Abstract
A few decades ago, researchers from the National Institute for Amazonian Research (INPA) started a pilot study to integrate the ecological studies of several organisms using monitoring plots, which then became the embryo for the creation of the RAPELD (Rapid Assessments and Long-term Ecological Research) system used by the Program for Biodiversity Research (PPBio) and the Long-term ecological research site POPA (PELD Western Pará). They installed and maintained permanent plots in an Amazonian-savanna patch near to the village of Alter do Chão. Amazonian savannas constitute a threatened ecosystem comprising only 6% of the Amazon biome. Most of the studies focused on three main long-term ecological research questions, but the site was also of importance for other inquiries and for the training of young researchers, contributing 71 articles so far and 32 masters and doctorate theses. Here, we present the experimental design and results of standardized studies in the savannas and forest fragments near Alter do Chão that have been carried out over the years. We discuss the future prospects and local threats to the area (e.g. soy crops and land speculation), and highlight the need to incorporate Alter do Chão villagers in land-use planning in the region.
Key words
Amazonian cerrado; ILTER (International Long-Term Ecological Research Network); PELD (Programa de Pesquisas Ecológicas de Longa Duração); multi-taxa
INTRODUCTION
When Tom Phillips, The Guardian’s Brazil correspondent, elected Alter do Chão among the ten most beautiful beaches of Brazil in 2009 (McOwan 2009MCOWAN G. 2009. Top 10 beaches in Brazil. The Guardian. https://www.theguardian.com/travel/2009/apr/15/beach-brazil-top-10. Accessed in 4 July 2020.
https://www.theguardian.com/travel/2009/...
), he did not anticipate that the former peaceful fishing village would become one of the main tourist destinations in the Amazon during the following decade. He was also unaware that the village had a long history of biodiversity research, which had started long before his arrival. The studies carried out there constitute the oldest Long-Term Ecological Research (LTER) site in Amazonian savannas, a unique and threatened ecosystem covering about 6% of the Amazon (Carvalho & Mustin 2017CARVALHO WD & MUSTIN K. 2017. The highly threatened and little-known Amazonian savannahs. Nat Ecol Evol 1: 0100.).
Alter do Chão is part of an International Long-Term Ecological Research Network comprising approximately 800 research sites, which aims to assess long-term changes in ecological patterns and processes that cannot be detected in short-term studies (Mirtl et al. 2018MIRTL M ET AL. 2018. Genesis, goals and achievements of Long-Term Ecological Research at the global scale: A critical review of ILTER and future directions. Sci Total Environ 626: 1439-1462.). The Alter do Chão region has supported typical savanna tree species for about 1.49 Ma (Buzatti et al. 2018BUZATTI RSO, PFEILSTICKER TR, DE MAGALHÃES RF, BUENO ML, LEMOS-FILHO JP & LOVATO MB. 2018. Genetic and historical colonization analyses of an endemic savanna tree, Qualea grandiflora, reveal ancient connections between amazonian savannas and cerrado core. Front Plant Sci 9: 1-16.), with a local landscape relatively stable for at least the last 7,000 to 6,000 years (Sanaiotti et al. 2002SANAIOTTI TM, MARTINELLI LA, VICTORIA RL, TRUMBORE SE & CAMARGO PB. 2002. Past vegetation changes in Amazon Savannas determined using carbon isotopes of soil organic matter. Biotropica 34: 2-16.), which coincides with the first records of people in the region (Maezumi et al. 2018MAEZUMI SY, ALVES D, ROBINSON M, DE SOUZA JG, LEVIS C, BARNETT RL, DE OLIVEIRA EA, URREGO D, SCHAAN D & IRIARTE J. 2018. The legacy of 4,500 years of polyculture agroforestry in the eastern Amazon. Nat Plants 4: 540-547.). The Alter do Chão LTER investigates the local and global drivers of biodiversity change, such as fire and global warming. Here we present a brief history of the savanna research, which was the forerunner of the RAPELD system (Magnusson et al. 2005MAGNUSSON WE, LIMA AP, LUIZÃO R, LUIZÃO F, COSTA FRC & VOLKMER C. 2005. Rapeld: a modification of the gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotrop 5: 19-24.), a modification of the Gentry Method for conducting rapid and long-term biodiversity surveys. This system is also used in the Tapajós National Forest (FLONA-Tapajós), which is also part of the LTER site locally known as PELD-POPA (Figure S1 - Supplementary Material), as well as by a network of scientists in Brazil and other countries for answering ecological questions at a variety of spatial scales. We present the experimental design and results of standardized studies in the savannas and forest fragments near Alter do Chão that have been carried out over more than twenty years, which were based on the first studies in the 1980s. We emphasize the future prospects and local threats to the area and highlight the need to incorporate the Alter do Chão villagers in land-use planning in the region.
Research history
Alter do Chão is an iconic place visited by naturalists since the 1850`s (Bates 1969BATES HW. 1969. The naturalist on the river Amazons. London: Everyman’s Library, J. M. Dent & Sons Ltd., 466 p.), and has been the site of continuous research since the early 1980s. Albertina Lima introduced William Magnusson to Alter do Chão in 1979 (Magnusson 2016MAGNUSSON WE. 2016. The fish and the frogs. New York, Open Science Publishers, 372 p.) and he invited Lyn Branch to undertake a study of the ethnobotanical knowledge of villagers (Branch & Silva 1983BRANCH LC & SILVA MFD. 1983. Folk medicine of Alter do Chão, Pará, Brazil. Acta Amaz 13: 737-797.). Alter do Chão became an important training ground for students studying frogs (Magnusson 2016MAGNUSSON WE. 2016. The fish and the frogs. New York, Open Science Publishers, 372 p.) and lizards (Magnusson 2019MAGNUSSON WE. 2019. Snakes and other lizards. New York: Open Science Publishers, 390 p.). In the mid 1980`s, Tânia Sanaiotti (a former student at the National Institute for Amazonian Research - INPA) arrived in Alter do Chão to conduct her master’s studies with a species of savanna bird, the Rusty-backed Antwren (Formicivora rufa). She would later study the plants and many species of animals from the region. INPA researchers returned to Alter do Chão repeatedly to carry out ecological research with various organisms, which resulted in a series of studies of the natural history of animals (Strussmann et al. 1984STRUSSMANN C, DOVALE MBR, MENEGHINI MH & MAGNUSSON WE. 1984. Diet and foraging mode of Bufo marinus and Leptodactylus ocellatus. J Herpetol 18: 138-146., Magnusson et al. 1985MAGNUSSON WE, PAIVA LJ, ROCHA RM, FRANKE CR, KASPER LA & LIMA AP. 1985. The correlates of foraging mode in a community of Brazilian lizards. Herpetologica 41: 324-332., 1986MAGNUSSON WE, FRANKE CR & KASPER LA. 1986. Factors affecting densities of Cnemidophorus lemniscatus. Copeia 3: 804-807., Magnusson & Sanaiotti 1987MAGNUSSON WE & SANAIOTTI TM. 1987. Dispersal of Miconia seeds by the rat Bolomys lasiurus. J Trop Ecol 3: 277-278.) and plants (Miranda 1993MIRANDA IS. 1993. Estrutura do estrato arbóreo do cerrado amazônico em Alter-do-Chão, Pará, Brasil. Rev Bras Bot 16: 143-150., 1995MIRANDA IS. 1995. Fenologia do estrato arbóreo de uma comunidade de cerrado em Alter-do-Chão, PA. Rev Bras Bot 18: 235-240.).
