and at pH 10 was low with short curing time and low LA ratio but high with long curing time and high LA ratio (Figure 1d and 1e).
Response surface of the model conditions of (a) moisture content and swelling in different solutions: (b) pH 2, (c) pH 4, (d) pH 7, (e) pH 10, (f) NaCl, and (g) KCl; and (h) Tg, (i) Tm, and (j) Td.
The actual equations used to predict each dependent variable related to swelling behavior are shown below. The equations for Y1, Y3, Y4, and Y5 were fitted to the linear mathematic model, while the other equations were fitted to the quadratic mathematical model (equations 1–7).
For thermal analysis parameters, Run 1 gave the lowest Tg value while Run 9 gave the highest Tg value, which seemed to relate with the moisture content (Y1). The highest Tm and Td values were found in Run 1, while the lowest Tm and Td values was found in Run 9 and Run 8, respectively. Figure 1h shows the response surface to predict the Tg value. It was low with short curing time and low to medium LA ratio but high with long curing time and high LA ratio. Figure 1i shows the response surface to predict the Tm value. It was low with long curing time and high LA ratio but high with short curing time and low LA ratio. Figure 1j shows the response surface to predict the Td value. It was low with long curing time and medium LA ratio but high with short curing time and low LA ratio.
The actual equations used to predict each dependent variable related to thermal properties are shown below. The equations for Y8 and Y10 were fitted to the quadratic mathematical model, while the equation for Y9 was fitted to the linear mathematic model (equations 8–10).
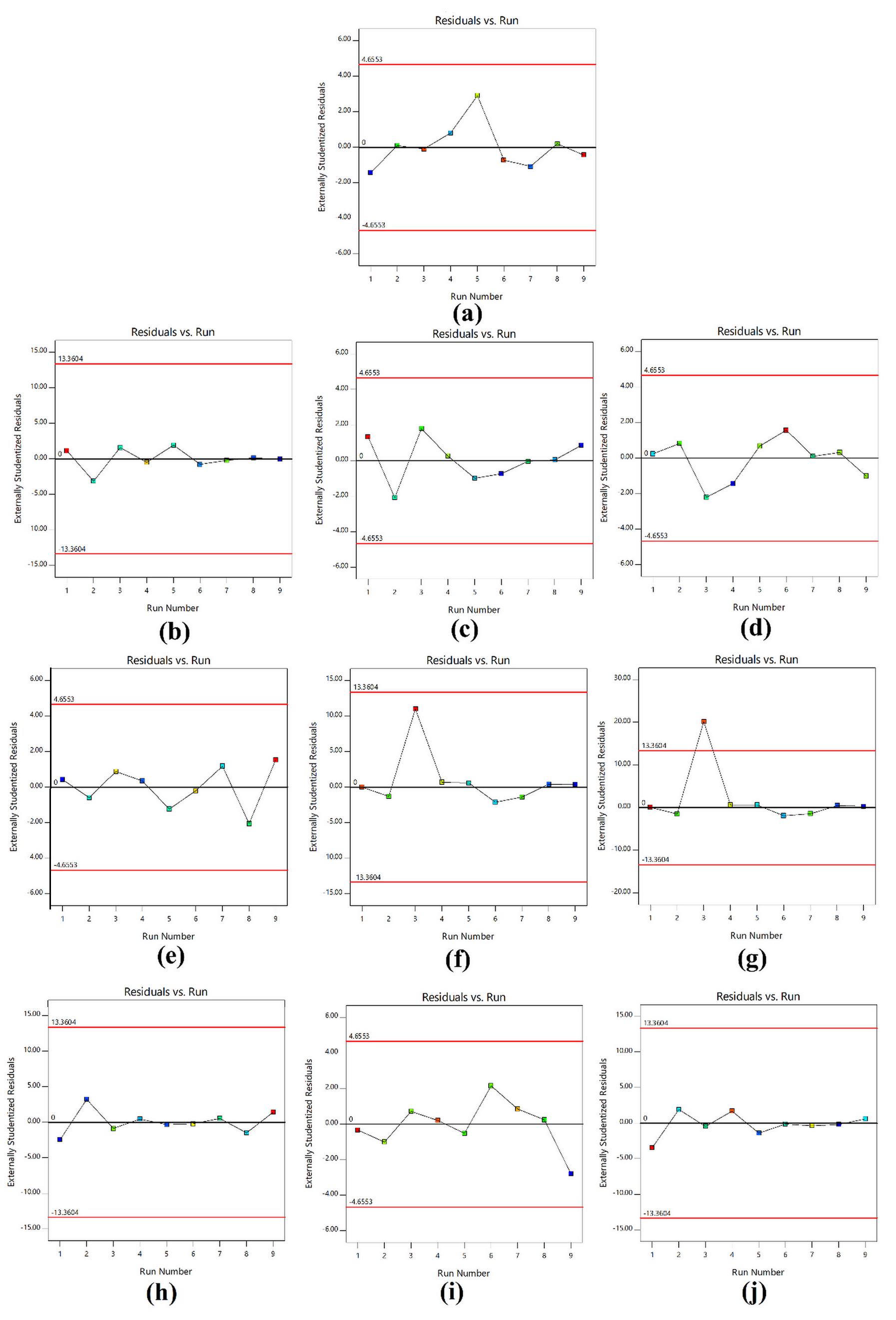
The predicted values from the quadratic mathematical model are summarized in Table II. The linearity plots of predicted value versus actual value of model conditions of moisture content; swelling in different solutions (pH 2, pH 4, pH 7, pH 10, NaCl, and KCl); and Tg, Tm, and Td are shown in Figure 2. All linearity plots had good correlation in the range of 0.875–0.902. The distributions between the internally studentized residuals and the run numbers of model conditions of moisture content; swelling in different solutions (pH 2, pH 4, pH 7, pH 10, NaCl, and KCl); and Tg, Tm, and Td are shown in Figure 3. The data distributions of all results ranged between the red borderlines within the 95% confidence interval. Thus, the prediction results from the Design-Expert® program had precision and reliability.
Predicted versus actual plots of model conditions of (a) moisture content; swelling in different solutions: (b) pH 2, (c) pH 4, (d) pH 7, (e) pH 10, (f) NaCl, and (g) KCl; and (h) Tg, (i) Tm, and (j) Td.
