Abstract
Health professionals working to mitigate the COVID-19 pandemic are one of the main risk groups for the disease, being prioritized for vaccination. Considering this, the aim of this study was to analyze the immune response of these professionals immunized with CoronaVac in the first and second doses. Blood samples were collected after the first and second doses of the vaccine (CoronaVac) and used to investigate hematological and biochemical parameters, analysis of immunoglobulin production, cytokines, and gene expression profile, as well as the identification of subsets of immune cells. Post-first dose immunological phenotypic memory (CD27+) profiles (T CD4+, TCD8+ and CD19+) showed a significant increase, as did Monocyte APCs (CD80+HLA-DR+) in relation to the second dose. The cytokines IL-2, IL-6 and IFN-° showed increased values in relation to the other analyzed cytokines. The Th2/Th17 profile in the second dose was characterized by gene expression analysis. The production of IgM and IgG after vaccination showed statistically significant values in the comparison between doses. CoronaVac showed activation of APCs monocytes, memory response of T and B lymphocytes, with immunoglobulins production. This set of responses is characterized by the Th2/Th17 immunological profile.
Key words
CoronaVac; health professionals; immunological memory; immunoglobulin production; COVID-19; SARS-CoV-2
INTRODUCTION
Coronavirus 2019 disease (COVID-19), which emerged in China, is currently considered the most serious outbreak of pneumonia in the last 100 years, affecting more than 200 countries around the world (Li et al. 2020aLI C, JI F, WANG L, WANG L, HAO J, DAI M & GU B. 2020. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis 26(7) 1626., Lu et al. 2020LU H, STRATTON CW & TANG YW. 2020. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 92(4): 401., Melo et al. 2020MELO CMD, SILVA GA, MELO AR & FREITAS AC. 2020. COVID-19 pandemic outbreak: the Brazilian reality from the first case to the collapse of health services. An Acad Bras Cienc 92: e20200709. DOI 10.1590/0001-3765202020200709.). According to the Johns Hopkins University Center for Systems Science and Engineering (CSSE) COVID-19 Panel, the disease had generated an estimated 195,767,462 cases and 4,183,074 deaths worldwide (on July 28, 2021), and 19,749,073 cases and 551,835 deaths from Brazil (JHU, 2021).
The transmissibility of COVID-19 is due to the high viral load in the upper respiratory tract, even in asymptomatic patients, which differentiates it from other respiratory diseases (Arons et al. 2020ARONS MM, HATFIELD KM, REDDY SC, KIMBALL A, JAMES A, JACOBS JR & JERNIGAN JA. 2020. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England J Med 382(22): 2081-2090., Li et al. 2020bLI Q, GUAN X, WU P, WANG X, ZHOU L, TONG Y & FENG Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England J Med 382: 1199-1207.). Virus propagation occurs through direct contact with contaminated surfaces, aerosols and respiratory droplets (Chan et al. 2020CHAN JFW ET AL. 2020. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395(10223): 514-523.). In the course of the infection, the patient may not present symptoms or develop different clinical manifestations, such as fever, cough, mild pneumonia, dyspnea, hypoxia, pulmonary impairment on imaging tests, respiratory failure, systemic shock or multiple organ failure and death (Buitrago-Garcia et al. 2020BUITRAGO-GARCIA D, EGLI-GANY D, COUNOTTE MJ, HOSSMANN S, IMERI H, IPEKCI AM & LOW N. 2020. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Medicine 17(9): e1003346., De Souza Silva et al. 2020DE SOUZA SILVA GA ET AL. 2020. SARS-CoV, MERS-CoV and SARS-CoV-2 infections in pregnancy and fetal development. J Gynec Obstetr Human Reprod 49(10): 101846., Furukawa et al. 2020FURUKAWA NW, BROOKS JT & SOBEL J. 2020. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis 26(7)., Merad & Martin 2020MERAD M & MARTIN JC. 2020. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature Rev Immunol 20(6): 355-362.).
Although the susceptibility to contagion is for everyone, healthcare workers are at high risk of contracting COVID-19 due to their direct or indirect contact with biological fluids from patients, visitors and/or other healthcare workers exposed to the disease. In China, about 29% of healthcare workers were infected with COVID-19 during the first months of the pandemic (Wang et al. 2020WANG D, HU B, HU C, ZHU F, LIU X, ZHANG J & PENG Z. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 323(11): 1061-1069.). At the same time, Spain and Italy had 22% and 20% of health workers infected, respectively (Arons et al. 2020ARONS MM, HATFIELD KM, REDDY SC, KIMBALL A, JAMES A, JACOBS JR & JERNIGAN JA. 2020. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England J Med 382(22): 2081-2090., Suárez-García et al. 2020SUÁREZ-GARCÍA I, DE ARAMAYONA LÓPEZ MM, VICENTE AS & ABASCAL PL. 2020. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect 106(2): 357-363.). According to the Pan American Health Organization, of the infected healthcare workers in all the Americas, Brazil had 54% of those infected, which corresponds to 307,000 professionals. The most affected were nursing professionals, especially technicians and assistants (OPAS 2020OPAS - ORGANIZAÇÃO PAN-AMERICANA DE SAÚDE. 2020. COVID-19 has infected some 570,000 health workers and killed 2,500 in the Americas, PAHO Director says. https://www.paho.org/en/news/2-9-2020-covid-19-has-infected-some-570000-health- workers-and-killed-2500-americas-paho. Acessado em 17 de julho de 2021.
https://www.paho.org/en/news/2-9-2020-co...
).
In this context, with the still insufficient supply of COVID-19 vaccines around the world, governments prioritized high-risk groups to receive the immunizer at the initial stage of availability. At first, these high-risk groups included healthcare workers, the elderly (especially those with chronic comorbid conditions), and those working in essential services. In Brazil, the first vaccines were approved for emergency use in January/2020 by the Brazilian Health Regulatory Agency, being an inactivated whole virus vaccine (CoronaVac) and a DNA vaccine carried by adenoviral vector (AstraZeneca) (ANVISA 2021ANVISA - AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. 2021. Covid-19: quadro de análises de vacinas pela Anvisa. Link: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/covid-19-quadro-de-analises-de-vacinas-pela-anvisa. Acessado em 6 de junho de 2021.
https://www.gov.br/anvisa/pt-br/assuntos...