However, data integration among the first studies was rare, as they were conducted at different places and spatial scales. In the 1990’s the INPA researchers obtained funding for a larger research project in the savannas and forests around the Alter do Chão village, with financing from the Pilot Program for Forest Protection - PPG7. By that time, they considered the region a natural laboratory for tackling longer-term ecological questions. Renato Cintra was the Principal Investigator, with William Magnusson and Ana Albernaz responsible for the sampling design. The project focused on three main long-term ecological research questions: 1) what are the ecological forces that maintain the borders between the savannas and forests?; 2) what are the short and long-term responses of fauna and flora to fire and other ecological filters?; and 3) what are the influences of natural forest fragmentation and landscape characteristics on the fauna and flora? Since Amazonian savannas have been historically neglected in conservation programs (Carvalho & Mustin 2017CARVALHO WD & MUSTIN K. 2017. The highly threatened and little-known Amazonian savannahs. Nat Ecol Evol 1: 0100.), these questions were important for the villagers and local decision makers (Magnusson et al. 2013MAGNUSSON W, BRAGA-NETO R, PEZZINI F, BACCARO F, BERGALLO H, PENHA J, RODRIGUES DJ, VERDADE LM, LIMA A & ALBERNAZ AL. 2013. Biodiversity and integrated environmental monitoring. Manaus: Attema Design, 351 p.). However, to answer these questions, they needed to decide how to sample the complex landscape of Alter do Chão formed by terra firme forests, inundated forests, sandy beaches, and savannas, and define the size and format of the research plots appropriate for integration of data from several taxonomic groups and ecological processes. We explore these decisions in the next section, and present some results to the main questions later in the Results section.
Study site and experimental design
The vegetation mosaic around Alter do Chão is easily distinguished on a satellite image or from a plane (Figure 1a). Within the 16,180 ha of the Environmental Protection Area of Alter do Chão, there is sparse herbaceous vegetation on sandy beaches (1.9%) (Figure 1b), fragmented semi-deciduous forests (6.4%) (Figure 1c), as well as continuous forests (59,3%) on higher ground, seasonally-flooded forests (Figure 1d) surrounding temporary lakes (2.6%), and predominantly white-sand savannas (15.7%) (Figure 1e). About 13.9% of the area has been converted to housing or agriculture. In the 1990s, researchers studying a diverse group of organisms (lizards, birds, plants, rodents) used a stratified sampling design to cover both forest and savanna sites, while the sandy beaches and seasonally-flooded forests were not included in standardized studies.
The landscape in Alter do Chão forms a vegetation mosaic (a) comprised of sand beaches (b), forest fragments (c), seasonally-flooded forests (d) and savannas (e). (Credits: a – Edson Varga Lopes; b, d and e – Rodrigo Fadini; c – Susan Aragón).
Forty plots were installed across the entire extent of savanna, generally with a separation of at least 500 m between plots. Plots were also installed in 26 forest fragments and nine areas of continuous forests (Figure 2). Plots were used to sample different organisms and environmental predictors such as fire, vegetation and soil properties (e.g., soil texture, macro- and micronutrients). The basic plot design consisted of four parallel 250 m transects distant 50 m from each other. This design was chosen to attain sampling sufficiency for several taxonomic groups such as birds, lizards, plants, rodents, etc. (Magnusson et al. 2013MAGNUSSON W, BRAGA-NETO R, PEZZINI F, BACCARO F, BERGALLO H, PENHA J, RODRIGUES DJ, VERDADE LM, LIMA A & ALBERNAZ AL. 2013. Biodiversity and integrated environmental monitoring. Manaus: Attema Design, 351 p.). The number and size of transects were different in some small irregularly-shaped forest fragments, but the total length was the same (1000 m). Plots were permanently marked with metal stakes (to resist fire) and, in the savanna, they were fixed in the ground every 10 m along transects. In the next section, we describe the data structure and summarize the LTER studies conducted in Alter do Chão since 1980. We focus on studies carried out in the standardized plots, but also list those with other spatial designs. A summary about the methods for sampling particular taxonomic groups can be found in the (Supplementary Material).
Alter do Chão LTER showing the savanna (S), forest-fragment (FF) and mature-forest (MF) plots. Some plots were inactivated and some became threatened due to a variety of problems (Table SI - Supplementary Material).
Data management, themes and taxonomic groups studied
Data management is an essential part of LTER studies. Basic data are used to generate hypotheses and set the scene for attacking major scientific questions. Data were collected on biotic and abiotic variables that can be used to describe habitat characteristics for organisms. Many of the studies at Alter do Chão have focused on three predictor variables: fire regime, vegetation structure/coverage and landscape features. Fire extent has been measured in savanna plots since 1998. The four-transects in each plot were surveyed at the beginning of the rainy period (December to June) in many years. A measuring tape was stretched along the transect and evidence of fire that occurred in the previous dry season (July to November) was recorded every 2 m.