Residuals versus run plots of model conditions of (a) moisture content; swelling in different solutions: (b) pH 2, (c) pH 4, (d) pH 7, (e) pH 10, (f) NaCl, and (g) KCl; and (h) Tg, (i) Tm, and (j) Td.
The three formulations with the highest desirability values were prepared and evaluate again. The curing time was fixed at 180 min, while the LA ratio was slightly varied. The moisture content, swelling, and thermal properties from these experiments are shown in Table III. The percent error of the prediction is shown in Table IV. Many publications reported the Tg and Tm of PVA to be 85–88°C and 209–226°C, respectively, and the degradation to be 230°C (Don et al. 2006DON T-M, KING C-F, CHIU W-Y & PENG C-A. 2006. Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr Polym 63: 331-339., Guirguis & Moselhey 2012GUIRGUIS O & MOSELHEY M. 2012. Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat Sci 4: 57-67., Raju et al. 2007RAJU CL, RAO JL, REDDY BCV & VEERA BRAHMAM K. 2007. Thermal and IR studies on copper doped polyvinyl alcohol. Bull Mater Sci 30: 215-218.). The prepared PVA-g-LA films had higher Tg, Tm, and Td values due to the increasing hydrocarbon chain compared to pure PVA. This suggested appreciable grafting attributable to physical and/or hydrogen bonding that increased the number of intramolecular interactions (Ding et al. 2011DING J, CHEN S-C, WANG X-L & WANG Y-Z. 2011. Preparation and rheological behaviors of thermoplastic poly(vinyl alcohol) modified by lactic acid. Ind Eng Chem Res 50: 9123-9130., Suksaeree et al. 2015SUKSAEREE J, LUPRASONG C, MONTON C, CHAROENCHAI L & PICHAYAKORN W. 2015. An eco-friendly synthesis of modified poly(vinyl alcohol)-graft-lactic acid by curing method. J Therm Anal Calorim 120: 929-936.). The results showed that the prediction of swelling behavior and thermal analysis parameters by response surface methodology was accurate due to the low value of percent error. In summary, the response surface methodology could be used as a tool to predict or optimize the process parameters for PVA-g-LA films in an accurate manner.
Scanning electron microscopy (SEM) study
The surface morphology of the three formulations (F1-F3) with the highest desirability values is shown in Figure 4. The films were compact and dense and had a greater strength interaction from esterification of hydroxyl groups of PVA and carboxyl groups of LA, leading to a decrease in the number of hydroxyl groups and the introduction of hydrophobic lactate (Suksaeree et al. 2015SUKSAEREE J, LUPRASONG C, MONTON C, CHAROENCHAI L & PICHAYAKORN W. 2015. An eco-friendly synthesis of modified poly(vinyl alcohol)-graft-lactic acid by curing method. J Therm Anal Calorim 120: 929-936., Xiao & Yang 2006XIAO C & YANG M. 2006. Controlled preparation of physical cross-linked starch-g-PVA hydrogel. Carbohydr Polym 64: 37-40.).