). Thus, the aim of this study is to analyze the immune response of healthcare workers immunized with the inactivated whole virus vaccine (CoronaVac) in the first and second doses.
MATERIALS AND METHODS
Blood Sample Collection
The study volunteers are 50 healthcare workers at Hospital das Clínicas of Federal University of Pernambuco (HC-UFPE) who worked throughout the COVID-19 pandemic and were vaccinated exclusively with the CoronaVac in two doses (Sinovac Research and Development Co®). Blood collection was performed in two stages: 15 days after the first dose and 15 days after the second dose. Laboratory and immunological analyzes were performed with blood samples collected in different tubes (BD Vacutainer®) for hematological, coagulogram, serological, biochemical and immunological exams. All volunteer health professionals who participated in this study signed a ‘Free and Informed Consent Form’ agreeing with the research approved by the Ethics Committee for Research with Human Beings of Federal University of Pernambuco (nº 4.206.047/2020, CAAE 30332120.8.3001.5191).
Laboratory analysis of blood samples
After blood collection from healthcare workers, the blood and serum samples were sent to the Laboratory of Analysis HC-UFPE for laboratory analysis. The blood count was performed on a Yumizen H2500® hematology analyzer, followed by review of the blood smear under an optical microscope. The coagulogram was analyzed in a STA Compact Max® automatic analyzer. Sample serum was used to evaluate serological and biochemical parameters using Architect i2000SR® fully automated immunopanels and CMD 800i automated biochemical analyzer (Wiener Lab®), respectively.
Immunological tests
Analysis of serum cytokines and immunoglobulins production
The serological samples collected, according to Blood Sample Collection section were used for cytokine measurements and for quantitative detection of anti-SARS-CoV-2 immunoglobulins produced by the vaccine. Th1/Th2/Th17 human cytokines were measured with a Cytometric Bead Array (CBA) kit (BD Bioscience®) with all data acquired by the Accuri cytometry platform and processed with the BD Accuri® software. Quantitative detection of IgG and IgM was performed using IchromaTM COVID-19 Ab kit (Boditech Med Incorporated®) by the fluorescent immunoassay technique. Qualitative detection of IgG and IgM was performed using SARS-CoV-2 antibody test kit by colloidal gold immunochromatography technique (Lepu Technology®).
Immunophenotyping Assay
The isolation of peripheral blood mononuclear cells (PBMC), previously collected in Blood Sample Collection section was performed by PBMC centrifugation (2,000 RPM, 30 min) under a Ficoll separation gradient (1.077 g/mL; GE Healthcare Life Sciences®), as cells were washed twice with 1X PBS (1600 RPM, 10 min) and stained with specific antibodies. Labeling was done using anti-CD3-FITC, anti-CD4-PercPCy5, anti-CD8-PE, anti-CD14-FITC, anti-CD19-FITC, anti-CD27-PECY7, anti-CD80-PE and Anti-HLA-DR-PercPCy5 (all from BD Biosciences®). Cell acquisition was performed in 10,000 events in a FACSVerse flow cytometer (BD Biosciences®) and fluorescence data analysis was performed using BD FACSuite® software.
Gene expression analysis
RNA extraction and cDNA synthesis
Total RNA extraction was performed using isolated PBMC, at a cell concentration of 5x106 and homogenized using 1 mL of liquid Trizol (Invitrogen®). Purification of isolated total RNA was performed using the RNeasy® Mini Kit (QIAGEN®) following the manufacturer’s instructions. RNA quality was assured by a NanoDrop 2000 spectrophotometer (Thermo Scientific® Wilmington, USA) and 1% agarose gel electrophoresis. Subsequently, 1μg of purified RNA of suitable quality (an OD260/280 of 1.8 to 2.1 and intact rRNA subunits - 28S and 18S) was used to synthesize cDNA using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR with dsDNase (Thermo Scientific® Wilmington, USA). Water was used as a negative control RT reaction for each sample.
Primer design and efficiency estimation for qPCR
The primers used to detect the STAT4, JAK2, STAT6, STAT3 and FOXO4 genes were designed based on previous studies (Park et al. 2019aPARK SY, LEE CJ, CHOI JH, KIM JH, KIM JW, KIM JY & NAM JS. 2019a. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res 38(1): 1-18., bPARK SJ, KIM H, KIM SH, JOE EH & JOU I. 2019b. Epigenetic downregulation of STAT6 increases HIF-1α expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells. Acta Neuropathol Comm 7(1): 1-19., Su et al. 2014SU L ET AL. 2014. The transcription factor FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer 14(1): 1-11., Usui et al. 2003USUI T, NISHIKOMORI R, KITANI A & STROBER W. 2003. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rβ2 chain or T-bet. Immunity 18(3): 415-428., Zhao et al. 2018ZHAO M ET AL. 2018. IL- 6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation. Nature Comm 9(1): 1-14.). The reference genes, ACTB and GAPDH, were used for relative quantification. These genes were previously validated in PBMC samples (Eyerich et al. 2009EYERICH S ET AL. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119(12): 3573-3585., Lewkowicz et al. 2011LEWKOWICZ N, KUR B, KURNATOWSKA A, TCHORZEWSKI H & LEWKOWICZ P. 2011. Expression of Th1/Th2/Th3/Th17-related genes in recurrent aphthous ulcers. Arch Immunol Ther Exp 59(5): 399-406., Mousset et al. 2019MOUSSET CM ET AL. 2019. Comprehensive phenotyping of T cells using flow cytometry. Cytometry Part A 95(6): 647-654.). A cDNA from a positive PBMC sample was used to exemplify a real test condition.
