Abstracts
This retrospective study assessed 17 DM1 pediatric patients (15.76 ± 4.5 years) submitted to 72h continuous glucose monitoring system (CGMS) (Medtronic, CA). The aim of this study was to evaluate the accuracy of CGMS in children and adolescents with type 1 diabetes mellitus (DM1) and the efficacy of this method to detect unrecognized hypoglycemia in this population. It were analyzed capillary glycemia (CG) and CGMS sensor’s value; glycemic excursions; postprandial hyperglycemia; unrecognized hypoglycemia; complications and therapeutic management after CGMS. A1c levels were measured at the start and after 3 months of the study. Correlation coefficient during hypo, hyper, and normoglycemia and sensitivity/specificity was determined. The mean CG values were 213.8 ± 63.4mg/dl vs. 209.7 ± 52.5mg/dl by sensor, with statistical significance by Pearson’s correlation (p< 0.001). There was no difference between CGMS and CG value in order to detect glycemic excursions (p= 0.32). The postprandial hyperglycemia and unrecognized hypoglycemia was detected in 66.7% and 56.2% of this patients, respectively. The correlation coefficient during hypoglycemia presented no statistical significance by Pearson’s correlation (p= 0.29) vs. during hyperglycemia (p= 0.001). The CGMS sensor presented low sensitivity (63.3%) to detect hypoglycemia. This data showed important decreased level of A1c in this population 3 months after CGMS with statistical significance (p= 0.03). The CGMS showed to be a very safe method, well tolerated, with high accuracy in glycemic values and low complications rate. This results suggest that CGMS is a good method to identify postprandial hyperglycemia, to improve metabolic changes in therapeutics with significant impact in A1c of diabetic pediatric patients. This data confirmed the low sensitivity of CGMS to detect unrecognized hypoglycemia in pediatric DM1 patients.
Continuous glucose monitoring system; Unrecognized hypoglycemia; Children; adolescents; Diabetes mellitus type 1
Foram estudados retrospectivamente 17 pacientes (15,76 ± 4,5 anos) submetidos a monitorização subcutânea contínua da glicemia (CGMS) (Medtronic; CA) por 72 horas. Buscou-se avaliar a acurácia da CGMS em crianças e adolescentes com diabetes mellitus tipo 1 (DM1) e sua eficácia na detecção de hipoglicemia assintomática nesse grupo de pacientes. Foram analisados os valores de glicemia capilar (GC) e pelo sensor CGMS; excursões glicêmicas; hiperglicemia pós-prandial; hipoglicemia assintomática; complicações e conduta após a CGMS. Os níveis de A1c foram determinados antes e 3 meses após a CGMS. A correlação sensor/glicemia capilar foi avaliada durante normo, hipo e hiperglicemia, bem como a sensibilidade e especificidade. A GC média durante a CGMS foi de 213,8 ± 63,4mg/dl vs. 209,7 ± 52,5mg/dl detectada pelo sensor, com correlação de Pearson significante (p< 0,001). Não houve diferença significante entre a CGMS e GC na detecção de excursões glicêmicas (p= 0,32). A hiperglicemia pós-prandial e hipoglicemia assintomática foram identificadas em 66,7% e 56,2% dos pacientes durante a CGMS, respectivamente. A correlação entre sensor CGMS e GC durante hipoglicemia assintomática mostrou-se não significante (p= 0,32) vs. durante hiperglicemia (p= 0,001). O sensor CGMS apresentou baixa sensibilidade (63,3%) para detecção de hipoglicemia assintomática. Observou-se redução significante da A1c três meses após a CGMS em crianças e adolescentes com DM1 (p= 0,03). A CGMS mostrou-se método seguro, bem tolerado, com alta acurácia nos valores glicêmicos detectados, com baixo índice de complicações. Esses dados comprovam a alta eficácia da CGMS na detecção de hiperglicemia pós-prandial, na redução da A1c e melhora do controle metabólico, com baixas sensibilidade para na identificação de hipoglicemia assintomática em crianças e adolescentes com DM1.