Loading of PVA-g-LA film with propranolol hydrochloride
In a preliminary study of the possibility of using PVA-g-LA film prepared by this technique as a transdermal film, propranolol hydrochloride was selected as a model drug and loaded into PVA-g-LA film (F1) so we could evaluate drug loading and in vitro drug release. A schematic representation of the possible reactions of propranolol hydrochloride–loaded PVA-g-LA film via thermal curing is shown in Figure 5a. The propranolol hydrochloride–loaded PVA-g-LA film was transparent in appearance. Tests were done to check the uniformity of thickness and weight. The thickness of the propranolol hydrochloride–loaded PVA-g-LA film was measured by digital Vernier calipers with the least count of 0.001 mm. To assess uniformity, the thickness was measured at five different sites, and the average of five readings with standard deviation was found to be 292 ± 32 µm. Squares of propranolol hydrochloride–loaded PVA-g-LA film measuring 1 cm x 1 cm were cut from five different sites and weighed on electronic balance to calculate the weight variation. It was found to be 86 ± 2.1 mg. The surface pH of the propranolol hydrochloride–loaded PVA-g-LA film was determined. Squares of propranolol hydrochloride–loaded PVA-g-LA film measuring 1 cm x 1 cm were allowed to swell by placing them in 1 mL distilled water in glass tubes for 1 hour. The surface pH was then noted by bringing a combined glass electrode near the surface of the film and allowing it to equilibrate for 1 min. The pH of propranolol hydrochloride–loaded PVA-g-LA film was in the range of 5.1–6.3, which is suitable for application to the skin. The content of propranolol hydrochloride in the PVA-g-LA film was 4.19 ± 1.05 mg/cm2. The in vitro release of propranolol hydrochloride from PVA-g-LA film is shown in Figure 5b. The cumulative release of propranolol hydrochloride was 2.60 ± 0.34 mg/cm2 (61.94 ± 8.03%) after 24 hours. The patterns of in vitro release of propranolol hydrochloride revealed low drug release. This might be due to the greater strength of the interaction with PVA-g-LA film, causing the drug to diffuse more slowly into the receptor medium. The in vitro skin permeation of the drug is shown in Figure 5c. The cumulative permeation of propranolol hydrochloride was 2.51 ± 0.28 mg/cm2 (59.96 ± 6.61%) after 24 hours. The patterns of in vitro permeation of propranolol hydrochloride are related to the in vitro study. In the future, this could be solved by mixing the film with plasticizer to decrease its flexibility and a penetration enhancer to increase drug release and permeation.
(a) Schematic representation of the possible reactions of propranolol hydrochloride-loaded PVA-g-LA film via thermally curing method, (b) in vitro release of propranolol hydrochloride from PVA-g-LA film (F1), and (c) in vitro permeation of propranolol hydrochloride from PVA-g-LA film (F1).
The behaviors of propranolol hydrochloride release and permeation from the film were described by kinetics models including Zero-order, First-order, and Higuchi models. These were calculated as following equations 11-13.
Where Q was the amount of drug release in time t, and Q0 was the amount of initial drug.
The drug’s in vitro release profile did not fit either zero-order (y = 0.0993x + 0.5523, R² = 0.8476) or first-order kinetics (y = -0.0166x + 0.5677, R² = 0.9399) but fitted to the Higuchi model (y = 0.5491x + 0.0276, R² = 0.9906). The drug’s in vitro permeation profile did not fit either first-order kinetic (y = -0.0161x + 0.6101, R² =0.9718) or Higuchi model (y = 0.5215x - 0.2313, R² = 0.9754) but fitted to the zero-order kinetic (y = 0.1017x + 0.2219, R² = 0.9979). Therefore, the behavior of drug release from PVA-g-LA film could be described by a diffusion-dominated mechanism of Higuchi’s model while the behavior of drug permeation was released at constant rate at a function of time, it would be possible to maintain the plasma drug concentration constant (Ritger & Peppas 1987RITGER PL & PEPPAS NA. 1987. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5: 37-42., Siepmann & Peppas 2001SIEPMANN J & PEPPAS NA. 2001. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 48: 139-157., Ubaidulla et al. 2007UBAIDULLA U, REDDY MVS, RUCKMANI K, AHMAD FJ & KHAR RK. 2007. Transdermal therapeutic system of carvedilol: Effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS PharmSciTech 8: E13-E20.).