Expression evaluation by qPCR
The RT-qPCR reaction was performed in a final volume of 10 µL using the Fast SYBR® Green Master Mix kit (Applied Biosystems®). For reading, the AriaMx Real-Time PCR System (Agilent Technologies®) was used according to the following parameters: 95 ºC for 20s for polymerase activation, 40 cycles of 95 ºC for 3s for denaturation and 60 ºC for 30s for annealing and extension. Thereafter, the geometric means of reference genes (ACTB and GAPDH) was used to calculate the relative expression of all targets (Livak & Schmittgen 2001LIVAK KJ & SCHMITTGEN TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2^(-ΔΔCT) method. Methods 25: 402-408.).
Statistical analysis
The D’Agostino test was applied to test the normality of the hypothesis and the statistical differences between groups were analyzed by one-way analysis of variance (ANOVA) with the student’s t-test confirmation. As for the results of genes expression, it was used the Mann-Whitey test. All results are expressed as the means of the values of the groups ± standard deviation and analyzed considering the value of p <0.05 as statistically significant. The graphs were processed by GraphPad Prism 9 Software®.
RESULTS
This study aimed to investigate, independently, the immunological profile promoted by the CoronaVac vaccine (Sinovac Research and Development Co®) in healthcare workers continuously exposed to patients contaminated by SARS-CoV-2, and symptomatic, in the scope of the Hospital’s Wards and ICU of the Clinics of the Federal University of Pernambuco. Although some articles that are being published demonstrate the effectiveness of the vaccine, including for some variants that have started to circulate around the world, our main focus was to observe the effects in a group at risk for continuous infection by the virus in the inter-dose and post-second dose period.
Blood parameters of vaccinated healthcare workers (red blood cells, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (HCM), mean corpuscular hemoglobin concentration (MHCM), red cell distribution width (RDW), leukocytes totals, neutrophils, eosinophils, basophils, lymphocytes, atypical lymphocytes, monocytes, and platelets) were evaluated comparing the results of individuals in the first and second doses of the vaccine. Most of the hematological parameters did not show changes in relation to the reference values nor in the comparison of the two doses. However, it was observed that healthcare workers vaccinated with the second dose had increased hematocrit, monocyte and lymphocyte parameters (Figure 1).
Hematological results of 50 healthcare workers vaccinated with CoronaVac. Gray vertical bars are the values observed 15 days after the first dose and black vertical bars are the values observed 15 days after the second dose.
The biochemical parameters investigated were creatinine, creatine phosphokinase (CPK), lactate dehydrogenase (LDH), ferritin, gamma glutamyl transferase (GGT), oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), urea, C-reactive protein (CRP), bilirubin, sodium, potassium, chloride and fibrinogen. Regarding these parameters, both groups did not present statistical differences between the first and the second dose.
The immunological study of vaccines followed two lines of reasoning, the elicitation of the cellular immune response (which investigates the cellular subtypes and activation pathways of the immune response directed by the T helper lymphocyte) and the humoral immune response (investigation of cytokines and immunoglobulins).
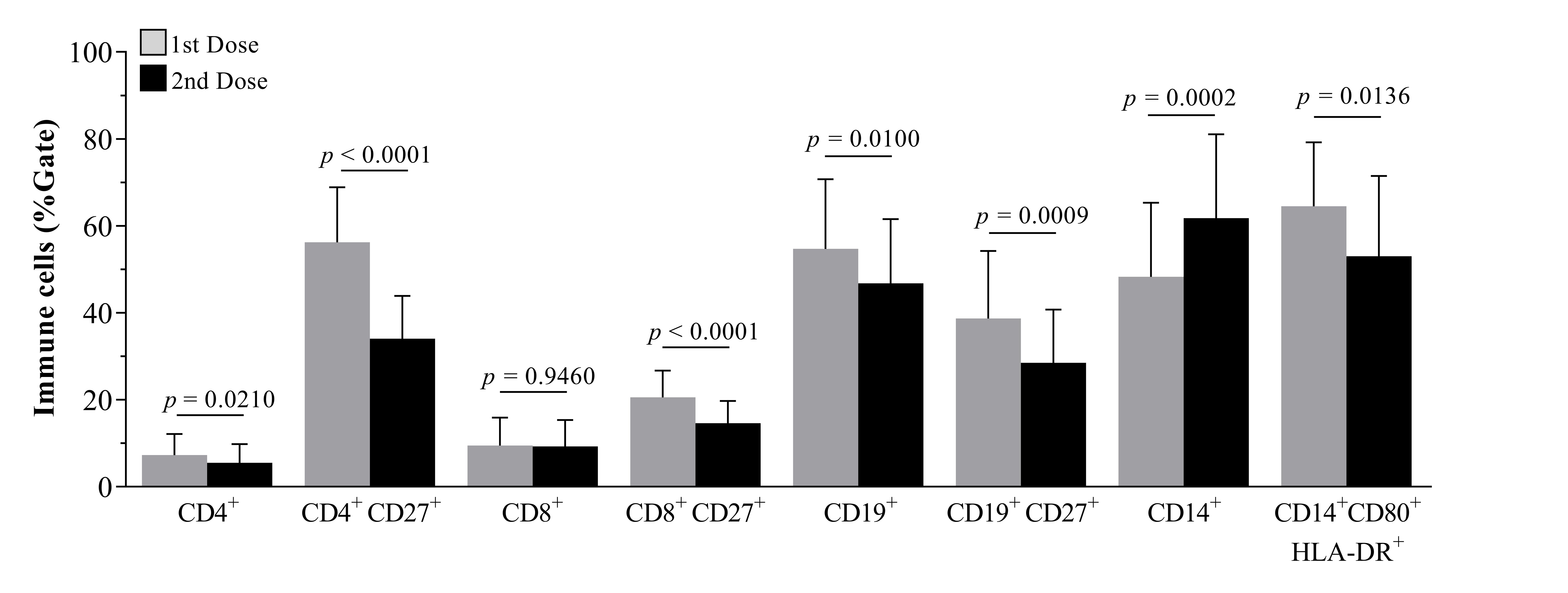
Cellular immunological analysis investigated the cellular memory (using the CD27 antibody) and monocytic effector action (CD80+HLA-DR+) profiles. In this sense, the results of immunophenotyping showed that there was a significant increase in monocytes and lymphocytes, with a decreasing prevalence of monocytes, B lymphocytes, CD8+ T lymphocytes and CD4+ T lymphocytes (Figure 2). When comparing doses, CD4+ and CD19+ lymphocytes (B lymphocytes) were increased after the first dose of the vaccine, but with no change to CD8+ T lymphocytes. Furthermore, this post-first dose increase was associated with phenotypic profiles of immunological memory (CD27+), where all sublines investigated (T CD4+, TCD8+ and CD19+) showed a significant increase in relation to the second dose. Monocyte lineage profiles corroborated the results of increased monocyte counts (Figure 1) for the second dose (CD14+). It is worth noting that about 60% of these monocytes had an activated profile, that is, an effector function for antigen presentation (CD80+HLA-DR+) in both the first and second doses of the vaccine, although the highest values were in the first dose (Figure 2).
