Abstracts
The enzyme 17β-hydroxysteroid dehydrogenase type 3 (17-β-HSD3) catalyzes the conversion of androstenedione to testosterone in the testes, and its deficiency is a rare disorder of sex development in 46,XY individuals. It can lead to a wide range of phenotypic features, with variable hormonal profiles. We report four patients with the 46,XY karyotype and 17-β-HSD3 deficiency, showing different degrees of genital ambiguity, increased androstenedione and decreased testosterone levels, and testosterone to androstenedione ratio < 0.8. In three of the patients, diagnosis was only determined due to the presence of signs of virilization at puberty. All patients had been raised as females, and female gender identity was maintained in all of them. Compound heterozygosis for c.277+2T>G novel mutation, and c.277+4A>T mutation, both located within the intron 3 splice donor site of the HSD17B3 gene, were identified in case 3. In addition, homozygosis for the missense p.Ala203Val, p.Gly289Ser, p.Arg80Gln mutations were found upon HSD17B3 gene sequencing in cases 1, 2, and 4, respectively. Arq Bras Endocrinol Metab. 2012;56(8):533-9
A enzima 17β-hidroxiesteroide desidrogenase tipo 3 (17-β-HSD3) catalisa a conversão de androstenediona a testosterona nos testículos, e sua deficiência é uma forma rara de distúrbio do desenvolvimento do sexo em indivíduos 46,XY. A desordem apresenta um amplo espectro de características fenotípicas e de resultados de dosagens laboratoriais. Neste trabalho, são relatados quatro casos de deficiência da 17-β-HSD3 com cariótipo 46,XY, ambiguidade genital em diversos graus, androstenediona aumentada, testosterona diminuída, e relação testosterona e androstenediona < 0,8. Em três das pacientes, o diagnóstico foi suspeitado devido à presença de sinais de virilização na puberdade. Todos os pacientes foram criados como mulheres, e a identidade de gênero feminino foi mantida em todas elas. A heterozigose composta da mutação nova c.277+2T>G e da mutação c.277+4A>T, ambas localizadas no sítio doador de splicing do íntron 3 do gene HSD17B3, foi identificada no caso 3. Além dessas, as mutações missense p.Ala203Val, p.Gly289Ser, p.Arg80Gln foram identificadas em homozigose pelo sequenciamento do gene HSD17B3 dos casos 1, 2 e 4, respectivamente. Arq Bras Endocrinol Metab. 2012;56(8):533-9
CASE REPORT
Clinical and molecular spectrum of patients with 17β-hydroxysteroid dehydrogenase type 3 (17-β-HSD3) deficiency
Espectro clínico e molecular de pacientes com deficiência de 17β-hidroxiesteroide desidrogenase tipo 2 (17-β-HSD3)
Carla Cristina Telles de Sousa CastroI; Guilherme Guaragna-FilhoI; Flavia Leme CalaisII; Fernanda Borchers CoeliII; Ianik Rafaela Lima LealIII; Erisvaldo Ferreira Cavalcante-JuniorIV; Isabella Lopes MonlleóIV; Silma Regina Ferreira PereiraIII; Roberto Benedito de Paiva e SilvaV,VI; José Roberto Erbolato GabiattiVII; Antonia Paula Marques-de-FariaVI,VIII; Andrea Trevas Maciel-GuerraVI,VIII; Maricilda Palandi De MelloII; Gil Guerra-JuniorI,VI
IUnidade de Endocrinologia Pediátrica, Departamento de Pediatria, Faculdade de Ciências Médicas, Universidade Estadual de Campinas (Unicamp), Campinas, SP, Brazil
IILaboratório de Genética Molecular Humana, Centro de Biologia Molecular e Engenharia Genética (CBMEG), Unicamp, Campinas, SP, Brazil
IIIDepartamento de Biologia, Universidade Federal do Maranhão (UFMA), São Luís, MA, Brazil
