Abstracts
Associated with elevated risk of cardiovascular events and cancer, obesity is a worldwide problem affecting developed and developing countries. Microcirculatory vessels, represented by arterioles, capillaries and venules (mean internal diameter < 100 µm), are the place where blood/tissue nutrition and exchange effectively take place. Microvascular dysfunction is an early event in obesity probably secondary to endothelial dysfunction and capillaries rarefaction. New research techniques allow the investigation of the microcirculation in different vascular beds in humans. Studies suggest a link between endothelial dysfunction and visceral obesity. Oxidative stress, inflammation and rennin-angiotensin system are among factors considered to be involved on microvascular dysfunction in obesity. Microcirculatory impairment present in obesity suggests that it could be an important causal factor in obesity-related disorders such as insulin resistance and hypertension.
Obesity; endothelial dysfunction; microcirculation; tissue perfusion; metabolic syndrome
Associada ao aumento do risco de eventos cardiovasculares e ao câncer, a obesidade é um problema mundial, que atinge tanto países desenvolvidos quanto em desenvolvimento. A microcirculação é composta por arteríolas, capilares e vênulas (diâmetro interno médio < 100 µm) e é o local onde ocorrem a oferta de nutrientes e as trocas entre o tecido e o sangue. A disfunção microcirculatória é um evento precoce na obesidade e este pode ser secundário à disfunção endotelial ou à redução no número de capilares (rarefação capilar). Novas técnicas em pesquisa permitem a avaliação da microcirculação em diferentes leitos vasculares em humanos. Estudos sugerem uma correlação entre disfunção endotelial e obesidade visceral. Acredita-se que o estresse oxidativo, a inflamação e a atividade aumentada do sistema renina-angiotensina estão entre os fatores envolvidos nessa associação. O comprometimento microcirculatório presente na obesidade sugere que esse pode ser um fator causal importante nas desordens relacionadas com a obesidade, como resistência insulínica e hipertensão.
Obesidade; disfunção endotelial; microcirculação; perfusão tecidual; síndrome metabólica
REVIEW
Metabolic disturbances linked to obesity: the role of impaired tissue perfusion
Alterações metabólicas associadas à obesidade: o papel da disfunção da microcirculação tecidual
Nivaldo Ribeiro VillelaI; Luiz Guilherme Kramer-AguiarI; Daniel Alexandre BottinoI; Nicolas WiernspergerII; Eliete BouskelaIII
ILaboratório de Pesquisas Clínicas e Experimentais em Biologia Vascular (BioVasc), Centro Biomédico, Universidade do Estado do Rio de Janeiro (Uerj), Rio de Janeiro, RJ, Brasil
IIFrench National Institute for Health and Medical Research (Inserm) UMR870, INSA Lyon, Lyon F69008, France
IIILaboratório de Pesquisas Clínicas e Experimentais em Biologia Vascular (BioVasc), Centro Biomédico, Universidade do Estado do Rio de Janeiro (Uerj), Rio de Janeiro, RJ, Brasil; French National Institute for Health and Medical Research (Inserm) UMR870, INSA Lyon, Lyon F69008, France
Correspondence to Correspondence to: Eliete Bouskela Laboratory for Clinical and Experimental Research on Vascular Biology Universidade do Estado do Rio de Janeiro, Pavilhão Reitor Haroldo Lisboa da Cunha, térreo Rua São Francisco Xavier, 524 20550-013 Rio de Janeiro, RJ, Brasil eliete_bouskela@yahoo.com.br
ABSTRACT
Associated with elevated risk of cardiovascular events and cancer, obesity is a worldwide problem affecting developed and developing countries. Microcirculatory vessels, represented by arterioles, capillaries and venules (mean internal diameter < 100 µm), are the place where blood/tissue nutrition and exchange effectively take place. Microvascular dysfunction is an early event in obesity probably secondary to endothelial dysfunction and capillaries rarefaction. New research techniques allow the investigation of the microcirculation in different vascular beds in humans. Studies suggest a link between endothelial dysfunction and visceral obesity. Oxidative stress, inflammation and rennin-angiotensin system are among factors considered to be involved on microvascular dysfunction in obesity. Microcirculatory impairment present in obesity suggests that it could be an important causal factor in obesity-related disorders such as insulin resistance and hypertension.