Vegetation structure has been measured twice (1998-2001 and 2017-2019) in 24 forest plots. Trees (and shrubs) 4 cm in circumference at breast height were identified and measured in 2-m wide strips along the four transects. Trees were also measured in the savanna plots in 2017. All trees 2 m in height with diameter 5 cm at 30 cm above soil level were measured in the first transect of each plot. The relative vegetation cover of plant species in savanna plots was estimated with point quadrats placed every 2 m along each of the four transects.
Landscape features for different studies, such as forest-fragment size, shape, connectivity (nearest-neighbor distance), distance from forest fragments to continuous forest and matrix characteristics (type of vegetation) were estimated from digitalized maps or classified LANDSAT satellite images with the aid of GIS tools such as ARCVIEW, QGIS and FRAGSTAT (Cintra et al. 2013CINTRA R, MAGNUSSON WE & ALBERNAZ A. 2013. Spatial and temporal changes in bird assemblages in forest fragments in an eastern Amazonian savannah. Ecol Evol 3: 3249-3262., Borges-Matos et al. 2016BORGES-MATOS C, ARAGÓN S, DA SILVA MNF, FORTIN MJ & MAGNUSSON WE. 2016. Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204: 417-425.).
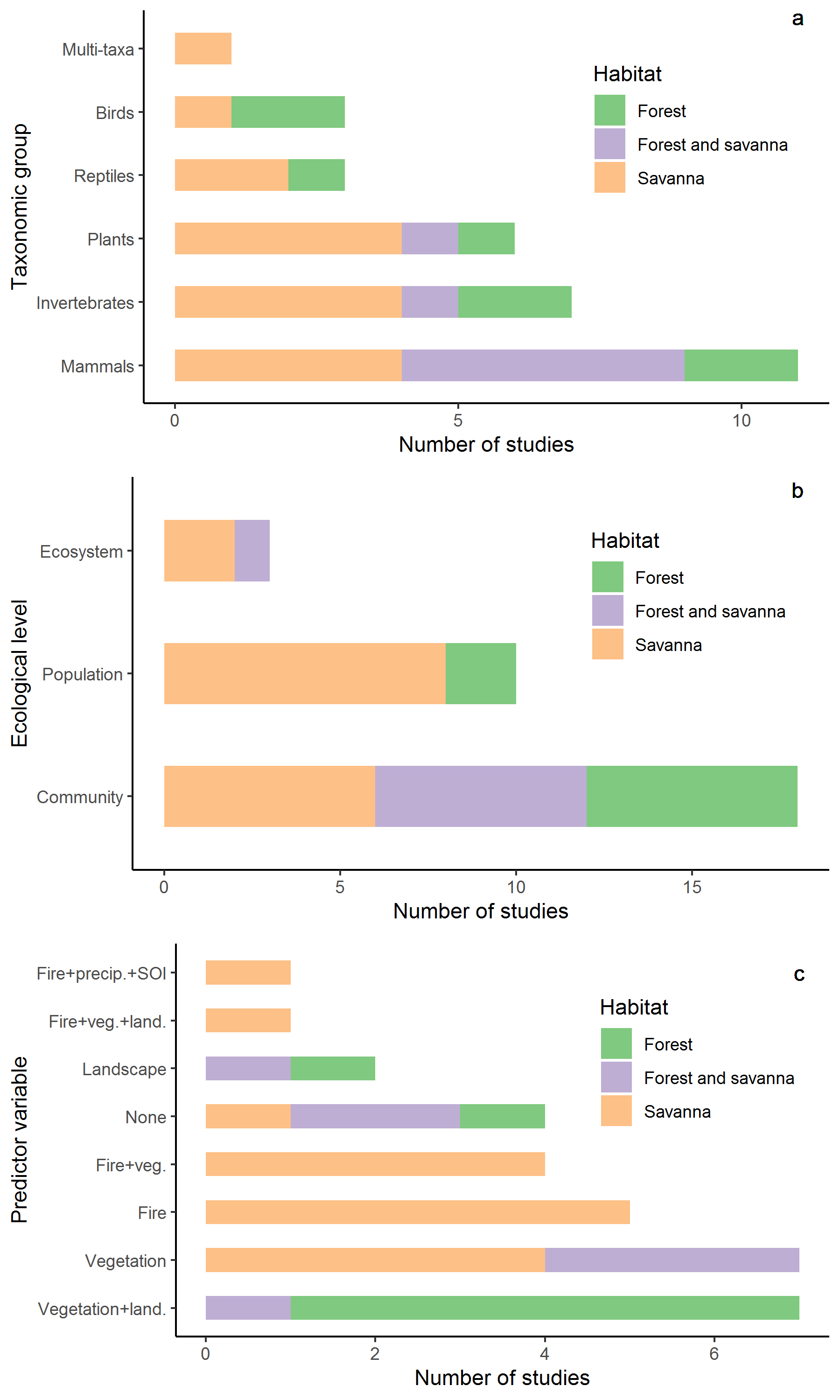
These predictor variables provide a solid foundation for conducting ecological studies with many organisms in our study site. Seventy-one scientific articles and thirty-two theses and dissertations were published between 1983 and 2021 (Table SI), with ~45% (of articles) carried out in the standardized plots. Of these, 86% used at least one of the predictor variables mentioned above. Nineteen studies (63%) used vegetation structure/coverage alone (or in combination) as a predictor variable, ~33% used fire extent, and another 33% used landscape measures (Figure 3a). Approximately 13% did not use any of these predictor variables, and only 23% of studies were conducted in both forests and savannas, independent of using such predictor variables or not.
Studies carried out in Alter do Chão LTER plots (published between 1999 and 2021) according to the taxonomic group (a), the ecological level (b), and the predictor variables used in the analyses (c) in three habitats.