IVCentro de Ciências da Saúde, Universidade Federal de Alagoas (UFAL), Maceió, AL, Brazil
VDepartamento de Desenvolvimento Humano e Reabilitação, FCM-Unicamp, Campinas, SP, Brazil
VIGrupo Interdisciplinar de Estudos da Determinação e Diferenciação do Sexo (GIEDDS), FCM-Unicamp, Campinas, SP, Brazil
VIIDepartamento de Tocoginecologia, FCM-Unicamp, Campinas, SP, Brazil
VIIIDepartamento de Genética Médica, FCM-Unicamp, Campinas, SP, Brazil
Correspondence Correspondence to: Gil Guerra-Junior Departamento de Pediatria, FCM-Unicamp 13083-100 Campinas, SP, Brazil gilguer@fcm.unicamp.br
SUMMARY
The enzyme 17β-hydroxysteroid dehydrogenase type 3 (17-β-HSD3) catalyzes the conversion of androstenedione to testosterone in the testes, and its deficiency is a rare disorder of sex development in 46,XY individuals. It can lead to a wide range of phenotypic features, with variable hormonal profiles. We report four patients with the 46,XY karyotype and 17-β-HSD3 deficiency, showing different degrees of genital ambiguity, increased androstenedione and decreased testosterone levels, and testosterone to androstenedione ratio < 0.8. In three of the patients, diagnosis was only determined due to the presence of signs of virilization at puberty. All patients had been raised as females, and female gender identity was maintained in all of them. Compound heterozygosis for c.277+2T>G novel mutation, and c.277+4A>T mutation, both located within the intron 3 splice donor site of the HSD17B3 gene, were identified in case 3. In addition, homozygosis for the missense p.Ala203Val, p.Gly289Ser, p.Arg80Gln mutations were found upon HSD17B3 gene sequencing in cases 1, 2, and 4, respectively. Arq Bras Endocrinol Metab. 2012;56(8):533-9
SUMÁRIO
A enzima 17β-hidroxiesteroide desidrogenase tipo 3 (17-β-HSD3) catalisa a conversão de androstenediona a testosterona nos testículos, e sua deficiência é uma forma rara de distúrbio do desenvolvimento do sexo em indivíduos 46,XY. A desordem apresenta um amplo espectro de características fenotípicas e de resultados de dosagens laboratoriais. Neste trabalho, são relatados quatro casos de deficiência da 17-β-HSD3 com cariótipo 46,XY, ambiguidade genital em diversos graus, androstenediona aumentada, testosterona diminuída, e relação testosterona e androstenediona < 0,8. Em três das pacientes, o diagnóstico foi suspeitado devido à presença de sinais de virilização na puberdade. Todos os pacientes foram criados como mulheres, e a identidade de gênero feminino foi mantida em todas elas. A heterozigose composta da mutação nova c.277+2T>G e da mutação c.277+4A>T, ambas localizadas no sítio doador de splicing do íntron 3 do gene HSD17B3, foi identificada no caso 3. Além dessas, as mutações missense p.Ala203Val, p.Gly289Ser, p.Arg80Gln foram identificadas em homozigose pelo sequenciamento do gene HSD17B3 dos casos 1, 2 e 4, respectivamente. Arq Bras Endocrinol Metab. 2012;56(8):533-9
INTRODUCTION
The isoenzyme 17β-hydroxysteroid dehydrogenase type 3 (OMIM *605573, 17-β-HSD3), also known as 17-ketosteroid reductase, catalyses the conversion of the weak androgen substrate, androstenedione (Δ4), to the more biologically active testosterone (T) in the testes. This conversion is essential for normal fetal development of male internal and external genitalia. The human gene, designated HSD17B3, contains 11 exons and is located on 9q22 (1-3).