Keywords: Obesity; endothelial dysfunction; microcirculation; tissue perfusion; metabolic syndrome
RESUMO
Associada ao aumento do risco de eventos cardiovasculares e ao câncer, a obesidade é um problema mundial, que atinge tanto países desenvolvidos quanto em desenvolvimento. A microcirculação é composta por arteríolas, capilares e vênulas (diâmetro interno médio < 100 µm) e é o local onde ocorrem a oferta de nutrientes e as trocas entre o tecido e o sangue. A disfunção microcirculatória é um evento precoce na obesidade e este pode ser secundário à disfunção endotelial ou à redução no número de capilares (rarefação capilar). Novas técnicas em pesquisa permitem a avaliação da microcirculação em diferentes leitos vasculares em humanos. Estudos sugerem uma correlação entre disfunção endotelial e obesidade visceral. Acredita-se que o estresse oxidativo, a inflamação e a atividade aumentada do sistema renina-angiotensina estão entre os fatores envolvidos nessa associação. O comprometimento microcirculatório presente na obesidade sugere que esse pode ser um fator causal importante nas desordens relacionadas com a obesidade, como resistência insulínica e hipertensão.
Descritores: Obesidade; disfunção endotelial; microcirculação; perfusão tecidual; síndrome metabólica
INTRODUCTION
Obesity is a worldwide problem, reaching epidemic proportions in several industrialized countries (1). Additionally, obesity is rising in many developing countries, resulting in changes in the policy and health maker's focus from undernutrition to obesity and obesity-related diseases (2,3), since it increases risks for diabetes mellitus, hypertension, dyslipidemia, coronary artery disease and some types of cancer (4).
The association of known cardiovascular risk factors, including abdominal obesity, impaired glucose tolerance or type 2 diabetes, dyslipidemia, raised blood pressure proinflamatory and prothrombotic factors, is often referred as metabolic syndrome (5) and it has been associated with high risk of cardiovascular events (> 20% for those with diabetes and 10% to 20% for those with two or more risk factors) (6). Metabolic syndrome is linked to endothelial dysfunction (7,8) and insulin resistance, a generalized metabolic disorder in which insulin actions are impaired (7).
Microvascular dysfunction is present in obese subjects and it is secondary to either endothelial dysfunction (9) or structural impairments in the microvasculature (10). Several conditions related to obesity such as hypertension (11), hypercholesterolemia (12) and hyperglycemia (13) are also associated with endothelial dysfunction, although endothelial dysfunction is present in obese subjects even in the absence of these conditions (14), suggesting obesity as a primary cause of microvascular dysfunction. Indeed, microvascular dysfunction is an important factor in metabolic disturbances linked to obesity, since it could influence both vascular resistance and insulin-mediated glucose disposal, contributing to hypertension and insulin resistance in obesity (15).
MICROCIRCULATION
Considering that the function of the cardiovascular system is to supply an appropriated milieu for tissues and organs, the microcirculation is the portion of the cardiovascular system in which blood/tissue nutrition and exchange effectively occur. Microvessels (mean internal diameter < 100 µm) are represented by arterioles, capillaries and venules the largest portion of vessels in the body subjected to multiple, fine-tuning regulations. This regulation is achieved by local nervous and humoral control with the main objective of supplying specific metabolic requirements for each tissue. Furthermore, it is in the microcirculation where the main resistance to blood flow takes place, since large and medium-sized arteries and veins offer little resistance to flow (16). Thus, microcirculatory functions can be summarized as delivery of oxygen and nutrients, removal of metabolic waste products from tissues, maintenance of tissue environment for cell survival and maintenance of peripheral resistance (17).
The mechanisms that regulate local blood flow are myogenic activity, in which arterioles respond to acutely increased pressure with vasoconstriction and local chemical and humoral factors, like interstitial PO2, PCO2 and pH, as well as local concentration of K+, lactic acid, ATP, ADP and adenosine. However, when high pressure in the microcirculation is maintained, vascular remodeling may occur and components of the vessel wall are rearranged in a process known as eutrophic inward remodeling. Increased production of reactive oxygen species (ROS), inflammation, extracellular matrix alterations and increased levels of apoptosis are some factors involved in remodeling (18,19). Angiotensin II is also thought to be an important factor involved in this process (20).
Microvascular rarefaction is defined as reduction on microvessel density in a given volume of tissue and it can be classified as a) functional when the number of perfused number of microvessels is maintained, or b) structural and when the actual number of microvessels is reduced (19). Research on spontaneously hypertensive rats showed that functional rarefaction may progress to structural one (21). Oxydative stress, endothelial dysfunction and apoptosis are important mechanisms implicated on microvascular rarefaction (22,23).