Monitorização subcutânea contínua da glicemia; Hipoglicemia assintomática; Crianças; adolescentes; Diabetes mellitus tipo 1
ARTIGO ORIGINAL
Efficacy of continuous glucose monitoring system to detect unrecognized hypoglycemia in children and adolescents with type 1 diabetes
Eficácia da monitorização subcutânea contínua da glicemia (CGMS) na detecção da hipoglicemia assintomática em crianças e adolescentes com diabetes tipo 1
Frederico F.R. Maia; Levimar R. Araújo
Department of Internal Medicine (FFRM) and Department of Endocrinology and Metabolism (LRA), Hospital Universitário São José, Faculdade de Ciências Médicas de Minas Gerais, Belo Horizonte, MG, Brazil
Correspondence Correspondence to Frederico Fernandes Ribeiro Maia Clínica Médica Hospital Universitário São José, FCMMG R. Nunes Vieira 299, ap. 702 30350-120 Belo Horizonte, MG Fax: (31) 3283-9772 E-mail: fredfrm@hotmail.com / frederico@diabetes.med.br
ABSTRACT
This retrospective study assessed 17 DM1 pediatric patients (15.76 ± 4.5 years) submitted to 72h continuous glucose monitoring system (CGMS) (Medtronic, CA). The aim of this study was to evaluate the accuracy of CGMS in children and adolescents with type 1 diabetes mellitus (DM1) and the efficacy of this method to detect unrecognized hypoglycemia in this population. It were analyzed capillary glycemia (CG) and CGMS sensors value; glycemic excursions; postprandial hyperglycemia; unrecognized hypoglycemia; complications and therapeutic management after CGMS. A1c levels were measured at the start and after 3 months of the study. Correlation coefficient during hypo, hyper, and normoglycemia and sensitivity/specificity was determined. The mean CG values were 213.8 ± 63.4mg/dl vs. 209.7 ± 52.5mg/dl by sensor, with statistical significance by Pearsons correlation (p< 0.001). There was no difference between CGMS and CG value in order to detect glycemic excursions (p= 0.32). The postprandial hyperglycemia and unrecognized hypoglycemia was detected in 66.7% and 56.2% of this patients, respectively. The correlation coefficient during hypoglycemia presented no statistical significance by Pearsons correlation (p= 0.29) vs. during hyperglycemia (p= 0.001). The CGMS sensor presented low sensitivity (63.3%) to detect hypoglycemia. This data showed important decreased level of A1c in this population 3 months after CGMS with statistical significance (p= 0.03). The CGMS showed to be a very safe method, well tolerated, with high accuracy in glycemic values and low complications rate. This results suggest that CGMS is a good method to identify postprandial hyperglycemia, to improve metabolic changes in therapeutics with significant impact in A1c of diabetic pediatric patients. This data confirmed the low sensitivity of CGMS to detect unrecognized hypoglycemia in pediatric DM1 patients.