17-β-HSD3 deficiency (OMIM #264300) is a rare autosomal recessive disorder form of male sex differentiation, characterized by hypoplastic-to-normal internal genitalia, absent or hypoplastic prostate, testes within the inguinal region, and female external genitalia at birth (4-9). Mutations in the HSD17B3 gene are responsible for the disease, in which homozygous or compound heterozygous affected 46,XY individuals are usually born with female genitalia, and the disorder remains undetectable until puberty (10), when virilization of the external genitalia occurs. This is probably due to the conversion of the abundant Δ4 to T by other extragonadal 17-β-HSD isoenzymes, or due to residual 17-β-HSD3 activity (11). Many individuals raised as females develop a male gender identity, and decide to be reassigned as males after puberty (4-9,12,13).
17-β-HSD3 deficiency in prepubertal patients is clinically indistinguishable from partial androgen insensitivity syndrome, 5α-reductase type 2 deficiency, and other disorders of T biosynthesis. Diagnosis can be established by elevated Δ4 and low T serum levels that result in a T/Δ4 ratio lower than 0.8. In addition, there is a poor response to hCG stimulation test. In the absence of suggestive signs during childhood, the disorder will only be diagnosed at puberty upon virilization of affected individuals (7,14).
To date, a total of 29 mutations in the HSD17B3 gene have been identified (Figure 1), including 21 missenses, one nonsense mutation, two frameshifs leading to downstream premature stop codon, four located at splice junctions leading to aberrant transcripts, and one duplication of exons 3-10 (4,15,16).
Here, we report the clinical and molecular pictures of four cases of 17-β-HSD3 deficiency.
MATERIALS AND METHODS
Genomic DNA was obtained from peripheral blood by proteinase K/phenol extraction method (17). The 11 exons and exon/intron junction sequences of HSD17B3 gene were amplified by polymerase chain reaction (PCR). Primers used for PCR were chosen with Prime 3 primer designing tool; primer sequences are available upon request. Purification of PCR products was carried out using the Wizard® SV Gel and PCR clean-up system (Promega, Madison, WI, USA). Direct PCR fragment sequencing with sense and antisense primers was performed using Big Dye® Terminator Cycle Sequencing Kit V3.1 Ready Reaction (ABI PRISM/PE Biosystems, Foster City, CA, USA). Sequences were obtained in an automatic sequencer ABI 3130 DNA Analyzer (ABI PRISM/PE Biosystems), and were compared with the HSD17B3 normal sequence (ENSEMBL ENSG00000130948) using Chromas (reduced version-free software) and CLC Sequence Viewer v.6.2 (free software).
CASE REPORTS
Case 1
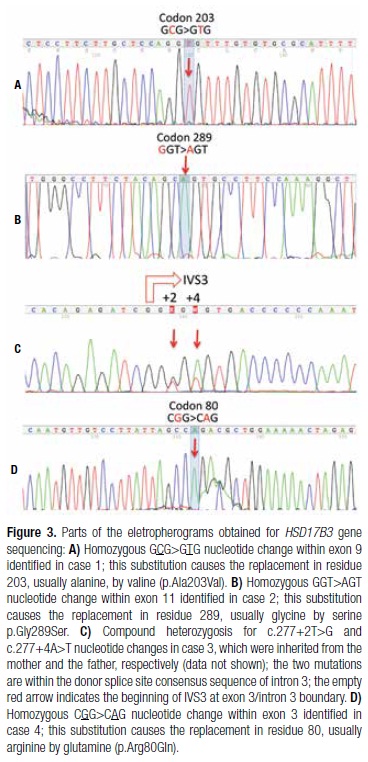
A 16-year-old girl from Pariconha (Alagoas Northeastern region of Brazil) was referred to us due to genital ambiguity and virilization at puberty. She was born at term by cesarean section to a 48-year-old mother, and weighted over 4.5 kg. Her parents were consanguineous (second cousins), and family history showed a sister with primary amenorrhea and absence of uterus, who had both gonadectomy and genitoplasty done during childhood. On physical examination, her weight and height were 68 kg and 168.4 cm, respectively; genitalia presented a 5.7-cm phallus and palpable gonads in labioscrotal folds with volumes of 15 cm3 and 10 cm3 for the right and the left gonad, respectively. A single perineal urethral opening with a short vagina were also observed. In addition, she presented facial hair and pubertal development Tanner 3 for breasts, and Tanner 5 for pubic hair (Figures 2A and 2B). The karyotype was 46,XY and laboratory data are shown in table 1. HSD17B3 gene sequencing revealed the homozygous p.Ala203Val missense mutation within exon 9 (Figure 3A). After psychological evaluation of the patient and the family, they decided to maintain the female gender. Bilateral gonadectomy and feminizing genitoplasty were performed, and hormonal replacement with estrogens was administrated. The analysis of both gonads indicated pubertal testes with discrete peritubular fibrosis. As she maintained the female gender, vaginal dilation will be performed as soon as she decides to initiate sexual activity.