CONCLUSION
A simplex lattice design was used to determine the properties of PVA-g-LA using Design-Expert® version 11. Two independent variables, curing time (X1) and LA ratio (X2), were investigated, each varied at three levels (–1, 0, and +1). The assigned code levels were 120 min, 150 min, and 180 min for X1 and 2.5 g, 5 g, and 7.5 g for X2. Swelling behavior in various swelling media and thermal analysis parameters of PVA-g-LA were monitored as dependent variables. The prediction results from the Design-Expert® program had precision and reliability. The formulations with the highest desirability values were prepared and re-evaluated. The percent error of the prediction had a low value. Morphologically, the selected formulation was compact and dense. To explore the possibility of using the prepared PVA-g-LA film for transdermal drug delivery, we loaded PVA-g-LA film (F1) with propranolol hydrochloride as a model drug and evaluate drug loading and in vitro drug release. The content of propranolol hydrochloride was 4.19 ± 1.05 mg/cm2 in PVA-g-LA film. The cumulative release of propranolol hydrochloride was 61.94 ± 8.03% after 24 hours. The mechanism of drug release from PVA-g-LA film could be described by the diffusion-dominated mechanism of Higuchi’s model. The cumulative permeation of propranolol hydrochloride 59.96 ± 6.61% after 24 hours. The mechanism of drug permeation from PVA-g-LA film could be described by the zero-order kinetic. In summary, the response surface methodology could be used as a tool to predict or optimize the process parameters for PVA-g-LA transdermal films in an accurate manner. PVA-g-LA could control the release of drug from the film layer. However, the release of propranolol hydrochloride from the film was low due to strong interactions with PVA-g-LA. To further develop the formulation, a plasticizer and a penetration enhancer might be added to decrease the flexibility of the film’s structure and to increase the drug release and permeation, respectively.
ACKNOWLEDGMENTS
We thank to the College of Pharmacy, Rangsit University. This paper was proofread and edited by Cambridge Proofreading LLC which supported by the Research Institute at Rangsit University.
REFERENCES
- ALEXY P, BAKOŠ D, CRKOŇOVÁ G, KRAMÁROVÁ Z, HOFFMANN J, JULINOVÁ M, CHIELLINI E & CINELLI P. 2003. Poly(vinyl alcohol)–collagen hydrolysate thermoplastic blends: II. Water penetration and biodegradability of melt extruded films. Polym Test 22: 811-818.
- AMORES S, DOMENECH J, COLOM H, CALPENA AC, CLARES B, GIMENO Á & LAUROBA J. 2014. An improved cryopreservation method for porcine buccal mucosa in ex vivo drug permeation studies using Franz diffusion cells. Eur J Pharm Sci 60: 49-54.
- AN N-M, KIM D-D, SHIN Y-H & LEE C-H. 2003. Development of a novel soft hydrogel for the transdermal delivery of testosterone. Drug Dev Ind Pharm 29: 99-105.
- CAVALCANTI BN, ZEITLIN BD & NÖR JE. 2013. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent Mater 29: 97-102.
- CERCHIARA T, LUPPI B, BIGUCCI F, ORIENTI I & ZECCHI V. 2002. Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drugs. J Pharm Pharmacol 54: 1453-1459.
- CID E, MELLA F, LUCCHINI L, CÁRCAMO M & MONASTERIO J. 1986. Plasma concentrations and bioavailability of propranolol by oral, rectal and intravenous administration in man. Biopharm Drug Dispos 7: 559-566.
- DING J, CHEN S-C, WANG X-L & WANG Y-Z. 2011. Preparation and rheological behaviors of thermoplastic poly(vinyl alcohol) modified by lactic acid. Ind Eng Chem Res 50: 9123-9130.
- DON T-M, KING C-F, CHIU W-Y & PENG C-A. 2006. Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr Polym 63: 331-339.
- FANG J-Y, HUNG C-F, CHI C-H & CHEN C-C. 2009. Transdermal permeation of selegiline from hydrogel-membrane drug delivery systems. Int J Pharm 380: 33-39.
- GANGA S, SINGH PN & SINGH J. 1992. Formulation, in-vitro release and therapeutic effect of hydrogels based controlled release tablets of propranolol hydrochloride. Drug Dev Ind Pharm 18: 2049-2066.