Immunophenotyping of immune cells of 50 healthcare workers vaccinated with CoronaVac. Gray vertical bars are the values observed 15 days after the first dose and black vertical bars are the values observed 15 days after the second dose.
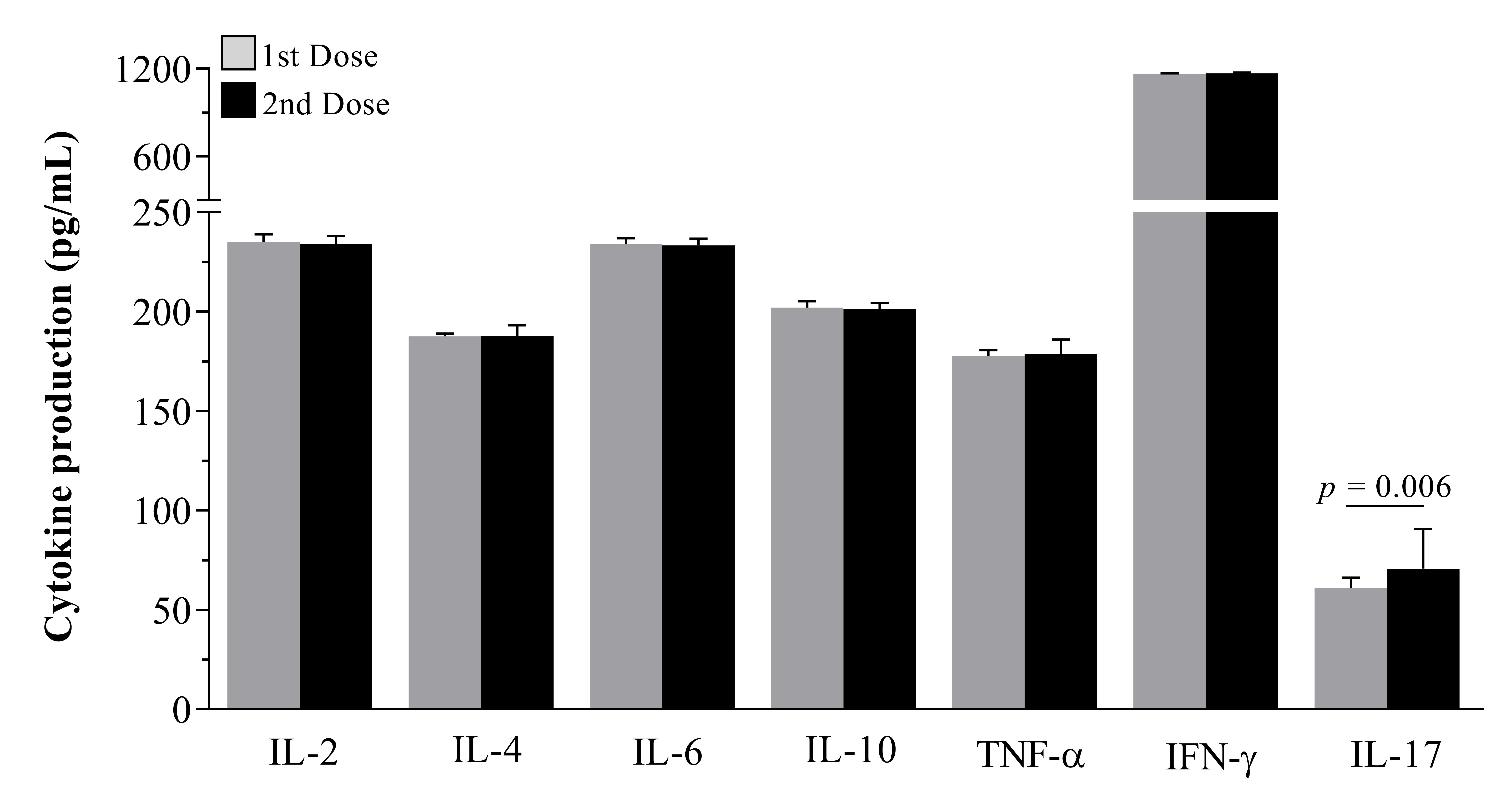
Regarding the results of the production of Th1, Th2 and Th17 cytokines, we could observe a certain balance in the values of the production of cytokines IL-2, IL-4, IL-6, IL-10 and TNF-α between the first and second dose (Figure 3). However, the cytokines IL-2, IL-6 and IFN-° showed increased values in relation to the other analyzed cytokines, with a predominant emphasis for IFN-. In addition, IL-17 cytokine values were statistically increased in the second dose (p = 0.0068) and TNF-α also showed a slight increase in the second dose (176.5±1.65; 179.3±7.5 for first and second doses, respectively) but with no statistical difference between them.
Levels of Th1, Th2 and Th17 cytokines found in the serum of 50 healthcare workers in the first and second doses with CoronaVac. Gray vertical bars are the values observed 15 days after the first dose and black vertical bars are the values observed 15 days after the second dose.
In the analysis of gene expression for the genes STAT4 (Th1), JAK2 (pleiotropic gene for Th1 and Th17 responses), STAT3 (Th17), STAT6 (Th2) and FOXO4 (Th22) there was an activation of all these genes in the second dose (Figure 4). However, the JAK2, STAT3 and STAT6 genes were predominant in relation to the others, characterizing a major T helper response of the Th2/Th17 type after the second dose.