In physiological conditions, autoregulatory mechanisms maintain tissue perfusion according to its metabolic needs, while in situations that vascular reactivity is impaired, as it occurs in obesity, microvascular dysfunction, mainly secondary to microvascular remodeling and rarefaction, reduces tissue perfusion and prevents blood-tissue exchange, producing tissue hypoxia in situations of high metabolic demand (24).
The microcirculation can be studied in humans by direct intravital videocapillaroscopy (Figure 1A) on skin, nailfold, lip or bulbar conjunctiva, or by laser Doppler measurements. Sidestream Dark Field (SDF) imaging is a new noninvasive method for assessment of human microcirculation and it was incorporated into a hand device called MicroScan (Figure 1B). In animal studies, these techniques can be used in different microvascular beds such as skeletal muscle, hamster cheek pouch or mesentery. Several microvessels are not perfused under resting conditions but can be activated during reactive hyperemia, as a result of recruitment one of the microcirculatory parameters often studied by videocapillaroscopy or skin flow in humans.
ENDOTHELIAL DYSFUNCTION
The endothelium maintains the vascular homeostasis regulating the vascular tonus by balancing the production of vasodilators, such as nitric oxide (NO), vasoconstrictors, like endothelin, direct action on blood fluidity and coagulation through production of factors that modulate platelet activity, clotting cascade and fibrinolysis (25). NO also regulates leukocyte-endothelium interaction (26).
Endothelial dysfunction, defined as loss of normal homeostatic function of the endothelium, partly secondary to a reduction of NO bioavailability, results in a defect on endothelium-blood interaction, abnormal vasomotor activity, development of a procoagulant endothelial surface, intimal growth and inflammation (27). Traditional cardiovascular risk factors such as hypertension (28), diabetes mellitus (29), hypercholesterolemia (12) and, recently, obesity (30) are associated with endothelial dysfunction. Endothelial dysfunction is an early marker of cardiovascular risk preceding any visible structural atheromatous plaques (31,32).
Endothelial function can be determined by either invasive or non-invasive methods in global, such as arm, limb or skin, or specific, like coronary, local techniques. Stimuli that increase production and release of endothelial NO, such as increased shear stress due to increased blood flow, or use of receptor-agonists, like acetylcholine, bradykinin or substance P (Figure 2), have been used to access endothelial-dependent vasodilation.
Several techniques have been proposed and used to access endothelial function each one of them with advantages and disadvantages. Thus, NO appears to be more related to flow-mediated dilation in large and small arteries than in arterioles (33) and associated to reactive hyperemia in skeletal muscle but not in skin (34). Moreover, reactive hyperemia after an ischemia of short duration is mainly secondary to NO release, while ischemia of long duration has additional factors playing a role, like prostaglandins and autonomic nervous system. Thus, depending on the technique or the tissue used, studies of endothelial function may give different results. Vasodilators, such as nitrates or sodium nitroprusside, directly induce vascular smooth muscle cell relaxation, independent of endothelium, and its use allow the evaluation of endothelium-independent vasodilation (35).
Vascular dysfunction does not depend only on structural and functional changes in feeding arteries but also and largely on the microcirculation. Thus, while endothelial dysfunction in conductance vessels (36,37) is a known predictor of cardiovascular risk, endothelial dysfunction on the microcirculation is emerging as an independent predictor of cardiovascular risk as well (38,39).
ENDOTHELIAL DYSFUNCTION AND OBESITY
Endothelial dysfunction is an early process in the evolution of atherosclerosis in obesity and it could be seen even in obese children aged 9 to 12 years, when obesity could be associated to impaired endothelium-dependent vasodilation, reduced arterial complacence and carotid artery intimal-medial thickening (40,41). Obesity also accelerates the atherosclerosis process observed in young persons (42,43).
Microvascular dysfunction is present in overweight and obese subjects, even in absence of hyperglycemia or hypertension (10,14). Obese subjects have blunted endothelial-dependent vasodilation in either skin or resistance vessels (44,45) and microcirculatory dysfunction could also be observed on these subjects, using nailfold videocapillaroscopy (46). Furthermore, obesity is associated with a decreased response to insulin-induced endothelium-dependent vasodilation (47).
Fat distribution has been found to be an important determinant in endothelial dysfunction (48) and body fatness is associated with microvascular dysfunction even in lean subjects (49). Thus, waist/hip ratio, a determinant of abdominal obesity, is a better marker of endothelial dysfunction than body mass index (BMI) by itself (50,51), as presented in Figure 3.