Keywords: Continuous glucose monitoring system; Unrecognized hypoglycemia; Children/adolescents; Diabetes mellitus type 1
RESUMO
Foram estudados retrospectivamente 17 pacientes (15,76 ± 4,5 anos) submetidos a monitorização subcutânea contínua da glicemia (CGMS) (Medtronic; CA) por 72 horas. Buscou-se avaliar a acurácia da CGMS em crianças e adolescentes com diabetes mellitus tipo 1 (DM1) e sua eficácia na detecção de hipoglicemia assintomática nesse grupo de pacientes. Foram analisados os valores de glicemia capilar (GC) e pelo sensor CGMS; excursões glicêmicas; hiperglicemia pós-prandial; hipoglicemia assintomática; complicações e conduta após a CGMS. Os níveis de A1c foram determinados antes e 3 meses após a CGMS. A correlação sensor/glicemia capilar foi avaliada durante normo, hipo e hiperglicemia, bem como a sensibilidade e especificidade. A GC média durante a CGMS foi de 213,8 ± 63,4mg/dl vs. 209,7 ± 52,5mg/dl detectada pelo sensor, com correlação de Pearson significante (p< 0,001). Não houve diferença significante entre a CGMS e GC na detecção de excursões glicêmicas (p= 0,32). A hiperglicemia pós-prandial e hipoglicemia assintomática foram identificadas em 66,7% e 56,2% dos pacientes durante a CGMS, respectivamente. A correlação entre sensor CGMS e GC durante hipoglicemia assintomática mostrou-se não significante (p= 0,32) vs. durante hiperglicemia (p= 0,001). O sensor CGMS apresentou baixa sensibilidade (63,3%) para detecção de hipoglicemia assintomática. Observou-se redução significante da A1c três meses após a CGMS em crianças e adolescentes com DM1 (p= 0,03). A CGMS mostrou-se método seguro, bem tolerado, com alta acurácia nos valores glicêmicos detectados, com baixo índice de complicações. Esses dados comprovam a alta eficácia da CGMS na detecção de hiperglicemia pós-prandial, na redução da A1c e melhora do controle metabólico, com baixas sensibilidade para na identificação de hipoglicemia assintomática em crianças e adolescentes com DM1.
Descritores: Monitorização subcutânea contínua da glicemia; Hipoglicemia assintomática; Crianças / adolescentes; Diabetes mellitus tipo 1
THE MAJOR INCONVENIENCE of self-monitoring of blood glucose (SMBG) in clinical practice is due to the fact that blood glucose is only intermittently measured by fingerstick capillary glycemia from which only a partial and therefore incomplete picture of blood glucose fluctuations can be made (1,2). Because of many factors, including pain and inconvenience, many children with diabetes do not accept frequent fingersticks for SMBG (3). The availability of the Continuous Glucose Monitoring System (CGMS) (Medtronic; Northridge, CA) offers the opportunity for pediatric type 1 diabetic patients to match the demands of intensive therapy with the intensive monitoring of blood glucose levels (1,2).
The DCCT (Diabetes Control and Complications Trial) established that intensive and multidisciplinary treatment of type 1 diabetes mellitus (DM1) improved metabolic control and reduces the complications of disease (4). Estimates showed that a significant damage in quality of life is frequently due to DM1 in children and adolescents, include function limitations, social stress, even mayor depression (2). Psychological aspects and childrens acceptance of DM1 may exercise some influence in their glycemic control (5).
Despite an excellent A1c levels and target preprandial glucose levels, pediatric diabetic patients often experience unrecognized hypoglycemia and postprandial hyperglycemia that are not evident with routine monitoring (6). In addition, families frequently do not measure blood glucose levels during the night and 55% of severe hypoglycemic events in the DCCT occurred during sleep (7). Several studies demonstrated the utility of the CGMS to improve metabolic control, to detect more glycemic excursions (hypo and hyperglycemia) and to detect more postprandial hyperglycemia than SMBG (8-12). The efficacy of CGMS in detecting hypoglycemia is not well established in medical literature (13-15).
There were no information in Brazil about the effects of CGMS in pediatric type 1 diabetic patients. This study aimed to determine the accuracy of CGMS and the efficacy of this method to detect unrecognized hypoglycemia in DM1 pediatric patients. The complications of CGMS in children and adolescents are still discussed.
SUBJECTS AND METHODS
Patients
This retrospective study assessed 17 diabetic patients (15.76 ± 4.52 years), duration of DM1: 2.0-18.0 years, mean duration of 7.42 ± 4.93 years, submitted to 72 hours CGMS (Medtronic; Northridge, CA). Each child had a mean A1c level > 7.0% (range: 7.0%-10.9%) for the 3 months before participating in the study. All participants were on intensive insulin treatment with 29.4% receiving continuous subcutaneous insulin infusion (insulin pump therapy) and 70.6% receiving multiple daily injections (MDIs). There were 64.7% (n= 11) of females and 35.3% (n= 6) of males.