Results of the immune response pathways in healthcare workers vaccinated in the first and second doses with CoronaVac. All genes showed a statistical increase between the first and second dose, value of p<0.001. Genes associated with the Th2/Th17 response (JAK2, STAT3 and STAT6) were seen at high values for healthcare workers who received the second dose of the vaccine.
For the analysis of immunoglobulins, three screening conditions for anti-SARS- CoV-2 antibodies were performed, the first condition occurred in the pre-vaccination period, the second in 15 days after the first dose and the third also 15 days after the second dose of the vaccine. The objective of the first screening was to elucidate whether professionals had contact with the virus in the pre-vaccination stage. With these results, we could elucidate whether the increase in immunoglobulins was due to the vaccine or whether the professionals already had an increased profile because they had previously been contaminated with the virus. In this case, two variables would need to be clarified: 1) the detection of pre-vaccination anti-SARS-CoV-2 immunoglobulins would increase or not the production of post-vaccination antibodies and 2) the detection of pre- vaccination anti-SARS-CoV-2 immunoglobulins-vaccination is a condition of better immune response for the post-vaccination phase. The results obtained were interpreted as follows: 1) individuals not detected by the kit and individuals negative for both immunoglobulins belong to the same line of reasoning, that is, they did not develop the disease. The results showed that these individuals presented production of both IgM and IgG after vaccination with statistically significant values in the comparison between doses. 2) The groups that showed detection of immunoglobulins in the pre-vaccination phase belong to another line of reasoning, that is, they had the disease, even if asymptomatically, as reported by all in their own questionnaire. In this case, despite the increased values in the second dose of the vaccine, only professionals reactive for IgG showed significant values in the production of IgG in the second dose.
The results of pre-vaccination screening detected 28 (56%) health professionals considered non-reactive or negative and 22 (44%) reagents to immunoglobulins (revealing previous contact with the virus). Figure 5 shows the comparison of immunoglobulin production in first and second doses between these two groups. The results demonstrate that there was no statistical difference in the production of immunoglobulins between negative individuals and those who presented an anti-SARS-CoV-2 antibody profile in any analyzed dose.
Production of anti-SARS-CoV-2 IgM and IgG immunoglobulins in non-reactive or negative healthcare workers 28 (56%) for immunoglobulins and positive 22 (44%) in the pre-vaccination screening. HW = Healthcare workers.
The results that consider only the 50 vaccinated individuals, with no relevance to the history of laboratory detection for previous infection (or not) with the SARS-CoV-2 virus, can be seen in Figure 6 and demonstrated a significant increase in IgM and IgG for the vaccinated in the second dose.
Index of serum immunoglobulin analysis of 50 healthcare workers vaccinated in the first and second doses with CoronaVac. Horizontal bars in gray represent the first dose and horizontal bars in black represent the second dose of the vaccine.
DISCUSSION
CoronaVac immunologically reproduces the same steps observed by wild virus, maintaining a cellular antigenic memory profile. In addition, we also needed to understand whether the individuals used as research subjects, healthcare workers exposed daily to patients with COVID-19, was a limiting factor in the action of the immunobiological, since we did not intend to study/question the efficacy and safety of vaccine that have already been proven in several multicenter studies (Faria et al. 2021FARIA E, GUEDES AR, OLIVEIRA MS, MOREIRA MVG, MAIA FL, BARBOZA AS & LEVIN AS. 2021. Performance of vaccination with CoronaVac in a cohort of healthcare workers (HCW)-preliminary report. https://doi.org/10.1101/2021.04.12.21255308.
https://doi.org/10.1101/2021.04.12.21255...
, Hitchings et al. 2021HITCHINGS MD ET AL. 2021. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P. 1 variant transmission in Brazil: A test-negative case-control study. https://doi.org/10.1016/j.lana.2021.100025.
https://doi.org/10.1016/j.lana.2021.1000...
, Tanriover et al. 2021TANRIOVER MD, DOĞANAY HL, AKOVA M, GÜNER HR, AZAP A, AKHAN S & AKSU K. 2021. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. The Lancet 398(10296): 213-222., Wu et al. 2021aWU K ET AL. 2021. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. DOI: 10.1101/2021.01.25.427948.
https://doi.org/10.1101/2021.01.25.42794...
, bWU Z, HU Y, XU M, CHEN Z, YANG W, JIANG Z & YIN W. 2021b. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo- controlled, phase 1/2 clinical trial. The Lancet Infect Dis 21(6): 803-812.).
The health professionals vaccinated in this study showed an increase in the number of immune cells, both for lymphocytes and monocytes after the second dose of the vaccine (Figure 1), these data were corroborated by the characterization of sublines with increased monocytes, CD19+ lymphocytes, CD8+ T lymphocytes and T CD4+ (Figure 4). The increase in monocytes and especially their M1 activated effector phenotype (CD14+/CD80+/HLA-DR+), observed in this study, reveals an important finding for mucosal protection (site of action of SARS-CoV-2), antiviral action and for the antigen presentation mechanism in COVID-19 (Burdo et al. 2015BURDO TH, WALKER J & WILLIAMS KC. 2015. Macrophage polarization in AIDS: dynamic interface between anti-viral and anti-inflammatory macrophages during acute and chronic infection. J Clin Cell Immunol 6: 333.).
