ABSTRACT
Purpose:
Administration of eye drops containing antihistamines or sodium cromoglycate and its derivatives for the treatment of allergic keratoconjunctivitis is often insufficient and usually requires the addition of corticosteroids. However, the risk of complications, such as glaucoma and cataract, limits the use of corticosteroids to short courses, resulting in inadequate long-term treatment response. Immunosuppressive drugs have been considered as a valid alternative to steroids for atopic keratoconjunctivitis and vernal keratoconjunctivitis. This study aimed to evaluate the use of topical tacrolimus (TCL) in improving the clinical signs of severe allergic keratoconjuctivitis in children.
Methods:
Patients with severe allergic keratoconjunctivitis associated with corneal epitheliopathy, gelatinous limbal infiltrates, and/or papillary reaction, along with a history of recurrences and resistance to conventional topical anti-allergy agents, were included in this open clinical trial. Patients were treated with 0.03% TCL ointment for ocular use. A severity score ranging from 0 to 9, with 9 being the highest and 0 being the lowest, was assigned based on signs observed on biomicroscopy prior to and following TCL treatment.
Results:
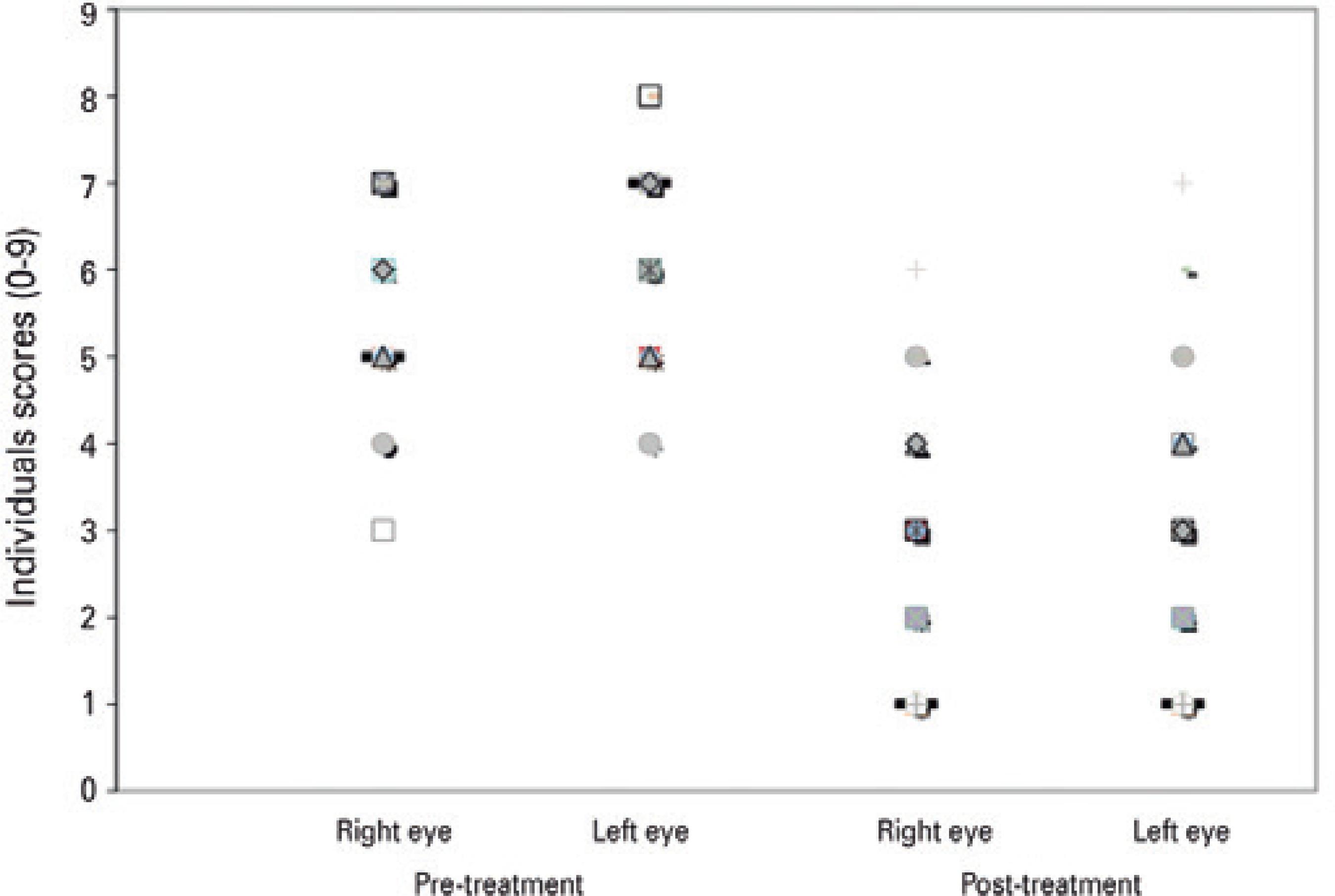
Analyses included 66 eyes of 33 patients. After a mean follow-up period of 13 months (range, 12-29 months), TCL treatment significantly decreased the mean symptom score severity for the right (from 5.56 ± 1.18 to 2.76 ± 1.5; p<0.001) and left (from 5.94 ± 1.16 to 2.86 ± 1.64; p<0.001).