Case 2
A 4‐year‐old girl, from Oliveira dos Brejinhos (Bahia Northeastern region of Brazil), was referred to us to investigate genital ambiguity with palpable gonads. She was the second child of non-consanguineous parents, and was born at term after an uneventful pregnancy. At physical examination, her weight was 16.2 kg and height 102 cm. A 3.7-cm phallus, single perineal opening, labioscrotal fusion, and bilateral palpable gonads within inguinal region were observed. Cytogenetic analysis indicated a 46,XY karyotype. Laboratory data are shown in table 1. HSD17B3 gene sequencing showed homozygosis for the p.Gly289Ser substitution in exon 11 (Figure 3B). Her family decided to maintain the female gender. Therefore, gonadectomy, clitoroplasty, and introitoplasty were performed. Histological analysis of the gonads showed normal pre‐pubertal testes.
Case 3
A 13‐year‐old girl from São Luís (Maranhão Northeastern region of Brazil) was referred to us due to signs of virilization such as acne, hirsutism, voice deepening, and phallus enlargement since the age of 11. She was born at term after an uneventful pregnancy. Her parents were healthy, non-consanguineous, and from small cities Maranhão State (Fortuna and Buriti Bravo). She had two sisters, both with telarche, and without signs of virilization, and one brother without sex ambiguity. On physical examination, the patient was 164 cm tall and weighed 59.8 kg. Her breasts were Tanner stage 1. She had increased hair on the abdomen and face, phallus enlargement (3.7 cm), and the skin of labioscrotal folds were pigmented and rugged. Pubic hair was Tanner stage 4. Her vagina ended in a 3-cm pouch, and no gonads were palpable (Figures 2C and 2D). Karyotype was 46,XY. Results of hormonal evaluation are shown in table 1. MRI of the abdomen and pelvis did not show an uterus or a prostate. However, seminal vesicles were identified, and both gonads were found at the inguinal region. HSD17B3 gene sequencing showed heterozygosis for two nucleotide changes within intron 3 splice donnor consensus sequence, c.277+4A>T, and the novel c.277+2T>G (Figure 3C). The analysis of the family indicated that the first variant was inherited from her father, and the second from her mother (data not shown). After female gender identity was confirmed by long-term psychological evaluation, video laparoscopy was performed to remove the gonads, and estrogen replacement therapy was initiated, followed by vaginal dilation. Histologic examination of both gonads showed bilateral testes with slight peritubular fibrosis, germ-cell aplasia, and Leydig cell hyperplasia, without evidence of malignancy.
Case 4
A 14‐year‐old girl from Alto do Araguaia (Mato Grosso Central-Western region of Brazil) was brought to us due to genital ambiguity and virilization at puberty. She was the second child of a consanguineous marriage (first cousins). Family history showed a 2-year-old female maternal cousin who presented clitoromegaly, and a 12-year-old female paternal cousin with hirsutism. Upon physical examination, the patient was 160 cm tall and weighed 41.4 kg. She had a 4.5-cm phallus, a single perineal opening, partial fusion of labioscrotal folds with pigmentation, and bilateral palpable gonads in the lower third of inguinal canal. Facial, axillary, abdominal, and pubic hair (Tanner 3) were observed, as well as Tanner 3 breast development. Karyotype was 46,XY. Hormonal evaluation data are shown in table 1. Pelvic ultrasonography did not show a uterus or a prostate. Genitography showed a urogenital sinus with a short vagina. HSD17B3 gene sequencing showed homozygosis for p.Arg80Gln mutation in exon 3 (Figure 3D). The patient decided to maintain the female gender; therefore, gonadectomy and feminizing genitoplasty were performed. Histological gonadal analysis indicated normal pubertal testes. Estrogen replacement therapy was initiated, and the patient decided to postpone vaginal dilation.