The healthcare workers vaccinated in this study showed an increase in the number of immune cells, both for lymphocytes and monocytes after the second dose of the vaccine (Figure 1), these data were corroborated by the characterization of sublines with increased monocytes, CD19+ lymphocytes, CD8+ T lymphocytes and T CD4+ (Figure 4). The increase in monocytes and especially their M1 activated effector phenotype (CD14+CD80+HLA-DR+), observed in this study, reveals an important finding for mucosal protection (site of action of SARS-CoV-2), antiviral action and for the antigen presentation mechanism in COVID-19 (Burdo et al. 2015BURDO TH, WALKER J & WILLIAMS KC. 2015. Macrophage polarization in AIDS: dynamic interface between anti-viral and anti-inflammatory macrophages during acute and chronic infection. J Clin Cell Immunol 6: 333.).
compared to the first. These cytokines are directly involved with the immune response against SARS-CoV-2, often so exacerbated that it can promote the cytokine storm in severe disease. An important point to be highlighted is the majority of IFN-° production that was observed, which, as previously mentioned, is suppressed by the virus during the infectious phase (Singh et al. 2020SINGH M, PRASAD N, RAI MK, BEHERA MR, AGARWAL V & MISRA DP. 2020. Role of IL-6 and IL-17 mediated inflammation amplifier loop in chronic antibody-mediated rejection (CABMR). Transplantation 104(S3): S54.). What is known so far is that, in COVID-19, IFN-° is activated and helps epithelial cells and macrophages in the intracellular antiviral defense and prevents the advance to severe COVID-19 (Singh et al 2020). Furthermore, IL-6 and IL-17 are cytokines that activate the JAK2/STAT3 pathway (Wu & Yang 2020WU D & YANG XO. 2020. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53(3): 368-370.), and IL-6 promotes the recruitment of neutrophils and cytotoxic T cells (Singh et. 2020, Wu & Yang 2020WU D & YANG XO. 2020. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53(3): 368-370.) and IL-17, a molecule associated with protection against a variety of pathogens on the mucosal surfaces of the respiratory tract, including influenza viruses and SARS-CoV-2 itself (Norton et al. 2012NORTON EB, LAWSON LB, MAHDI Z, FREYTAG LC D & CLEMENTS JD. 2012. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun 80(7): 2426-2435., Gallorini et al. 2014GALLORINI S, TACCONE M, BONCI A, NARDELLI F, CASINI D, BONIFICIO A, KOMMAREDDY S, BERTHOLETA S, O’HAGAN DT & BAUDNER BC. 2014. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine 32(20): 2382-2388., Wu et al. 2020WU C ET AL. 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med 180(7): 934-943. Doi: 10.1001/jamainternmed.2020.0994.).
The results of the analyzed immunological pathways corroborate all the studies mentioned above, with specific activation JAK2/STAT3/STAT6 corresponding to a Th2/Th17 response, especially overexpressed in the second dose of the vaccine (Figure 5). Since the Th2 response observed in this study (which was not confirmed by the prevalent production of IL-4) is not a response directed towards antiviral action, we suggest that it is being confirmed by only one gene (STAT6), with others being prevalent two canonical genes confirming the Th17 pathway (JAK2 and STAT3). In this case, it is possible that STAT6 elicitation is associated with the CoronaVac vaccine adjuvant and not with the inactivated virus components. Similar to the findings of this study with CoronaVac, Miskulin et al. (2021)MISKULIN D & COMBE C. 2021. mRNA COVID-19 Vaccine for People with Kidney 517 Failure. Clin J Am Soc Nephrol 16(7): 996-998. analyzing the immune response of an mRNA vaccine (Pfizer/BNT162b2) observed that there is activation of CD4+ and CD8+ T lymphocytes, inductors of robust production of IFN-° and, consequently, with a Th1 profile (Vogel et al. 2020VOGEL AB, KANEVSKY I, CHE Y, SWANSON KA, MUIK A, VORMEHR M & SAHIN U. 2020. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. BioRxiv 2020.09. 08.280818., Cascella et al. 2021CASCELLA M, RANJNIK M, ALEEM A, DULEBOHN SC & NAPOLI RD. 2021. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls., Wu et al. 2021aWU K ET AL. 2021. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. DOI: 10.1101/2021.01.25.427948.
https://doi.org/10.1101/2021.01.25.42794...
, b).
This same response linked to a Th1 profile can also be observed for other vaccines against SARS-CoV-2, such as the single- dose adenoviral vector-based vaccine (Janssen and Beth Israel Deaconess Medical Center/JNJ-78436735/Ad26.COV2.S) (Mercado et al. 2020MERCADO NB ET AL. 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586(7830): 583-588.) and the adenoviral vector- based booster dose vaccine (AstraZeneca/Oxford University/AZD1222) (Graham et al. 2020GRAHAM SP ET AL. 2020. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines 5(1): 1-6., Silva-Cayetano et al. 2021SILVA-CAYETANO A ET AL. 2021. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med 2(3): 243-262. E8., Watanabe et al. 2021WATANABE Y ET AL. 2021. Native-like SARS-CoV-2 spike glycoprotein expressed by ChAdOx1 nCoV-19/AZD1222 vaccine. ACS Central Science 7(4): 594-602.).
Studies show that the response of anti-SARS-CoV-2 T cells during COVID-19 may not depend on circulating antibodies (Sewell et al. 2020SEWELL HF, AGIUS RM, STEWART M & KENDRICK D. 2020. Cellular immune responses to covid-19. Bmj 370: m3018. DOI: 10.1136/bmj.m3018.) and have pointed to the need for the formulation of immunobiological that strongly activate T lymphocytes and B, memory, since these cells are critical for the protection and maintenance of the individual’s defense system against reinfections and the establishment of COVID-19 (Grifoni et al. 2020GRIFONI A ET AL. 2020. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181: 1489–501.e15., Sekine et al. 2020SEKINE T, PEREZ-POTTI A, RIVERA-BALLESTEROS O, STRÅLIN K, GORIN JB, OLSSON A & BUGGERT M. 2020. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183(1): 158-168.) By specifically analyzing patients recovered from COVID-19, it is possible to identify a robust immune response of neutralizing antibodies (IgG and IgA) Spike-specific, which can decay around two months after the absence of symptoms, memory B cells and cells TFH (follicular T lymphocytes), which indicates elicitation of plasma cells (effector B lymphocytes) and maturation of the humoral immune response (Cox & Brokstad 2020COX RJ & BROKSTAD KA. 2020. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nature Rev Immunol 20(10): 581-582., Huang et al. 2020HUANG C, WANG Y, LI X, REN L, ZHAO J, HU Y, ZHANG L, FAN G, XU J & GU X. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395(10223): 497-506.). All these parameters were observed and elicited by CoronaVac in health professionals immunized in this study.