Most studies used a community (60%) rather than a population (30%) or ecosystem approach (10%) (Figure 3b). While there is a concentration of population studies in savannas, community and ecosystem studies were equitably distributed across the habitats. The savanna has a concentration of population studies, mainly due to the population monitoring of the rodent Necromys lasiurus, carried out from 1983 to the present time, and population studies of lizards that were the main focus of the first years of study in the region. In contrast to Carvalho & Mustin (2017)CARVALHO WD & MUSTIN K. 2017. The highly threatened and little-known Amazonian savannahs. Nat Ecol Evol 1: 0100., who concluded that plants were the most studied group in the Amazonian savannas as a whole, mammals were the focus of most studies at Alter do Chão, though many of these used vegetation as a predictor variable (Figure 3c), with bats and small mammals (especially one species: Necromys lasiurus, Rodentia) representing 50% and 40% of the studies in this group, followed by large mammals (10%). Multi-taxa studies were scarce (~3%). Some vertebrates such as fishes and amphibians were rarely studied in the standardized plots. (but see Fróis et al. 2021FRÓIS RPS, RIBEIRO BO, ZUANON J & MORTATI AF. 2021. Fish fauna of small-order streams of savannah and forest fragments landscape in the lower Tapajós River basin, Amazonia. Biota Neot 21(4): e20201179., Supplementary Material).
RESULTS
What keeps the forest-savanna borders stable at Alter do Chão? Sanaiotti et al. (2002)SANAIOTTI TM, MARTINELLI LA, VICTORIA RL, TRUMBORE SE & CAMARGO PB. 2002. Past vegetation changes in Amazon Savannas determined using carbon isotopes of soil organic matter. Biotropica 34: 2-16. showed that local forest soil profiles are more clayey than that of the savanna profiles, which suggests that soil texture partly determines the forest-savanna boundary. In addition, Lloyd et al. (2015)LLOYD J ET AL. 2015. Edaphic, structural and physiological contrasts across Amazon Basin forest–savanna ecotones suggest a role for potassium as a key modulator of tropical woody vegetation structure and function. Biogeosciences 12(22): 6529-6571. used Alter do Chão as one of the study sites to compare several measures of soil structure and chemistry between forest and savanna, and found that exchangeable potassium content [K]sa, in conjunction with precipitation and soil plant-available water-storage capacity, was a key predictor of vegetation structure (i.e. canopy cover) at forest-savanna boundaries. Finally, fire is probably of cornerstone importance for maintaining the forest-savanna borders in the Alter do Chão region (Lima et al. 2020LIMA JM, CASTRO AB, LIMA AP, MAGNUSSON WE, LANDEIRO VL & FADINI RF. 2020. Influência do regime de queimadas sobre a composição florística de uma savana isolada na Amazônia - PELD Oeste do Pará. Oecol Aust 24: 301-316.), as it is globally (Hoffmann et al. 2012HOFFMANN WA, GEIGER EL, GOTSCH SG, ROSSATTO DR, SILVA LCR, LAU OL, HARIDASAN M & FRANCO AC. 2012. Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett 15: 759-768.).
As a persistent ecological force through time, fire has both an ecological as well as an evolutionary effect on the native fauna and flora of Alter do Chão. However, the studies conducted in the last 20 years in the savanna patches detected no evidence of direct effect of seasonal fire on the population density of either lizards or the rodent N. lasiurus in either the short (1-5 years) or long terms (more than 17 years) (Faria et al. 2004FARIA AS, LIMA AP & MAGNUSSON WE. 2004. The effects of fire on behaviour and relative abundance of three lizard species in an Amazonian savanna. J Trop Ecol 20: 591-594., Layme et al. 2004LAYME VMG, LIMA AP & MAGNUSSON WE. 2004. Effects of fire, food availability and vegetation on the distribution of the rodent Bolomys lasiurus in an Amazonian savanna. J Trop Ecol 20: 183-187., Ghizoni et al. 2005GHIZONI IR, LAYME VMG, LIMA AP & MAGNUSSON WE. 2005. Spatially explicit population dynamics in a declining population of the tropical rodent, Bolomys lasiurus. J Mammal 86: 677-682., Souza et al. 2021SOUZA E, LIMA AP, MAGNUSSON WE, KAWASHITA-RIBEIRO R, FADINI R, GHIZONI JR IR, GANANÇA PHS & FRAGA R. 2021. Short-and long-term effects of fire and vegetation cover on four lizard species in Amazonian savannas. Can J Zool 99(3): 173-182.). Global climate appears to affect N. lasiurus population dynamics at local scale (i.e. plot scale) but not at the scale of all the Alter do Chão savannas (Magnusson et al. 2010MAGNUSSON WE, LAYME VMG & LIMA AP. 2010. Complex effects of climate change: population fluctuations in a tropical rodent are associated with the southern oscillation index and regional fire extent, but not directly with local rainfall. Glob Chang Biol 16: 2401-2406., 2021MAGNUSSON WE, ROSA C, LAYME VMG, GHIZONI JR IR & LIMA AP. 2021. Local effects of global climate on a small rodent Necromys lasiurus. J Mammal 102(1): 188-194.). These short and long-term studies have shown that rodents and lizards probably have strategies (e.g. underground shelter, avoidance of high sun exposure, seasonal changes in diet) to thrive in environments with frequent fires, which suggests that fires limit the animal species that occur in these savannas rather than inducing large fluctuations in susceptible species.
Several vegetation properties, such as structure, species richness and composition were correlated with fire regime during the last few decades (Lima et al. 2020LIMA JM, CASTRO AB, LIMA AP, MAGNUSSON WE, LANDEIRO VL & FADINI RF. 2020. Influência do regime de queimadas sobre a composição florística de uma savana isolada na Amazônia - PELD Oeste do Pará. Oecol Aust 24: 301-316.). Species composition of unburned plots or plots with very low fire frequency was different from with plots that burn frequently. Open savannas were encroached by woody savannas or invaded by forests in the few sites unburned for more than 20 years (Lima et al. 2020LIMA JM, CASTRO AB, LIMA AP, MAGNUSSON WE, LANDEIRO VL & FADINI RF. 2020. Influência do regime de queimadas sobre a composição florística de uma savana isolada na Amazônia - PELD Oeste do Pará. Oecol Aust 24: 301-316.). Therefore, by affecting vegetation dynamics and diversity, fire could have an indirect effect on vertebrates by changing vegetation structure and cover. Fires reduce the cover of shrubs used as food by frugivorous birds in the savanna, favoring the spread of native grasses (Sanaiotti & Magnusson 1995SANAIOTTI TM & MAGNUSSON WE. 1995. Effects of annual fires on the production of fleshy fruits eaten by birds in a Brazilian Amazonian savanna. J Trop Ecol 11(1): 53-65.), and fire could reduce the shrubby vegetation used by some species for shelter and/or thermoregulation (Souza et al. 2021SOUZA E, LIMA AP, MAGNUSSON WE, KAWASHITA-RIBEIRO R, FADINI R, GHIZONI JR IR, GANANÇA PHS & FRAGA R. 2021. Short-and long-term effects of fire and vegetation cover on four lizard species in Amazonian savannas. Can J Zool 99(3): 173-182.).