Glucose Sensor
The MiniMed Meditronic (Northridge, CA) CGMS, the first model approved by FDA (Food And Drug Administration, EUA) was used for subcutaneous glucose monitoring. The glucose is measured by an electrochemical assay of glucose-oxidize detecting values range from 40 to 400mg/dl. The system consists of a subcutaneous sensor connected by a cable to a pager-sized glucose monitor. Glucose readings are acquired by the monitor every 10 seconds and an average glucose value is stored in the monitor memory once every 5 minutes (up to 288 measurements per day and 864 in all exam). Each glucose sensor provides glucose information for up to 72 hours. After the initial 60 minutes, the electrical current in nanoamper is converted in glucose value after the information of this measured in the monitor. The stored values in the monitor are downloaded by the MiniMed Com-Station and presented in graphical and statistical form via a computer program and the sensor is eliminated. The original Medtronic MiniMed glucose sensor was modified in November 2002. This modification resulted in improved accuracy. In this study was utilized the new modified sensors.
Procedure
All patients were submitted to basic orientations of CGMS function and the register of all events in a "patient diary", by one person (F.F.R.M). During the CGMS, all participants had to perform at least four capillary glycemic tests per day and enter these values into the CGMS monitor to obtain correlation coefficients between the SMBG and the CGMS values. All SMBG tests were performed using the digital glucometer (Accu-Chek Active; Roche Diagnosis). The first capillary glycemia entered in the monitor were realized after 60 minutes of CGMS. Families were asked to not change their dietary practices during the study.
It were analyzed: correlation coefficient (%); mean absolute difference (MAD); number of sensors readings; duration of exam (h); mean capillary glycemia (CG) and mean CGMS sensors glycemic value; glycemic excursions; postprandial hyperglycemia (NR< 140mg/dl); unrecognized hypoglycemia; A1c levels at start and after 3 months; complications (trauma, local infection, disconnection); dropped the method; therapeutic management after CGMS.
The correlation coefficient and MAD were calculated by Medtronic software analysis and defined by > 0.79% and < 28%, respectively, to meet the optimal accuracy criteria. The MAD was determined by the average value of differences between sensors glucose values and blood glucose values in percentage (%) for a given day. The number of measurements by CGMS was considered significant higher than 80% (> 640 readings/72h).
The glycemic excursions were based on patients information and correlated by CGMS register. Hypo and hyperglycemia were defined as blood glucose < 70mg/dl and > 180mg/dl, respectively. The duration of hypo, hyper and normoglycemia were registered in hours/percent for comparison effect. The postprandial hyperglycemia was considered when blood glucose values were over than 140mg/dl two hours after lunch. The unrecognized hypoglycemia was detected by glycemia < 70mg/dl without clinical symptoms. The accuracy of CGMS sensor was based on comparison of capillary glycemic values and sensors values by the Pearsons correlation during hypo, normo and hyperglycemia, with P value < 0.05. The sensitivity and specificity of sensors value for hypo, hyper and normoglycemia was determined by statistical analysis.
The A1c values were determined at the start and at the 3-month after the CGMS in 13 patients. A1c values were determined using the HPLC instrument, with a normal range of 4.3% to 6.9%.
The complications during the CGMS were based in medical observation and patients information. It were analyzed the complications during the sensor implantation (bleeding and pain) and during the exam (trauma, local infection, disconnection, psychological aversion, technical deficiency, others "alarms"), dropped of the method and therapeutic management after CGMS.
Statistics
The data was collected and analyzed by Minitab software, by t test, Qui-square test, Pearsons correlation and regression test. It was considered significant an p value < 0.05.
RESULTS
The number of glucose readings during CGMS was 799.8 ± 189.9 (VR > 680), with significant value in 88.3% of patients. The mean number of hours per sensor was 71.8 ± 9.7 hours. The correlation coefficient between SBGM and CGMS values was 0.93 ± 0.04 (normal range, NR > 0.79), with ideal values in most of 94.12% of patients studied. About the MAD, the mean value was 13.6 ± 3.5% (NR < 28%), significant in 88.3% of patients. The main reason for the higher numbers of usable CGMS data were patient compliance, with successful to enter the ideal minimum four SMBG levels each day.