DISCUSSION
We report here four cases of 17-β-HSD3 deficiency, a rare disorder that was described for the first time in 1971 (18,19). It is a rare form of 46,XY DSD that affects testosterone biosynthesis (4). Its prevalence is higher among the Arab population, ranging from 1:100 to 1:300, due to a high frequency of consanguineous marriages (20). Two out of the four cases described here were born to consanguineous parents (cases 1 and 4), and showed homozygosis for HSD17B3 mutations. On the other hand, case 2, who has non-consanguineous parents, also showed a homozygous mutation in the HSD17B3 gene. In this case, the patient and her parents were born at a small city of the countryside of the State of Bahia.
Different HSD17B3 gene mutations confer a wide range of phenotypic characteristics to 46,XY affected individuals. These may vary from predominantly female genitalia, as in case 3; to mild virilized female genitalia, as in case 2; to evident genital ambiguity with palpable gonads, as in cases 1 and 4; to predominantly male genitalia with microphallus and hypospadias (4-8). This variability in phenotypes may correlate with partial activity of mutated 17-β-HSD3 in the testes, or to an extragonadal conversion of Δ4 to T by other 17-β-HSD isoenzymes (11).
As the most common clinical presentations of 17-β-HSD3 deficiency are female or mild virilized female genitalia, most patients do not have the diagnosis of 46,XY DSD at birth, and are registered and raised as females (4,5,8). Therefore, diagnosis is only established at puberty for most patients (4-8). Conversely, some cases may be diagnosed early, because they seek medical care during childhood due to some degree of virilization with palpable gonads (4,5,7).
If 17-β-HSD3 deficiency is not diagnosed in childhood, and gonadectomy is not performed, patients may present virilization at puberty (4,5,7). Main signs of virilization are increased hair growth all over the body and face, deepening of the voice, and android fat distribution, in addition to phallus elongation reaching 5-8 cm in length, which may also be observed in response to peripheral T conversion. However, the phallus will always be shorter than a normal-sized penis (4,5,7).
The diagnosis of 17-β-HSD3 deficiency may be suspected upon laboratory investigation, and may be confirmed with molecular analysis. As verified in all cases reported here, patients have, respectively, high and low to normal Δ4 and T serum concentrations (4,5,7). When T/Δ4 ratio is less than 0.8, 17-β-HSD3 deficiency is suggested (7,14). The literature also refers to increased serum concentrations of DHEA and DHEA sulfate (4,5,7). However, these elevated values are not always observed.
The mutation p.Arg80Gln identified in case 4 is the most common one in Mediterranean (21) and Brazilian patients (6). McKeever and cols. (22) demonstrated that p.Arg80Gln causes a significant decrease in the rate of enzymatic reaction leading to approximately 5% of residual enzyme activity, and a 1/60 reduction in the binding affinity of the mutant protein to NADPH cofactor. Therefore, it is suggested that the R in residue 80 of the protein structure should be critical in maintaining appropriate levels of enzymatic activity, and to promote normal human male development. This mutation was first described in a Palestinian family from the Gaza Strip (20). Further, it was also identified in Brazilian families with no Palestinian ancestry, and of probable Portuguese origin (6). However, its recurrence, not only in patients of Arab origin, but also in Dutch patients, was reported for individuals who were homo- or heterozygous for this mutation (5). Therefore, p.Arg80Gln may represent a founder effect, common among Arabs from different regions of Israel, Lebanon, and Syria (23). This fact led to the hypothesis that it has been introduced in Portugal and Spain by Phoenicians who migrated from Syria, Lebanon, and Israel around 750 B.C. (24,25). Its introduction in the Netherlands could have occurred during the Spanish domination in the sixteenth and seventeenth centuries and, in Brazil, by Portuguese colonizers and during the Dutch invasion of the Northeastern region of the country.