Insulin resistance is associated with endothelial dysfunction. High levels of lipids and glucose could be associated to reduction on NO availability (52,53). These findings suggest that endothelial dysfunction might be treated as cause and consequence of the metabolic disturbance observed in states of insulin resistance (54). In fact, insulin cross the endothelial barrier to reach its receptor on the cell membrane, and impairment on insulin diffusion across the capillary bed may represent a rate-limiting step in peripheral insulin action (54,55).
These observations, associated with the fact that weight loss improves endothelial function (56), establish a strong association between obesity and microvascular dysfunction in different tissues.
MICROVASCULAR DYSFUNCTION AND OBESITY
Studies on obese Zucker rat, in which a defective receptor gene causes obesity, type 2 diabetes and hypertension, showed microvascular remodeling and rarefaction in skeletal muscle, even before any elevation of blood pressure could be observed (57). In humans, Gavin and cols. (58) demonstrated a reduction on capillary density in skeletal muscle of obese subjects when compared to lean individuals. Obesity was also associated with lower capillary density in the skin (46).
Although of doubtful physiological importance, insulin dilates resistance vessels and increases skeletal muscle blood flow, promoting the delivery of glucose and insulin to this tissue (59,60). Besides these actions on resistance vessels, insulin also has one secondary action, named capillary recruitment that redirects blood flow from non-nutritive to nutritive vessels, increasing functional capillary density in skeletal muscle and, consequently, the delivery of glucose and insulin (59).
Obesity produces a blunted response to vasodilation induced by oral glucose loading (61). This blunted response probably is due to impaired capillary recruitment in response to an increase on plasma insulin level (45,62). Also, there is a reduction in transcapillary delivery of insulin to muscle in obese subjects (63).
Although studies suggest that chronic reduction on vascular NO bioavailability is the main mechanism underlying microvascular rarefaction in the metabolic syndrome, this is not completely clarified (64). One possibility is that insulin, acting on insulin and IGF receptors, associated with angiotensin II, stimulates vascular remodeling (65).
Obesity also leads to formation of hypoxic areas in the adipose tissue; Regazzetti and cols. (66) showed that adipocytes from human and murine origins, under hypoxic conditions, developed a state of insulin resistance, pointing to hypoxia as one of the mechanisms participating in insulin resistance in adipose tissue of obese subjects.
MECHANISMS INVOLVED IN MICROCIRCULATORY DISTURBANCES IN OBESITY
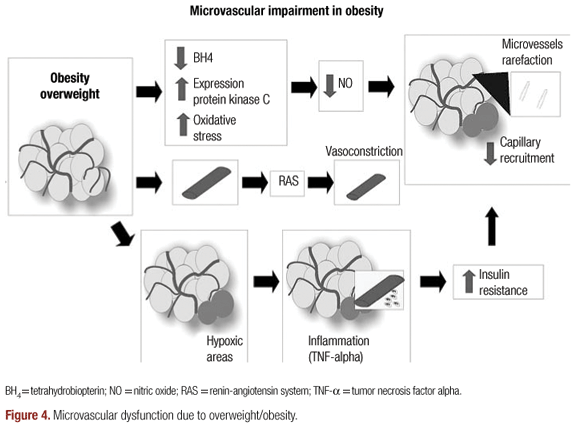
Studies on obese Zucker rats also showed that microvessel rarefaction in obesity is closely related to a chronic reduction in NO bioavailability (57). Several factors might contribute to the observed rarefaction: increased oxidative stress (67), with its NO scavenging affect (68); increased activity and expression of protein kinase C, that reduce NO bioavailability in mesenteric microvessels of Zucker diabetic fatty rats (69); and reduction in tetrahydrobiopterin (BH4), a necessary cofactor for NO production (70).
Excess of adiposity produces chronic vascular inflammation with production of several inflammatory cytokines such as tumor necrosis factor-α (TNF-α) (71). Ijzerman and cols. have showed a negative correlation between TNF-α and capillary recruitment in adults, suggesting a relationship between TNF- α and insulin resistance (72).
Ijzerman and cols. (72) proposed that increased adipose mass generates cytokine signals to blood vessels, by perivascular fat, resulting on impaired perfusion and insulin resistance.
The rennin-angiotensin system (RAS) can also be an important component for microvascular dysfunction viewed in obesity since all necessary components needed to generate the vasoconstrictor angiotensin II are present in the adipose tissue (73) and increased activity in RAS is present in obesity (74) (Figure 4).