As previously reported, there was a large elicitation of B lymphocyte response, especially memory, and in the first dose of the vaccine. However, the proof of effector activity of these cells (activated plasma cells) occurred through the increasing immunoglobulin release profile between prime and boost (Figure 6). Other independent studies like this one corroborate our findings. Özdemir et al. (2021)ÖZDEMIR İH, ÖZLEK B, ÖZEN MB, GÜNDÜZ R & BAYTURAN Ö. 2021. Type 1 Kounis syndrome induced by inactivated SARS-CoV-2 vaccine. J Emerg Med 61(4): e71-e76. demonstrated that 91.6% of the 264 healthcare workers vaccinated with CoronaVac had anti-SARS-CoV-2 IgG titres after the second-dose, Calil et al. (2021)CALIL VMLT, PALMEIRA P, ZHENG Y, KREBS VLJ, CARVALHO WBD & CARNEIRO-SAMPAIO M. 2021. CoronaVac can induce the production of anti- SARS-CoV-2 IgA antibodies in human milk. Clinics 76. investigating breast milk samples from 16 vaccinated mothers showed a significant increase in anti-SARS-CoV-2 IgA and no decay for one month after the second vaccine dose and Bochnia-Bueno et al. (2021)BOCHNIA-BUENO L, DE ALMEIDA SM, RABONI SM, ADAMOSKI D, AMADEU LLM, CARSTENSEN S & NOGUEIRA MB. 2021. SARS-CoV-2 vaccination with CoronaVac: seroconversion rate in healthcare workers after 40 days. Available at SSRN 3831124., investigating the seroconversion of antibodies from 133 healthcare workers vaccinated with CoronaVac showed that after 20 days of the second dose, 97% of the sera had anti- Spike IgG and 52% had anti-N SARS-CoV-2 IgG.
CONCLUSIONS
The healthcare workers in this study had two doses of the CoronaVac vaccine and did not show side effects. The immunological response balance was interesting, showing memory activation in the first dose (increasing of CD4+CD27+, CD8+CD27+, and CD19+CD27+ subsets) and effector activation (increasing of CD4+ and CD19+ lymphocytes, CD80+HLA-DR+ monocytes, and IgM and IgG immunoglobulins) in the second dose. Thus, the results point to the fact that the second dose of CoronaVac enhanced the immunological response through the increase of the Th2/Th17 immunological profile, linked to a response of T lymphocytes, B lymphocytes, monocytes, memory formation, and immunoglobulin production.
ACKNOWLEDGMENTS
We are grateful to all healthcare professionals to agree with our study and help us with their experience in hospital service. In addition, to the Clinical Hospital of the Federal University of Pernambuco for its technical assistance, staff collaboration and laboratory exams performed. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, process 88887.507421/2020-00) 402 and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process 308489/2019-5). The authors declare that they have no conflicts of interest.
REFERENCES
- ANVISA - AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA. 2021. Covid-19: quadro de análises de vacinas pela Anvisa. Link: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/covid-19-quadro-de-analises-de-vacinas-pela-anvisa Acessado em 6 de junho de 2021.
» https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/covid-19-quadro-de-analises-de-vacinas-pela-anvisa - ARONS MM, HATFIELD KM, REDDY SC, KIMBALL A, JAMES A, JACOBS JR & JERNIGAN JA. 2020. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. New England J Med 382(22): 2081-2090.
- BOCHNIA-BUENO L, DE ALMEIDA SM, RABONI SM, ADAMOSKI D, AMADEU LLM, CARSTENSEN S & NOGUEIRA MB. 2021. SARS-CoV-2 vaccination with CoronaVac: seroconversion rate in healthcare workers after 40 days. Available at SSRN 3831124.
- BUITRAGO-GARCIA D, EGLI-GANY D, COUNOTTE MJ, HOSSMANN S, IMERI H, IPEKCI AM & LOW N. 2020. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Medicine 17(9): e1003346.
- BURDO TH, WALKER J & WILLIAMS KC. 2015. Macrophage polarization in AIDS: dynamic interface between anti-viral and anti-inflammatory macrophages during acute and chronic infection. J Clin Cell Immunol 6: 333.
- CALIL VMLT, PALMEIRA P, ZHENG Y, KREBS VLJ, CARVALHO WBD & CARNEIRO-SAMPAIO M. 2021. CoronaVac can induce the production of anti- SARS-CoV-2 IgA antibodies in human milk. Clinics 76.
- CASCELLA M, RANJNIK M, ALEEM A, DULEBOHN SC & NAPOLI RD. 2021. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls.
- CHAN JFW ET AL. 2020. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet 395(10223): 514-523.
- COX RJ & BROKSTAD KA. 2020. Not just antibodies: B cells and T cells mediate immunity to COVID-19. Nature Rev Immunol 20(10): 581-582.
- DE SOUZA SILVA GA ET AL. 2020. SARS-CoV, MERS-CoV and SARS-CoV-2 infections in pregnancy and fetal development. J Gynec Obstetr Human Reprod 49(10): 101846.
- EYERICH S ET AL. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119(12): 3573-3585.
- FARIA E, GUEDES AR, OLIVEIRA MS, MOREIRA MVG, MAIA FL, BARBOZA AS & LEVIN AS. 2021. Performance of vaccination with CoronaVac in a cohort of healthcare workers (HCW)-preliminary report. https://doi.org/10.1101/2021.04.12.21255308
» https://doi.org/10.1101/2021.04.12.21255308 - FURUKAWA NW, BROOKS JT & SOBEL J. 2020. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis 26(7).
- GALLORINI S, TACCONE M, BONCI A, NARDELLI F, CASINI D, BONIFICIO A, KOMMAREDDY S, BERTHOLETA S, O’HAGAN DT & BAUDNER BC. 2014. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization, but also induces local IgA and TH17 responses. Vaccine 32(20): 2382-2388.
- GRAHAM SP ET AL. 2020. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. NPJ Vaccines 5(1): 1-6.
- GRIFONI A ET AL. 2020. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181: 1489–501.e15.