Studies conducted in the forest plots generally used patch size and landscape characteristics as predictors of biodiversity change, rather than fire. A diversity of responses was found, depending on the group studied. Continuous-forest plots had significantly higher species richness than fragments in studies of trees (Amaral et al. 2017AMARAL IL, MAGNUSSON WE, DE ALMEIDA MATOS FD, ALBERNAZ ALK, FEITOSA YO & GUILLAUMET J-L. 2017. Disentangling structural patterns of natural forest fragments in a savanna matrix in the eastern Brazilian Amazon. Acta Amaz 47: 111-122.), ants (Vasconcelos et al. 2006VASCONCELOS HL, VILHENA JMS, MAGNUSSON WE & ALBERNAZ ALK. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. J Biogeogr 33: 1348-1356.), large mammals (Sampaio et al. 2010SAMPAIO R, LIMA AP, MAGNUSSON WE & PERES CA. 2010. Long-term persistence of midsized to large-bodied mammals in Amazonian landscapes under varying contexts of forest cover. Biodivers Conserv 19: 2421-2439.) and birds (Cintra et al. 2013CINTRA R, MAGNUSSON WE & ALBERNAZ A. 2013. Spatial and temporal changes in bird assemblages in forest fragments in an eastern Amazonian savannah. Ecol Evol 3: 3249-3262.), but not for small non-volant mammals (Borges-Matos et al. 2016BORGES-MATOS C, ARAGÓN S, DA SILVA MNF, FORTIN MJ & MAGNUSSON WE. 2016. Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204: 417-425.) or bats (Bernard & Fenton 2007BERNARD E & FENTON MB. 2007. Bats in a fragmented landscape: species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biol Conserv 134: 332-343.). In addition, patch size was positively related to species richness of ants (Vasconcelos et al. 2006VASCONCELOS HL, VILHENA JMS, MAGNUSSON WE & ALBERNAZ ALK. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. J Biogeogr 33: 1348-1356.) and birds (Cintra et al. 2013CINTRA R, MAGNUSSON WE & ALBERNAZ A. 2013. Spatial and temporal changes in bird assemblages in forest fragments in an eastern Amazonian savannah. Ecol Evol 3: 3249-3262.), but not of bats (Bernard & Fenton 2007BERNARD E & FENTON MB. 2007. Bats in a fragmented landscape: species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biol Conserv 134: 332-343.), small non-volant mammals (Borges-Matos et al. 2016BORGES-MATOS C, ARAGÓN S, DA SILVA MNF, FORTIN MJ & MAGNUSSON WE. 2016. Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204: 417-425.), larger mammals (Sampaio et al. 2010SAMPAIO R, LIMA AP, MAGNUSSON WE & PERES CA. 2010. Long-term persistence of midsized to large-bodied mammals in Amazonian landscapes under varying contexts of forest cover. Biodivers Conserv 19: 2421-2439.) or dung beetles (Vulinec et al. 2008VULINEC K, LIMA AP, CARVALHO JR EC & MELLOW DJ. 2008. Dung beetles and long-term habitat fragmentation in Alter do Chão, Amazônia, Brazil. Trop Conserv Sci 1: 111-121.). Patch isolation, on the other hand, was negatively related to the species richness of dung beetles (Vulinec et al. 2008VULINEC K, LIMA AP, CARVALHO JR EC & MELLOW DJ. 2008. Dung beetles and long-term habitat fragmentation in Alter do Chão, Amazônia, Brazil. Trop Conserv Sci 1: 111-121.), and the basal area of trees (Amaral et al. 2017AMARAL IL, MAGNUSSON WE, DE ALMEIDA MATOS FD, ALBERNAZ ALK, FEITOSA YO & GUILLAUMET J-L. 2017. Disentangling structural patterns of natural forest fragments in a savanna matrix in the eastern Brazilian Amazon. Acta Amaz 47: 111-122.). These results demonstrate that the response to long-term, natural fragmentation may depend on the group´s traits, such as the capacity to cross or use the matrix (Bernard & Fenton 2007BERNARD E & FENTON MB. 2007. Bats in a fragmented landscape: species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biol Conserv 134: 332-343., Sampaio et al. 2010SAMPAIO R, LIMA AP, MAGNUSSON WE & PERES CA. 2010. Long-term persistence of midsized to large-bodied mammals in Amazonian landscapes under varying contexts of forest cover. Biodivers Conserv 19: 2421-2439.), the availability of resources (Carvalho et al. 2008CARVALHO EARJ, LIMA AP, MAGNUSSON WE & ALBERNAZ ALKM. 2008. Long-term effect of forest fragmentation on the Amazonian gekkonid lizards, Coleodactylus amazonicus and Gonatodes humeralis. Austral Ecol 33: 723-729.), the effects of matrix type (Borges-Matos et al. 2016BORGES-MATOS C, ARAGÓN S, DA SILVA MNF, FORTIN MJ & MAGNUSSON WE. 2016. Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204: 417-425.), and human pressure (Sampaio et al. 2010SAMPAIO R, LIMA AP, MAGNUSSON WE & PERES CA. 2010. Long-term persistence of midsized to large-bodied mammals in Amazonian landscapes under varying contexts of forest cover. Biodivers Conserv 19: 2421-2439., Amaral et al. 2017AMARAL IL, MAGNUSSON WE, DE ALMEIDA MATOS FD, ALBERNAZ ALK, FEITOSA YO & GUILLAUMET J-L. 2017. Disentangling structural patterns of natural forest fragments in a savanna matrix in the eastern Brazilian Amazon. Acta Amaz 47: 111-122.).