The mean capillary glucose values were 213.8 ± 63.4mg/dl vs. 209.7 ± 52.5mg/dl by CGMS sensor, with statistical significance detected by Pearsons correlation (p< 0.001) (figure 1).
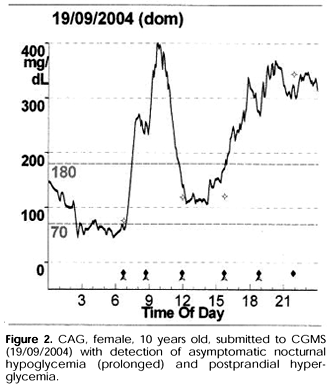
About the glycemic excursions, the CGMS was not more efficient in detecting glycemic excursion than fingerstick capillary glycemia (p= 0.32). The postprandial hyperglycemia was identified in 66.7% of pediatric type 1 diabetic patients with mean value of 187.0 ± 77.4mg/dl (NR< 140mg/dl). The unrecognized hypoglycemia was detected in 56.3% of this patients (figure 2). During the 72h CGMS, the patients were in state of hyperglycemia during 57.3 ± 29.4% of the register (table 1).
The correlation of blood glucose values and CGMS sensors value during hypoglycemia showed low rate of concordance and presented no statistical significance by Pearsons correlation (p= 0.29) vs. during hyperglycemia (p< 0.001) or normoglycemia (p= 0.004). The CGMS sensor presented low sensitivity (63.3%) and high specificity (96.9%) to detect hypoglycemia (table 2).
The evaluation of A1c levels in 13 (76.5%) patients before the CGMS and after 3 months showed significant decreased level of A1c in this population (8.30 ± 1.1 vs. 7.32 ± 0.9; p= 0.03).
No complications were registered in 94.1% of patients. The disconnection sign was the most common in this case (11.8%). No trauma, local infection, allergy, bleeding or other were registered during CGMS in this study. The psychological aversion and technical deficiency were not observed in this population. All patients (100%) completed the CGMS integrally.
The therapeutic management of pediatric type 1 diabetic patients was changed in 100% of patients, including insulin adjust dose, change of insulin type, institute nutritional and psychology support and physical activity approach.
DISCUSSION
This study is the first report of accuracy, utility and complications of CGMS in pediatric patients in Brazil. In the present study, all patients who used the CGMS showed some decline in A1c after 3 months, in agreement to Ludvigsson et al. (15). After 3 months, the decrease in mean A1c levels for the CGMS group was from 8.18% to 7.28%, similar to Ludvigsson et al. findings (15). In this data, we observed a total adherence to CGMS, with no interruption during the procedure.
This data showed high accuracy of CGMS vs. capillary glycemia, similar to medical literature (8-12). Sachedina & Pickup (2003) demonstrated the correlation of sensors and fingersticks values in 18 DM1 patients submitted to 72h CGMS. The CGMS showed to be better than intensive CG (8 times per day) in detection of asymptomatic hypoglycemia and postprandial hyperglycemia, in agreement with our data (16). We verify a correlation coefficient of 0.93, with significant value in more than 90% of patients, corroborated by Djakoure-Platonoff et al. (2003) (17). They obtained correlation coefficient of 0.92, with significant value in 93% of cases and MAD of 25%. This group considered the 72h CGMS method of high accuracy and gold standard in determination of glycemic profile in diabetic persons.
Guerci et al. reported the accuracy, performance, and reproducibility of the CGMS in 18 type 1 diabetic patients submitted to 72-h CGMS, with mean duration of CGMS recording of 63 ± 12h, 692 pairs of data for glucose meter readings and CGMS, correlation coefficient ranging from 0.87 to 0.92 (NR > 0.79) (18).
In pediatric patients, the CGMS showed to be a very safe method and an important alternative to promote decrease of A1c levels, therapeutic adjustment, education and motivation of patients (17). In EUA, 12 diabetic adolescents (A1c > 8%) were studied and submitted to 72h CGMS. The CGMS promoted detection of glycemic excursions in all patients, postprandial hyperglycemia in 10/12 cases and nocturnal hypoglycemia in 30% of patients. After two months, they observed a significant decrease of A1c levels (6,20). This data were very similar to our findings in Brazilian pediatric type 1 diabetic patients.