The p.Ala203Val mutation identified in case 1 was first described by Geissler and cols. (26), in a patient from São Paulo Brazil. This mutation was assayed for the ability to convert Δ4 into T, and it completely inactivated the enzyme 17-β-HSD3, indicating a good correlation with the phenotype of case 1.
Moghrabi and cols. (27) reported the missense substitution p.Gly289Ser (SNP CM023631). This mutation has been considered a polymorphism since it is frequent in all populations reported in the screening of 1,000 genomes (28). It also did not alter the in vitro enzymatic activity. However, it was identified in a compound heterozygous patient who also carried the well-known deleterious p.Asn130Ser mutation. It was supposed that this patient carried alterations in regulatory regions of the HSD17B3 gene that may explain the phenotype (5). In addition, p.Gly289Ser amino acid substitution has been associated with increased risk of developing prostate cancer, and to hypospadias in individuals carrying the S289 allele in homozygosis, once mRNA expression levels were significantly lower for the mutant S289 than for the wild-type G289, indicating that it may not be as neutral as it had been initially considered (29,30). Since we did not evaluate either 5' or 3' regulatory regions, we can speculate that the homozygous p.Gly289Ser in case 2 may be associated with the phenotype, and some other nucleotide change in regulatory regions could act synergistically with the mutation.
Case 3 was found to be compound heterozygous for two nucleotide changes affecting intron 3 splice donor region. The c.277+4A>T mutation was reported in 1996 by Andersson and cols. (9), who identified it in homozygosis in three families, and in heterozygosis in two other families. Those results indicated it as one of the most prevalent mutations found in subjects with 17-β-HSD3 deficiency. It is located within the intron 3 canonical splice donor site, and was shown to disrupt normal splicing by Boehmer and cols. (5), who analyzed the HSD17B3 cDNA prepared from testis mRNA of a homozygous patient. The result of cDNA sequencing showed a transcript where exon 3 had been skipped and, in minor amounts, a transcript with deletion of both exons 3 and 4 (5,31).
The mutation c.277+4A>T is found in populations worldwide, including Dutch, Germans, white Australians and white Americans, who share the same marker genotype, and are likely to be identical by descent (5). The c.277+2T>G mutation was also identified in case 3. It changes the almost invariant GT at the splice donor site to GG in intron 3. This mutation was identified here for the first time, and it probably suppresses the normal splicing so that it can either activate a cryptic splice site, or lead to exon 4 skipping. The in silico search for splicing sites using the online Splice Site Prediction NNSPLICE 0.9 version indicated that the mutation eliminated the normal donor splice at that position (data not shown). As testicular samples are not accessible for in vitro studies, the construction of mini-genes will be an alternative to test an aberrant splicing process in this case.
Patients with 17-β-HSD3 deficiency undergo virilization if gonadectomy is not performed before puberty, and some may adopt the male gender (4-8,12,13). Such a decision usually occurs in late adolescence or early adulthood, and the frequency varies between 39% and 64% (13). The explanation for the male option to occur is still unclear. Conversely, most patients who undergo prepubertal gonadectomy remain in the female gender (4-8). According to the literature, patients who choose to remain as females must undergo gonadectomy not only to avoid virilization, but also because of the 28% risk of malignant germ cell tumors (32). These patients should receive estrogen replacement therapy for the development of secondary sexual characteristics (4). All patients reported here, even those diagnosed at puberty, decided to maintain female identity after psychological evaluation of the patient and family members.
In conclusion, we report mutations in HSD17B3 gene, including the novel c.277+2T>G mutation, in four cases of 17-β-HSD3 deficiency with different clinical laboratorial and presentations.