Present and future
The cumulative knowledge and practical expertise provided by the studies at Alter do Chão have turned the region into a laboratory for scientific investigation of ecological processes of savannas and fragmented tropical areas. Many researchers were trained in the area and formed a new generation of scientists, most of which remained in the Amazon. The region is now one of the 161 sites of the Program for Biodiversity Research (PPBio), a program present in all Brazilian biomes to monitoring biodiversity across space and through time (Rosa et al. 2021ROSA C ET AL. 2021. The program for biodiversity research in Brazil: the role of regional networks for biodiversity knowledge, dissemination, and conservation. An Acad Bras Cienc 93: e20201604.). In addition, the creation of the Universidade Federal do Oeste do Pará, in Santarém, and the ongoing partnership with INPA, were of fundamental importance for the maintenance of infrastructure and continuity of ecological research in the region.
Results produced by the studies triggered the creation of a Protected Area (Albernaz et al. 2004ALBERNAZ ALK, MAGNUSSON WE, CINTRA R, LIMA A & SANAIOTTI TM. 2004. Proteção para a savana amazônica. Ciência Hoje 35: 61-63.), though with only half the area originally proposed (Santarém 2003SANTARÉM. 2003. https://www.semas.pa.gov.br/2003/07/02/9839/ Accessed in 4 July 2020.
https://www.semas.pa.gov.br/2003/07/02/9...
). The Environmental Protection Area of Alter do Chão is a municipally protected area that has the aim of the sustainable use of natural resources by the resident community, in addition to activities with low environmental impact, such as tourism. Despite the establishment of this Protected Area, several factors threaten the ecological integrity of the Alter do Chão mosaic and endanger the legacy of the long-term studies (Figure 4). Expansion of soy and cattle agribusiness is advancing and modifying the landscape and livelihoods of the people at regional scales (Walker et al. 2009WALKER R, BROWDER J, ARIMA E, SIMMONS C, PEREIRA R, CALDAS M, SHIROTA R & DE ZEN S. 2009. Ranching and the new global range: Amazonia in the 21st century. Geoforum 40: 732-745., Withey et al. 2018WITHEY K ET AL. 2018. Quantifying immediate carbon emissions from El Niño-mediated wildfires in humid tropical forests. Philos Trans R Soc B Biol Sci 373: 20170312.), while land speculation and urban expansion are the major threats at the local level. Most of these threats come from activities that are not carried out for the benefit of local communities, which have tourism as the main source of sustainable income. Being a tourist destination that is renowned nationally and internationally, money from agribusiness enterprises is being used to buy real estate in the coveted tourist areas. Rampant deforestation and fires are becoming common in the areas along the main road to Santarem. A large, allegedly criminal, fire in 2019 may have been related to land speculation (Watts 2019WATTS J. 2019. Someone has declared war’: Brazil activists fear crackdown after arrests. The Guardian. https://www.theguardian.com/world/2019/dec/05/brazil-ngos-crackdown-raids-amazon-fires. Accessed in 4 July 2020.
https://www.theguardian.com/world/2019/d...
). Furthermore, climate change might increase the frequency and intensity of fires (Barlow et al. 2020BARLOW J, BERENGER E, CARMENTA R & FRANÇA F. 2020. Clarifying Amazonia’s burning crisis. Glob Chang Biol 26: 319-320.), affecting the forest fragments and accelerating the degradation of savannas and riparian forests.
Local threats to the Alter do Chão LTER: allotments (a and d); stream interruption for recreation (b); mining for civil construction (c); forest fires (e). (Credits: a, c and d – Rodrigo Fadini; b – Clarissa Rosa; and e - Instituto Aquífero de Alter do Chão).
From a research perspective, the long-term effects of fire on invertebrates (e.g. dung beetles, ants) and vertebrates (i.e. birds, bats) in the Alter do Chão region needs further investigation. Multi-taxa studies are necessary to determine which mechanisms are involved in species adaptation to the savanna environment. These studies can help us model future scenarios of Amazonian deforestation and climate change (the Alter do Chão LTER initiative, i.e. PELD-POPA). In addition, the conversion of natural areas to intensive human-modified ecosystems is occurring at ever-increasing speed in Alter do Chão, so, more than ever, the long-term ecological studies need to be in line with society, especially local communities. The Alter do Chão LTER studies can become a strategic program with the capacity to expand knowledge about biodiversity in the Amazon, integrating and training local communities and future scientists, encouraging the use of biodiversity knowledge for income-generating activities and fostering an alliance for the stewardship of this iconic landscape. Conservation of biodiversity and the ecosystem processes that maintain the savanna mosaic will require the participation of all local stakeholders as well as government authorities.
ACKNOWLEDGMENTS
We deeply appreciate the dedication of researchers, students and the local staff that participated of the Alter do Chão LTER in the last decades. Tânia Sanaiotti and Renato Cintra compiled part of the information about the theses and dissertations of this study. CR thanks the Programa de Capacitação Institucional - Conselho Nacional de Desenvolvimento Científico e Tecnológico (PCI-CNPq) from Ministério da Ciência, Tecnologia e Inovações (MCTIC) for the postdoctoral fellowship. CRB received a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance code 001). SA thanks CAPES and CNPq for support as a postdoctoral researcher. Collaboration for this publication was facilitated by the Programa de Pesquisa em Biodiversidade (PPBio) and Instituto Nacional de Ciência e Tecnologia (de Estudos Integrados da Biodiversidade Amazônica (INCT-CENBAM). This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), PROCAD-AM, [Process 88881.314420/2019-01]; and PELD (LTER) POPA by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [Proc. 441443/2016-8].
REFERENCES
- ALBERNAZ ALK, MAGNUSSON WE, CINTRA R, LIMA A & SANAIOTTI TM. 2004. Proteção para a savana amazônica. Ciência Hoje 35: 61-63.
- AMARAL IL, MAGNUSSON WE, DE ALMEIDA MATOS FD, ALBERNAZ ALK, FEITOSA YO & GUILLAUMET J-L. 2017. Disentangling structural patterns of natural forest fragments in a savanna matrix in the eastern Brazilian Amazon. Acta Amaz 47: 111-122.
- BARLOW J, BERENGER E, CARMENTA R & FRANÇA F. 2020. Clarifying Amazonia’s burning crisis. Glob Chang Biol 26: 319-320.