CONCLUSIONS
Obesity is associated with reduction on tissue perfusion secondary to either endothelial dysfunction or capillary rarefaction. Endothelial dysfunction is an early process in obesity, present even in the absence of hypertension or hyperglycemia, associated with visceral obesity, suggesting that obesity per se is an important risk for endothelial dysfunction. The microcirculatory impairment present in obesity may result from increased levels of oxidative stress, inflammatory cytokines or increased activity of RAS, suggesting an association between obesity and obesity-related disorders to insulin resistance and hypertension.
Acknowledgments: The authors wish to thank Ms. Fatima Zely Garcia de Almeida Cyrino and Ms. Priscila Alves Maranhão for excellent technical help. Grants: The study has been supported by grants from the National Research Council (CNPq) 474116/2008-5 and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj) E-26/110.318/2007.
Disclosure: No potential conflict of interest relevant to this article was reported.
Received in Feb/16/2009
Accepted in Feb/17/2009
-
1[No authors listed]. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894: i-xii,1-253.
- 2. Martorell R, Khan LK, Hughes ML, Grummer-Strawn LM. Obesity in women from developing countries. Eur J Clin Nutr. 2000;54(3):247-52.
- 3. Popkin BM. The nutrition transition in low-income countries: an emerging crisis. Nutr Rev. 1994;52(9):285-98.
- 4. Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8(5):395-408.
- 5. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97.
- 6. Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709-16.
- 7. Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112(1):32-8.
- 8. Quinones MJ, Hernandez-Pampaloni M, Schelbert H, Bulnes-Enriquez I, Jimenez X, Hernandez G, et al. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140(9):700-8.
- 9. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601-10.
- 10. Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol. 2008;294:H1685-92.
- 11. Taddei S, Virdis A, Ghiadoni L, Sudano I, Salvetti A. Effects of antihypertensive drugs on endothelial dysfunction: clinical implications. Drugs. 2002;62(2):265-84.
- 12. Vogel RA. Cholesterol lowering and endothelial function. Am J Med. 1999;107(5): 479-87.
- 13. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695-701.
- 14. Van Guilder GP, Hoetzer GL, Dengel DR, Stauffer BL, DeSouza CA. Impaired endothelium-dependent vasodilation in normotensive and normoglycemic obese adult humans. J Cardiovasc Pharmacol. 2006;47(2):310-3.
- 15. Jonk AM, Houben AJ, de Jongh RT, Serne EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda). 2007;22:252-60.
- 16. DeLano FA, Schmid-Schonbein GW, Skalak TC, Zweifach BW. Penetration of the systemic blood pressure into the microvasculature of rat skeletal muscle. Microvasc Res. 1991;41(1):92-110.
- 17. Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001;104(6):735-40.
- 18. Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension. 2006;48(6):1012-17.
- 19. Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118(9):968-76.
- 20. Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90(4):449-55.
- 21. Prewitt RL, Chen, II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Physiol. 1982;243(2):H243-251.
- 22. DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schonbein GW. Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation. 2006;13(7):551-66.
- 23. Vogt CJ, Schmid-Schonbein GW. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation. 2001;8(2):129-39.
- 24. McGuire BJ, Secomb TW. A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J Appl Physiol. 2001;91(5):2255-65.
- 25. Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20(11 Suppl 2):II-3-10.
- 26. Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88(11):4651-5.
- 27. Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149-60.
- 28. Yang Z, Venardos K, Jones E, Morris BJ, Chin-Dusting J, Kaye DM. Identification of a novel polymorphism in the 3'UTR of the Larginine transporter gene SLC7A1: contribution to hypertension and endothelial dysfunction. Circulation. 2007;115(10):1269-74.
- 29. Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27(3):567-74.
- 30. Al Suwaidi J, Higano ST, Holmes DR Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37(6):1523-8.
- 31. Quyyumi AA. Prognostic value of endothelial function. Am J Cardiol. 2003;91(12A):19H-24H.
- 32. Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899-906.
- 33. Pohl U, De Wit C, Gloe T. Large arterioles in the control of blood flow: role of endothelium-dependent dilation. Acta Physiol Scand. 2000;168(4):505-10.
- 34. Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95(2):504-10.
- 35. Caballero AE, Arora S, Saouaf R, Lim SC, Smakowski P, Park JY, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48(9):1856-62.
- 36. Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, et al. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multiethnic cohort. Atherosclerosis. 2007;192(1):197-203.
- 37. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390-97.
- 38. Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15(5):259-64.