Disclosure: no potential conflict of interest relevant to this article was reported.
Received on Aug/2/2012
Accepted on Sept/20/2012
- 1. Andersson S, Moghrabi N. Physiology and molecular genetics of 17beta-hydroxysteroid dehydrogenases. Steroids. 1997;62:143-47.
- 2. Lukacik P, Kavanagh KL, Oppermann U. Structure and function of human 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2006;248:61-71.
- 3. Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, et al. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 1997;62:148-58.
- 4. George MM, New M I, Tem S, Sultan C, Bhangoo A. The clinical and molecular heterogeneity of 17βHSD3 enzyme deficiency. Horm Res Paediatr. 2010;74:229-40.
- 5. Boehmer AL, Brinkmann AO, Sandkuijl LA, Halley DJ, Niermeijer MF, Andersson S, et al. 17beta-hydroxysteroid dehydrogenase-3 deficiency: diagnosis, phenotypic variability, population genetics, and worldwide distribution of ancient and de novo mutations. J Clin Endocrinol Metab. 1999;84:4713-21.
- 6. Mendonça BB, Inacio M, Arnhold IJ, Costa EM, Bloise W, Martin RM, et al. Male pseudohermaphroditism due to 17beta-hydroxysteroid dehydrogenase 3 deficiency. Diagnosis, psychological evaluation, and management. Medicine (Baltimore). 2000;79:299-309.
- 7. Lee YS, Kirk JM, Stanhope RG, Johnston DI, Harland S, Auchus RJ, et al. Phenotypic variability in 17beta-hydroxysteroid dehydrogenase-3 deficiency and diagnostic pitfalls. Clin Endocrinol (Oxf). 2007;67:20-8.
- 8. Faienza MF, Giordani L, Delvecchio M, Cavallo L. Clinical, endocrine, and molecular findings in 17beta-hydroxysteroid dehydrogenase type 3 deficiency. J Endocrinol Invest. 2008;31:85-91.
- 9. Andersson S, Geissler WM, Wu L, Davis DL, Grumbach MM, New MI, et al. Molecular genetics and pathophysiology of 17 beta-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab. 1996;81:130-6.
- 10. Mendonca BB, Arnhold IJ, Bloise W, Andersson S, Russell DW, Wilson JD. 17Beta-hydroxysteroid dehydrogenase 3 deficiency in women. J Clin Endocrinol Metab. 1999;84:802-4.
- 11. Prehn C, Möller G, Adamski J. Recent advances in 17beta-hydroxysteroid dehydrogenases. J Steroid Biochem Mol Biol. 2009;114:72-7.
- 12. Hiort O, Reinecke S, Thyen U, Jurgensen M, Holterhus PM, Schon D, et al. Puberty in disorders of somatosexual differentiation. J Pediatr Endocrinol Metab. 2003;16(suppl 2):297-306.
- 13. Cohen-Kettenis PT. Gender change in 46,XY persons with 5alpha-reductase-2 deficiency and 17beta-hydroxysteroid dehydrogenase-3 deficiency. Arch Sex Behav. 2005;34:399-410.
- 14. Faisal Ahmed S, Iqbal A, Hughes IA. The testosterone: androstenedione ratio in male undermasculinization. Clin Endocrinol (Oxf). 2000;53:697-702.
- 15. Ben Rhouma B, Belguith N, Mnif MF, Kamoun T, Charfi N, Kamoun M, et al. A novel nonsense mutation in HSD17B3 gene in a Tunisian patient with sexual ambiguity. J Sex Med. 2012 [Epub ahead of print]
- 16. Neocleous V, Sismani C, Shammas C, Efstathiou E, Alexandrou A, Ioannides M, et al. Duplication of exons 3-10 of the HSD17B3 gene: a novel type of genetic defect underlying 17β-HSD-3 deficiency. Gene. 2012;499:250-5.
- 17. Sambrook J, Fritsch EF, Maniatis TE. Molecular cloning, a laboratory manual New York: Cold Spring Harbor; 1989.