- BATES HW. 1969. The naturalist on the river Amazons. London: Everyman’s Library, J. M. Dent & Sons Ltd., 466 p.
- BERNARD E & BROCK FENTON M. 2002. Species diversity of bats (Mammalia: Chiroptera) in forest fragments, primary forests, and savannas in central Amazonia, Brazil. Can J Zool 80: 1124-1140.
- BERNARD E & FENTON MB. 2007. Bats in a fragmented landscape: species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biol Conserv 134: 332-343.
- BORGES-MATOS C, ARAGÓN S, DA SILVA MNF, FORTIN MJ & MAGNUSSON WE. 2016. Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204: 417-425.
- BRANCH LC & SILVA MFD. 1983. Folk medicine of Alter do Chão, Pará, Brazil. Acta Amaz 13: 737-797.
- BUZATTI RSO, PFEILSTICKER TR, DE MAGALHÃES RF, BUENO ML, LEMOS-FILHO JP & LOVATO MB. 2018. Genetic and historical colonization analyses of an endemic savanna tree, Qualea grandiflora, reveal ancient connections between amazonian savannas and cerrado core. Front Plant Sci 9: 1-16.
- CARVALHO EARJ, LIMA AP, MAGNUSSON WE & ALBERNAZ ALKM. 2008. Long-term effect of forest fragmentation on the Amazonian gekkonid lizards, Coleodactylus amazonicus and Gonatodes humeralis. Austral Ecol 33: 723-729.
- CARVALHO WD & MUSTIN K. 2017. The highly threatened and little-known Amazonian savannahs. Nat Ecol Evol 1: 0100.
- CINTRA R, MAGNUSSON WE & ALBERNAZ A. 2013. Spatial and temporal changes in bird assemblages in forest fragments in an eastern Amazonian savannah. Ecol Evol 3: 3249-3262.
- CINTRA R & SANAIOTTI TM. 2006. Fire effects on the composition of a bird community in an Amazonian savanna (Brazil). Brazilian J Biol 65: 683-695.
- FADINI RF & LIMA AP. 2012. Fire and host abundance as determinants of the distribution of three congener and sympatric mistletoes in an Amazonian savanna. Biotropica 44: 27-34.
- FARIA AS, LIMA AP & MAGNUSSON WE. 2004. The effects of fire on behaviour and relative abundance of three lizard species in an Amazonian savanna. J Trop Ecol 20: 591-594.
- FRANCISCO AD, MAGNUSSON WE & SANAIOTTI TM. 1995. Variation in growth and reproduction of Bolomys lasiurus (Rodentia, Muridae) in an Amazonian savanna. J Trop Ecol 11: 419-428.
- FRÓIS RPS, RIBEIRO BO, ZUANON J & MORTATI AF. 2021. Fish fauna of small-order streams of savannah and forest fragments landscape in the lower Tapajós River basin, Amazonia. Biota Neot 21(4): e20201179.
- GHIZONI IR, LAYME VMG, LIMA AP & MAGNUSSON WE. 2005. Spatially explicit population dynamics in a declining population of the tropical rodent, Bolomys lasiurus. J Mammal 86: 677-682.
- HOFFMANN WA, GEIGER EL, GOTSCH SG, ROSSATTO DR, SILVA LCR, LAU OL, HARIDASAN M & FRANCO AC. 2012. Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett 15: 759-768.
- LAYME VMG, LIMA AP & MAGNUSSON WE. 2004. Effects of fire, food availability and vegetation on the distribution of the rodent Bolomys lasiurus in an Amazonian savanna. J Trop Ecol 20: 183-187.
- LIMA JM, CASTRO AB, LIMA AP, MAGNUSSON WE, LANDEIRO VL & FADINI RF. 2020. Influência do regime de queimadas sobre a composição florística de uma savana isolada na Amazônia - PELD Oeste do Pará. Oecol Aust 24: 301-316.
- LLOYD J ET AL. 2015. Edaphic, structural and physiological contrasts across Amazon Basin forest–savanna ecotones suggest a role for potassium as a key modulator of tropical woody vegetation structure and function. Biogeosciences 12(22): 6529-6571.
- LOUZADA J, LIMA AP, MATAVELLI R, ZAMBALDI L & BARLOW J. 2010. Community structure of dung beetles in Amazonian savannas: role of fire disturbance, vegetation and landscape structure. Landsc Ecol 25: 631-641.
- MAEZUMI SY, ALVES D, ROBINSON M, DE SOUZA JG, LEVIS C, BARNETT RL, DE OLIVEIRA EA, URREGO D, SCHAAN D & IRIARTE J. 2018. The legacy of 4,500 years of polyculture agroforestry in the eastern Amazon. Nat Plants 4: 540-547.
- MAGNUSSON W, BRAGA-NETO R, PEZZINI F, BACCARO F, BERGALLO H, PENHA J, RODRIGUES DJ, VERDADE LM, LIMA A & ALBERNAZ AL. 2013. Biodiversity and integrated environmental monitoring. Manaus: Attema Design, 351 p.
- MAGNUSSON WE. 2016. The fish and the frogs. New York, Open Science Publishers, 372 p.
- MAGNUSSON WE. 2019. Snakes and other lizards. New York: Open Science Publishers, 390 p.
- MAGNUSSON WE, FRANCISCO ADL & SANAIOTTI TM. 1995. Home-range size and territoriality Bolomys lasiurus (Rodentia: Muridae) in an Amazonian savanna. J Trop Ecol 11: 179-188.
- MAGNUSSON WE, FRANKE CR & KASPER LA. 1986. Factors affecting densities of Cnemidophorus lemniscatus. Copeia 3: 804-807.
- MAGNUSSON WE, LAYME VMG & LIMA AP. 2010. Complex effects of climate change: population fluctuations in a tropical rodent are associated with the southern oscillation index and regional fire extent, but not directly with local rainfall. Glob Chang Biol 16: 2401-2406.
- MAGNUSSON WE, LIMA AP, ALBERNAZ ALKM, SANAIOTTI TM & GUILLAUMET J-L. 2008. Composição florística e cobertura vegetal das savanas na região de Alter do Chão, Santarém - PA. Rev Bras Botânica 31: 165-177.