About the CGMS sensor efficacy in detecting glycemic excursions, this results corroborated to many studies in medical literature (21-24). This data showed that CGMS is very useful to detect postprandial hyperglycemia. Recent data of 91 DM1 patients estimated that the accuracy of CGMS sensor is more effective in elevated glycemic levels than hypoglycemic state (21).
The efficacy of CGMS in detecting hypoglycemia is not well established in medical literature (13-15). Boland et al. (2001) detected unrecognized hypoglycemia in 70% of 56 type 1 diabetic children submitted to 72h CGMS and considered this method a gold standard in hypoglycemia management in pediatric population underwent only to SMBG (23). Guerci et al. observed a low sensitivity of CGMS sensor to detect hypoglycemic levels (33%) in diabetic patients (18).
According to Mcgowan et al. (2002), the CGMS sensor presented low efficacy in detecting hypoglycemia in adolescents with DM1. The correlation coefficient during hypoglycemic episodes showed only 0.76 (NR > 0.79) of concordance and has to be analyzed with attention (14). Kovatchev et al. (2004) revealed that the accuracy of sensor readings were lower in hypoglycemia (73,5%) versus euglycemia (99%) and hyperglycemia (95.4%), with failure to detect hypoglycemia like the most common error during the test (13). In this data, the correlation between capillary glucose and sensors glucose values during hypoglycemia showed no significance (p= 0.29) vs. during hyperglycemia (p< 0.001), with low sensitivity (63.3%) of CGMS sensor for hypoglycemic state, corroborating value to Mcgowan, Guerci and Kovatchev groups (13,14,18).
Chico et al. (2003) reported the useful of CGMS in detecting unrecognized hypoglycemia and improving metabolic control in 70 diabetic patients submitted to 72h CGMS. The CGMS detected unrecognized hypoglycemia in 62.5% of the type 1 diabetic patients and in 46.6% of the type 2 diabetic patients and in both were observed significant decrease levels of A1c after 3 months, similar to our data. This findings suggest that CGMS is useful for detecting unrecognized hypoglycemia in diabetic patients and improve metabolic control (24).
In a recent study of nocturnal hypoglycemia in 50 children with diabetes who were hospitalized overnight, it was found that 47% had a blood glucose level below 60mg/dL (< 3.3mmol/L) during the night (using hourly blood glucose determinations) (25). They found that 49% of cases were asymptomatic. The data from Ludvigsson et al. suggest that the incidence of asymptomatic episodes is closer to 85% (17 of 20) (15).
No complications were registered in almost 95 percent of patients. No trauma, local infection or bleeding were registered. The insulin therapeutic regimen was adjusted in 100% of patients. In Guerci et al. analisis, the disconnection was the most common problem detected during CGMS, without any side effects reported at the site of sensor implantation (18). In this study, the disconnection was the most common problem detected during CGMS in this data, agreed with Guerci et al. Ludvigsson et al. observed some failed to enter the minimum 4 SBGM values during the day and that usable data were not obtained (15). In this data, all the children completed at least 4 fingerstick values entered to the monitor, promoting high accuracy of CGMS values.
CONCLUSIONS
This study suggest that CGMS is a very good method to identify postprandial hyperglycemia and to improve metabolic changes in therapeutics with significant impact in A1c levels of pediatric type 1 diabetic patients. The CGMS is a very safe method, well tolerated by patients, with low accuracy in detecting hypoglycemic state. This data confirmed the low efficacy of CGMS in detecting unrecognized hypoglycemia in pediatric type 1 diabetic patients.
Recebido em 19/11/04
Revisado em 22/02/05
Aceito em 18/03/05
- 1. Ferraz DP, Maia FFR, Araújo LR. Glicemia capilar em ponta do dedo versus lóbulo de orelha: estudo comparativo dos valores resultantes e preferências dos pacientes. Arq Bras Endocrinol Metab 2004;48:389-93.