- 39. Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27(10): 2113-9.
- 40. Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117(5):1560-7.
- 41. Whincup PH, Gilg JA, Donald AE, Katterhorn M, Oliver C, Cook DG, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112(12):1789-97.
- 42. McGill HC Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105(23):2712-8.
- 43. McGill HC Jr, McMahan CA, Zieske AW, Tracy RE, Malcom GT, Herderick EE, et al. Association of Coronary Heart Disease Risk Factors with microscopic qualities of coronary atherosclerosis in youth. Circulation. 2000;102(4):374-79.
- 44. De Filippis E, Cusi K, Ocampo G, Berria R, Buck S, Consoli A, et al. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91(12):4903-10.
- 45. de Jongh RT, Serne EH, RG IJ, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesityassociated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109(21):2529-35.
- 46. Kraemer-Aguiar LG, Laflor CM, Bouskela E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metabolism. 2008;57(12):1740-6.
- 47. Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85(6):1844-52.
- 48. Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, et al. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disord. 1998;22(5):477-84.
- 49. de Jongh RT, Ijzerman RG, Serné EH, Voordouw JJ, Yudkin JS, de Waal HA, et al. Visceral and truncal subcutaneous adipose tissue are associated with impaired capillary recruitment in healthy individuals. J Clin Endocrinol Metab. 2006;91(12):5100-06.
- 50. Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88(11):1264-9.
- 51. Villela NR, Aguiar LG, Bahia L, Bottino D, Bouskela E. Does endothelial dysfunction correlate better with waist-to-hip ratio than with body mass index or waist circumference among obese patients? Clinics. 2006;61(1):53-8.
- 52. Lundman P, Eriksson M, Schenck-Gustafsson K, Karpe F, Tornvall P. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation. 1997;96(10):3266-8.
- 53. Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45(5):623-34.
- 54. Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22(6):423-36.
- 55. Sjöstrand M, Gudbjörnsdottir S, Holmäng A, Lönn L, Strindberg L, Lönnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51(9):2742-8.
- 56. Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105(7):804-9.
- 57. Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12(5):383-92.
- 58. Gavin TP, Stallings HW 3rd, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol. 2005;98(1):315-21.
- 59. Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267(2 Pt 1):E187-202.
- 60. Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284(2):E241-258.
- 61. Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab. 1990;70(6):1525-33.
- 62. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55(5):1436-42.
- 63. Sjöstrand M, Gudbjörnsdottir S, Strindberg L, Lönnroth P. Delayed transcapillary delivery of insulin to muscle interstitial fluid after oral glucose load in obese subjects. Diabetes. 2005;54(1):152-7.
- 64. Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2006;291(5):H2483-2492.
- 65. Horiuchi M, Mogi M, Iwai M. Signaling crosstalk angiotensin II receptor subtypes and insulin. Endocr J. 2006;53(1):1-5.
- 66. Regazzetti C, Peraldi P, Grémeaux T, Najem-Lendom R, Ben-Sahra I, Cormont M, et al. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58(1):95-103.
- 67. Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004;89(10):4963-71.
- 68. Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5Pt 1):C1424-37.
- 69. Bohlen HG. Protein kinase betaII in Zucker obese rats compromises oxygen and flow-mediated regulation of nitric oxide formation. Am J Physiol Heart Circ Physiol. 2004;286(2): H492-7.
- 70. Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, et al. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J. 2004;18(15):1900-2.
- 71. Fain JN, Nesbit AS, Sudlow FF, Cheema P, Peeples JM, Madan AK, et al. Release in vitro of adipsin, vascular cell adhesion molecule 1, angiotensin 1-converting enzyme, and soluble tumor necrosis factor receptor 2 by human omental adipose tissue as well as by the nonfat cells and adipocytes. Metabolism. 2007;56(11):1583-90.
- 72. Ijzerman RG, Voordouw JJ, Van Weissenbruch MM, Yudkin JS, Serné EH, Delemarre-van de Waal HA, et al. TNF-alpha levels are associated with skin capillary recruitment in humans: a potential explanation for the relationship between TNF-alpha and insulin resistance. Clin Sci (Lond). 2006;110(3):361-8.
- 73. Karlsson C, Lindell K, Ottosson M, Sjöström L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83(11):3925-9.
- 74. Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depotspecific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab. 2004;286(6): E891-5.
Correspondence to:
Publication Dates
-
Publication in this collection
15 May 2009 -
Date of issue
Mar 2009
History
-
Accepted
17 Feb 2009 -
Received
16 Feb 2009