- MAGNUSSON WE, LIMA AP, LUIZÃO R, LUIZÃO F, COSTA FRC & VOLKMER C. 2005. Rapeld: a modification of the gentry method for biodiversity surveys in long-term ecological research sites. Biota Neotrop 5: 19-24.
- MAGNUSSON WE, PAIVA LJ, ROCHA RM, FRANKE CR, KASPER LA & LIMA AP. 1985. The correlates of foraging mode in a community of Brazilian lizards. Herpetologica 41: 324-332.
- MAGNUSSON WE & SANAIOTTI TM. 1987. Dispersal of Miconia seeds by the rat Bolomys lasiurus. J Trop Ecol 3: 277-278.
- MAGNUSSON WE, ROSA C, LAYME VMG, GHIZONI JR IR & LIMA AP. 2021. Local effects of global climate on a small rodent Necromys lasiurus. J Mammal 102(1): 188-194.
- MCOWAN G. 2009. Top 10 beaches in Brazil. The Guardian. https://www.theguardian.com/travel/2009/apr/15/beach-brazil-top-10 Accessed in 4 July 2020.
» https://www.theguardian.com/travel/2009/apr/15/beach-brazil-top-10 - MIRANDA IS. 1993. Estrutura do estrato arbóreo do cerrado amazônico em Alter-do-Chão, Pará, Brasil. Rev Bras Bot 16: 143-150.
- MIRANDA IS. 1995. Fenologia do estrato arbóreo de uma comunidade de cerrado em Alter-do-Chão, PA. Rev Bras Bot 18: 235-240.
- MIRTL M ET AL. 2018. Genesis, goals and achievements of Long-Term Ecological Research at the global scale: A critical review of ILTER and future directions. Sci Total Environ 626: 1439-1462.
- ROSA C ET AL. 2021. The program for biodiversity research in Brazil: the role of regional networks for biodiversity knowledge, dissemination, and conservation. An Acad Bras Cienc 93: e20201604.
- SAMPAIO R, LIMA AP, MAGNUSSON WE & PERES CA. 2010. Long-term persistence of midsized to large-bodied mammals in Amazonian landscapes under varying contexts of forest cover. Biodivers Conserv 19: 2421-2439.
- SANAIOTTI TM & MAGNUSSON WE. 1995. Effects of annual fires on the production of fleshy fruits eaten by birds in a Brazilian Amazonian savanna. J Trop Ecol 11(1): 53-65.
- SANAIOTTI TM, MARTINELLI LA, VICTORIA RL, TRUMBORE SE & CAMARGO PB. 2002. Past vegetation changes in Amazon Savannas determined using carbon isotopes of soil organic matter. Biotropica 34: 2-16.
- SANTARÉM. 2003. https://www.semas.pa.gov.br/2003/07/02/9839/ Accessed in 4 July 2020.
» https://www.semas.pa.gov.br/2003/07/02/9839/ - SOUZA E, LIMA AP, MAGNUSSON WE, KAWASHITA-RIBEIRO R, FADINI R, GHIZONI JR IR, GANANÇA PHS & FRAGA R. 2021. Short-and long-term effects of fire and vegetation cover on four lizard species in Amazonian savannas. Can J Zool 99(3): 173-182.
- STARK S ET AL. 2020. Reframing tropical savannization: linking changes in canopy structure to energy balance alterations that impact climate. Ecosphere 11(9): e03231.
- STRUSSMANN C, DOVALE MBR, MENEGHINI MH & MAGNUSSON WE. 1984. Diet and foraging mode of Bufo marinus and Leptodactylus ocellatus. J Herpetol 18: 138-146.
- VASCONCELOS HL, LEITE MF, VILHENA JMS, LIMA AP & MAGNUSSON WE. 2008. Ant diversity in an Amazonian savanna: relationship with vegetation structure, disturbance by fire, and dominant ants. Austral Ecol 33(2): 221-231.
- VASCONCELOS HL, VILHENA JMS, MAGNUSSON WE & ALBERNAZ ALK. 2006. Long-term effects of forest fragmentation on Amazonian ant communities. J Biogeogr 33: 1348-1356.
- VULINEC K, LIMA AP, CARVALHO JR EC & MELLOW DJ. 2008. Dung beetles and long-term habitat fragmentation in Alter do Chão, Amazônia, Brazil. Trop Conserv Sci 1: 111-121.
- WALKER R, BROWDER J, ARIMA E, SIMMONS C, PEREIRA R, CALDAS M, SHIROTA R & DE ZEN S. 2009. Ranching and the new global range: Amazonia in the 21st century. Geoforum 40: 732-745.
- WATTS J. 2019. Someone has declared war’: Brazil activists fear crackdown after arrests. The Guardian. https://www.theguardian.com/world/2019/dec/05/brazil-ngos-crackdown-raids-amazon-fires Accessed in 4 July 2020.
» https://www.theguardian.com/world/2019/dec/05/brazil-ngos-crackdown-raids-amazon-fires - WITHEY K ET AL. 2018. Quantifying immediate carbon emissions from El Niño-mediated wildfires in humid tropical forests. Philos Trans R Soc B Biol Sci 373: 20170312.
- WORRELL R & APPLEBY MC. 2000. Stewardship of natural resources: definition, ethical and practical aspects. J Agric Enrivonmental Ethics 12: 263-277.
SUPPLEMENTARY MATERIAL
A summary of the methods used for sampling different groups of animals and plants in the standardized LTER plots at Alter do Chão. Details can be consulted in the articles cited in the reference list.
Figure S1. The POPA PELD (Box 1) accommodates plots from the Alter do Chão LTER (black box above) and the RAPELD plots located in the Tapajós National Forest. Plots are distributed along a gradient of soils, vegetation, and human disturbance.
Table SI. Articles published, dissertations and theses concluded, species list, plot status and online dataset.
https://www.dropbox.com/s/s497v7ldup6t5sl/Sheet_supplement_Fadini%20et%20al.%202021_Anais.xls?dl=0
Publication Dates
-
Publication in this collection
08 Dec 2021 -
Date of issue
2021
History
-
Received
16 June 2021 -
Accepted
14 Aug 2021