- 18. Saez JM, De Peretti E, Morera AM, David M, Bertrand J. Familial male pseudohermaphroditism with gynecomastia due to a testicular 17-ketosteroid reductase defect. I. Studies in vivo. J Clin Endocrinol Metab. 1971;32:604-10.
- 19. Saez JM, Morera AM, De Peretti E, Bertrand J. Further in vivo studies in male pseudohermaphroditism with gynecomastia due to a testicular 17-ketosteroid reductase defect (compared to a case of testicular feminization). J Clin Endocrinol Metab. 1972;34:598-600.
- 20. Rösler A, Silverstein S, Abeliovich D. A (R80Q) mutation in 17 beta-hydroxysteroid dehydrogenase type 3 gene among Arabs of Israel is associated with pseudohermaphroditism in males and normal asymptomatic females. J Clin Endocrinol Metab. 1996;81:1827-31.
- 21. Rösler A. 17 beta-hydroxysteroid dehydrogenase 3 deficiency in the Mediterranean population. Pediatr Endocrinol Rev. 2006;3(suppl 3):455-61.
- 22. Mckeever BM, Hawkins BK, Geissler WM, Wu L, Sheridan RP, Mosley RT, et al. Amino acid substitution of arginine 80 in 17β-hidroxysteroide dehydrogenase 3 and its effect on NADPH cofator binding and oxidation/reduction kinetics. Biochim Biophys Acta. 2002;1601:29-37.
- 23. Rosler A, Belanger A, Labrie F. Mechanisms of androgen production in male pseudohermaphroditism due to 17b-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 1992;75:773-8.
- 24. Culigan W. Phoenicia and Phoenician colonization. In: Boardman J, Edwards IE, Hammond NG, Sollberger E, Walker CB, eds. The Cambridge ancienty history, 2nd Ed. Cambridge University Press; 1991. p. 461-546.
- 25. Cavalli-Sforza LL, Menozzi P, Piazza A. The history and geography of human genes. Princeton: Princeton University Press; 1994. p. 217, 242-245, 260.
- 26. Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonça BB, et al. Male pseudohermaphroditism caused by mutations of testicular 17β-hidroxysteroide dehydrogenase 3. Nat Genet. 1994;7:34-9.
- 27. Moghrabi N, Hughes IA, Dunaif A, Andersson S. Deleterious missense mutations and silent polymorphism in the human 17b-hydroxysteroid dehydrogenase 3 gene (hsd17b3). J Clin Endocrinol Metabol. 1998;83(8):2855-60.
-
28http://www.ensembl.org/Homo_sapiens/Variation/Population?db=core;g=ENSG00000130948;r=9:98997588-99064434;t=ENST00000375263;v=rs2066479;vdb=variation;vf=16374979 Accessed on: Sept 30, 2012.
» link - 29. Margiotti K, Kim E, Pearce CL, Spera E, Novelli G, Reichardt JK. Association of the G289S single nucleotide polymorphism in the HSD17B3 gene with prostate cancer in Italian men. Prostate. 2002;53:65-8.
- 30. Sata F, Kurahashi N, Ban S, Moriya K, Tanaka KD, Ishizuka M, et al. Genetic polymorphisms of 17 β-hydroxysteroid dehydrogenase 3 and the risk of hypospadias. J Sex Med. 2010;7(8):2729-38.
- 31. Mains LM, Vakili MB, Lacassie Y, Andersson S, Lindqvistc A, Rock JA. 17beta hydroxysteroid dehydrogenase 3 deficiency in a male Pseudohermaphrodite. Fertil Steril. 2008;89(1):228.e13-228.e17.
- 32. Lee PA, Houk CP, Faisal A, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Pediatrics. 2006;118:488-500.
Correspondence to:
Publication Dates
-
Publication in this collection
02 Jan 2013 -
Date of issue
Nov 2012
History
-
Received
02 Aug 2012 -
Accepted
20 Sept 2012