- 2. Glasgow RE, Ruggiero l, Eakin EG. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care 1997;20:562-7.
- 3. The DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177-88.
- 4. The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-86.
- 5. Maia FFR, Araújo LR. Aspectos psicológicos e controle glicêmico de um grupo de pacientes com diabetes mellitus tipo 1 de Minas Gerais. Arq Bras Endocrinol Metab 2004;48:261-6.
- 6. The Diabetes Research in Children Network (DirecNet) Study Group. Accuracy of the GlucoWatch G2 Biographer and the Continuous Glucose Monitoring System during hypoglycemia. Experience of the Diabetes Research in Children Network (DirecNet). Diabetes Care 2004;27:722-6.
- 7. The DCCT Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med 1991;90:450-9.
- 8. Anderson RJ, Freedland KE, Clouse RE, Iustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069-78.
- 9. Kaufman FR. Role of continuous glucose monitoring in pediatric patients. Diabetes Technol Ther 2000;2:S49-52.
- 10. Boland EA, Tamborlane VW. Continuous glucose monitoring in youth with type 2 diabetes: overcoming barriers to successful treatment. Diabetes Technol Ther 2000;2:S53-9.
- 11. Caduff A, Hirt E, Feldman Y, Ali Z, Heinemann L. First human experiments with a non-invasive, non-optical continuous glucose monitoring system. Biosens Bioelecton 2003;19:207-9.
- 12. Aussedat B, Dupire-Angel M, Gifford R, et al. Interstitial glucose concentration and glycemia: impiclations for continuous subcutaneous glucose monitoring. Endocrinol Metab 2000;278:E716-28.
- 13. Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors. Diabetes Care 2004; 27:1922-8.
- 14. McGowan K, Tomas W, Moran A. Spurius reporting of nocturnal hypoglycemia by CGMS in patients with thigthtyle controlled diabetes. Diabetes Care 2002;25:1499-503.
- 15. Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933-8.
- 16. Sachedina N, Pickup JC. Performance assessment of the Medtronic-Minimed Continuous Glucose Monitoring System and its use for measurement of glycaemic control in Type 1 diabetic subjects. Diabet Metab 2003;20:1012-5.
- 17. Djakoure-Platonoff C, Radermercker R, Reach G, Slama G, Selam JI. Accuracy of the continuous glucose monitoring system in inpatient and outpatient conditions. Diabetes Metab 2003;29:159-62.
- 18. Guerci B, Floriot M, Bohme P, et al. Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analogs. Diabetes Care 2003;26:582-9.
- 19. Schaepelynck-Belicar P, Bague P, Simonin G, Lassmann-Vague V. Improved metabolic control in diabetic adolescents using the continuous glucose monitoring system (CGMS). Diabet Metab 2003;29:608-12.
- 20. The Accuracy of the CGMS in children with type 1 diabetes: results of the Diabetes Research in Children Network (DirecNet). Diabetes Technol Ther 2003;5:781-9.
- 21. Steil GM, Rebrin K, Mastrototaro J, Bernaba B, Saad MF. Determination of plasma glucose during rapid glucose excursions with a subcutaneous glucose sensor. Diabetes Technol Ther 2003;5:27-31.
- 22. Metzger M, Leibowitz G, Waistein J, et al. Reproductibility of glucose measurements using the glucose sensor. Diabetes Care 2002;25:1185-91.
- 23. Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane VW. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001;24:1858-62.
- 24. Chico A, Vidal-Rios P, Subira M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care 2003;26:1153-7.
- 25. Boyne M, Silver D, Kaplan J, Saudek C. Timing of changes in interstitial and blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes Care 2000;49(suppl 1):398.
Publication Dates
-
Publication in this collection
19 Oct 2005 -
Date of issue
Aug 2005
History
-
Accepted
18 Mar 2005 -
Reviewed
22 Feb 2005 -
Received
19 Nov 2004