- GIL EC, COLARTE AI, BATAILLE B, PEDRAZ JL, RODRÍGUEZ F & HEINÄMÄKI J. 2006. Development and optimization of a novel sustained-release dextran tablet formulation for propranolol hydrochloride. Int J Pharm 317: 32-39.
- GUIRGUIS O & MOSELHEY M. 2012. Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat Sci 4: 57-67.
- GULREZ SKH & AL-ASSAF S. 2011. Hydrogels: Methods of Preparation, Characterisation and Applications. In: Progress in Molecular and Environmental Bioengineering - From Analysis and Modeling to Technology Applications. Croatia: InTech.
- GWON H-J, LIM Y-M, CHANG H-N & NHO Y-C. 2010. Reduction of postsurgical adhesion formation with CM-chitosan hydrogel barriers prepared by using γ-irradiation. J Appl Polym Sci 116: 3682-3687.
- HENNINK WE & VAN NOSTRUM CF. 2002. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 54: 13-36.
- HOFFMAN AS. 2002. Hydrogels for biomedical applications. Adv Drug Deliv Rev 54: 3-12.
- INOUE T, CHEN G, NAKAMAE K & HOFFMAN AS. 1997. A hydrophobically-modified bioadhesive polyelectrolyte hydrogel for drug delivery. J Controlled Release 49: 167-176.
- KUMAR S, SASMAL D & PAL S. 2008. Rheological characterization and drug release studies of gum exudates of Terminalia catappa Linn. AAPS PharmSciTech 9: 885-890.
- MORIMOTO K, NAGAYASU A, FUKANOKI S, MORISAKS K, HYON S-H & IKADA Y. 1990. Evaluation of polyvinyl alcohol hydrogel as sustained-release vehicle for transdermal sytem of bunitrolol-HCl. Drug Dev Ind Pharm 16: 13-29.
- ORIENTI I, DI PIETRA A, LUPPI B & ZECCHI V. 2000. Crosslinked polyvinylalcohol hydrogels as vehicles for hydrophilic drugs. Archiv der Pharmazie 333: 421-424.
- ORIENTI I, TRERÉ R & ZECCHI V. 2001. Hydrogels formed by cross-linked polyvinylalcohol as colon-specific drug delivery systems. Drug Dev Ind Pharm 27: 877-884.
- PATEL V, PRAJAPATI B & PATEL M. 2007. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS Pharm Sci Tech 8: E147-E154.
- RAJU CL, RAO JL, REDDY BCV & VEERA BRAHMAM K. 2007. Thermal and IR studies on copper doped polyvinyl alcohol. Bull Mater Sci 30: 215-218.
- RITGER PL & PEPPAS NA. 1987. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5: 37-42.
- ROSIAK JM & YOSHII F. 1999. Hydrogels and their medical applications. Nucl Instrum Meth B 151: 56-64.
- SCHMEDLEN RH, MASTERS KS & WEST JL. 2002. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 23: 4325-4332.
- SIEPMANN J & PEPPAS NA. 2001. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev 48: 139-157.
- SUKSAEREE J & CHUCHOTE C. 2018. Swelling properties of propranolol HCl-loaded polyvinyl alcohol-graft-lactic acid hydrogel films. MATEC Web Conf 237: 02004.
- SUKSAEREE J, LUPRASONG C & MONTON C. 2016. Swelling behavior of polyvinyl alcohol and lactic acid hydrogel films. Asian J Pharm Sci 11: 102-103.
- SUKSAEREE J, LUPRASONG C, MONTON C, CHAROENCHAI L & PICHAYAKORN W. 2015. An eco-friendly synthesis of modified poly(vinyl alcohol)-graft-lactic acid by curing method. J Therm Anal Calorim 120: 929-936.
- UBAIDULLA U, REDDY MVS, RUCKMANI K, AHMAD FJ & KHAR RK. 2007. Transdermal therapeutic system of carvedilol: Effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS PharmSciTech 8: E13-E20.
- XIAO C & YANG M. 2006. Controlled preparation of physical cross-linked starch-g-PVA hydrogel. Carbohydr Polym 64: 37-40.
- ZHENG SHU X, LIU Y, PALUMBO FS, LUO Y & PRESTWICH GD. 2004. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 25: 1339-1348.
- ZHOU HY, CHEN XG, KONG M, LIU CS, CHA DS & KENNEDY JF. 2008. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr Polym 73: 265-273.
Publication Dates
-
Publication in this collection
22 Nov 2021 -
Date of issue
2021
History
-
Received
20 Mar 2021 -
Accepted
8 Aug 2021