- HITCHINGS MD ET AL. 2021. Effectiveness of CoronaVac in the setting of high SARS-CoV-2 P. 1 variant transmission in Brazil: A test-negative case-control study. https://doi.org/10.1016/j.lana.2021.100025
» https://doi.org/10.1016/j.lana.2021.100025 - HUANG C, WANG Y, LI X, REN L, ZHAO J, HU Y, ZHANG L, FAN G, XU J & GU X. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395(10223): 497-506.
- LEWKOWICZ N, KUR B, KURNATOWSKA A, TCHORZEWSKI H & LEWKOWICZ P. 2011. Expression of Th1/Th2/Th3/Th17-related genes in recurrent aphthous ulcers. Arch Immunol Ther Exp 59(5): 399-406.
- LI C, JI F, WANG L, WANG L, HAO J, DAI M & GU B. 2020. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, Xuzhou, China. Emerg Infect Dis 26(7) 1626.
- LI Q, GUAN X, WU P, WANG X, ZHOU L, TONG Y & FENG Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England J Med 382: 1199-1207.
- LIVAK KJ & SCHMITTGEN TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2^(-ΔΔCT) method. Methods 25: 402-408.
- LU H, STRATTON CW & TANG YW. 2020. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 92(4): 401.
- MELO CMD, SILVA GA, MELO AR & FREITAS AC. 2020. COVID-19 pandemic outbreak: the Brazilian reality from the first case to the collapse of health services. An Acad Bras Cienc 92: e20200709. DOI 10.1590/0001-3765202020200709.
- MERAD M & MARTIN JC. 2020. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature Rev Immunol 20(6): 355-362.
- MERCADO NB ET AL. 2020. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586(7830): 583-588.
- MISKULIN D & COMBE C. 2021. mRNA COVID-19 Vaccine for People with Kidney 517 Failure. Clin J Am Soc Nephrol 16(7): 996-998.
- MOUSSET CM ET AL. 2019. Comprehensive phenotyping of T cells using flow cytometry. Cytometry Part A 95(6): 647-654.
- NORTON EB, LAWSON LB, MAHDI Z, FREYTAG LC D & CLEMENTS JD. 2012. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun 80(7): 2426-2435.
- OPAS - ORGANIZAÇÃO PAN-AMERICANA DE SAÚDE. 2020. COVID-19 has infected some 570,000 health workers and killed 2,500 in the Americas, PAHO Director says. https://www.paho.org/en/news/2-9-2020-covid-19-has-infected-some-570000-health- workers-and-killed-2500-americas-paho Acessado em 17 de julho de 2021.
» https://www.paho.org/en/news/2-9-2020-covid-19-has-infected-some-570000-health- workers-and-killed-2500-americas-paho - ÖZDEMIR İH, ÖZLEK B, ÖZEN MB, GÜNDÜZ R & BAYTURAN Ö. 2021. Type 1 Kounis syndrome induced by inactivated SARS-CoV-2 vaccine. J Emerg Med 61(4): e71-e76.
- PARK SY, LEE CJ, CHOI JH, KIM JH, KIM JW, KIM JY & NAM JS. 2019a. The JAK2/STAT3/CCND2 Axis promotes colorectal Cancer stem cell persistence and radioresistance. J Exp Clin Cancer Res 38(1): 1-18.
- PARK SJ, KIM H, KIM SH, JOE EH & JOU I. 2019b. Epigenetic downregulation of STAT6 increases HIF-1α expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells. Acta Neuropathol Comm 7(1): 1-19.
- SEKINE T, PEREZ-POTTI A, RIVERA-BALLESTEROS O, STRÅLIN K, GORIN JB, OLSSON A & BUGGERT M. 2020. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183(1): 158-168.
- SEWELL HF, AGIUS RM, STEWART M & KENDRICK D. 2020. Cellular immune responses to covid-19. Bmj 370: m3018. DOI: 10.1136/bmj.m3018.
- SILVA-CAYETANO A ET AL. 2021. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med 2(3): 243-262. E8.
- SINGH M, PRASAD N, RAI MK, BEHERA MR, AGARWAL V & MISRA DP. 2020. Role of IL-6 and IL-17 mediated inflammation amplifier loop in chronic antibody-mediated rejection (CABMR). Transplantation 104(S3): S54.
- SU L ET AL. 2014. The transcription factor FOXO4 is down-regulated and inhibits tumor proliferation and metastasis in gastric cancer. BMC Cancer 14(1): 1-11.
- SUÁREZ-GARCÍA I, DE ARAMAYONA LÓPEZ MM, VICENTE AS & ABASCAL PL. 2020. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect 106(2): 357-363.
- TANRIOVER MD, DOĞANAY HL, AKOVA M, GÜNER HR, AZAP A, AKHAN S & AKSU K. 2021. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. The Lancet 398(10296): 213-222.
- USUI T, NISHIKOMORI R, KITANI A & STROBER W. 2003. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rβ2 chain or T-bet. Immunity 18(3): 415-428.
- VOGEL AB, KANEVSKY I, CHE Y, SWANSON KA, MUIK A, VORMEHR M & SAHIN U. 2020. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. BioRxiv 2020.09. 08.280818.
- WANG D, HU B, HU C, ZHU F, LIU X, ZHANG J & PENG Z. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 323(11): 1061-1069.
- WATANABE Y ET AL. 2021. Native-like SARS-CoV-2 spike glycoprotein expressed by ChAdOx1 nCoV-19/AZD1222 vaccine. ACS Central Science 7(4): 594-602.
- WU C ET AL. 2020. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med 180(7): 934-943. Doi: 10.1001/jamainternmed.2020.0994.
- WU D & YANG XO. 2020. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53(3): 368-370.
- WU K ET AL. 2021. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. DOI: 10.1101/2021.01.25.427948.
» https://doi.org/10.1101/2021.01.25.427948 - WU Z, HU Y, XU M, CHEN Z, YANG W, JIANG Z & YIN W. 2021b. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo- controlled, phase 1/2 clinical trial. The Lancet Infect Dis 21(6): 803-812.
- ZHAO M ET AL. 2018. IL- 6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation. Nature Comm 9(1): 1-14.
Publication Dates
-
Publication in this collection
27 June 2022 -
Date of issue
2022
History
-
Received
16 Dec 2021 -
Accepted
7 Feb 2022