Conclusion:
Topical TCL was effective and significantly improved the clinical signs of allergic keratoconjuctivitis in children. Thus, it is a potential new option for severe and challenging cases of ocular allergy.
Keywords:
Cornea; Tacrolimus therapy; Conjunctivitis, Allergic drug therapy
RESUMO
Objetivos:
O tratamento da ceratoconjuntivite alérgica baseado em colírios que contenham anti-histamínicos ou cromoglicato de sódio e seus derivados geralmente são insuficientes. A adição de corticosteróides geralmente é mandatória. No entanto, o risco de complicações como glaucoma e catarata limita o uso dos corticosteróides em curtos períodos de tratamento resultando em respostas inadequadas a longo prazo. Drogas imunossupressoras vem sendo consideradas como uma opção terapêutica alternativa válida para as ceratoconjuntivite atópica (AKC) e ceratoconjuntivite vernal (VKC). Este trabalho tem como objetivo avaliar a melhora nos sinais clínicos durante o uso de tacrolimus (TCL) tópico em crianças com ceratoconjuntivites alérgicas.
Métodos:
Pacientes com ceratoconjuntivite alérgica severa associada a ceratites, infiltrados limbares gelatinosos e/ou papilas gigantes, com história de recorrências e resistência ao tratamento anti-alérgico tópico convencional foram incluídos neste estudo. Os pacientes foram tratados com TCL 0,03% pomada tópica para uso ocular. Um escore variando de 0 a 9 foi atribuído para os sinais observados na biomicroscopia antes e depois do tratamento. Quanto maiores os escores, mais severos eram os sinais.
Resultados:
Foram estudados 66 olhos de 33 pacientes. Antes do tratamento a média do escore para o olho direito foi 5,56 ± 1,18 e para o olho esquerdo 5,94 ± 1,16. Após o tratamento com TCL a média do escore para o olho direito foi 2,76 ± 1,5 e para o olho esquerdo 2,86 ± 1,64 (p<0.001 para os dois olhos). O tempo de seguimento médio foi de 13 meses (12-29 meses).
Conclusão:
O presente estudo sugere que o TCL tópico foi efetivo e demonstrou resultado satisfatório, com melhora nos sinais clínicos na ceratoconjuntivite alérgica em crianças, constituindo uma nova opção para o tratamento de casos severos de alergia ocular.
Descritores:
Córnea; Tacrolimo/uso terapêutico; Conjuntivite alérgica/quimioterapia
INTRODUCTION
Ocular allergies constitute a heterogeneous group of frequently recurring inflammatory diseases of the ocular surface, with manifestations that range from mild to severe. Allergic conjunctivitis encompasses seasonal conjunctivitis, perennial conjunctivitis, atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC), and giant papillary conjunctivitis11 Barney NP. Vernal and atopic keratoconjunctivitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. Fundamentals, diagnosis and management. 2nd ed. Philadelphia (PA): Elsevier Mosby; 2005. p. 667-73.. AKC and VKC are the most severe forms because of the possibility of visual impairment caused by corneal scarring, irregular astigmatism, cataract, and glaucoma22 Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153 Suppl 1:17-21.,33 Singh S, Pal V, Dhull CS. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J Ophthalmol. 2001;49(4):241-5.. Glaucoma and cataract secondary to corticosteroid therapy are potential causes of blindness, which is a concern especially in young patients.
The treatment of ocular allergies involves a stepwise approach starting with preventative measures, such as avoidance of allergens, cold compress, and maintenance of a cool dry environment. Medical treatment, including preservative-free artificial tears, topical nonsteroidal anti-inflammatory drugs, and topical antihistamines or mast cell stabilizers are used for milder cases. In contrast, more severe cases are treated with a combination of antihistamines or mast cell stabilizers and topical or systemic corticosteroids, which is given either as an intensive short-term therapy or as a long-term treatment regimen44 Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004;18(4):345-51.. The use of immunomodulators, such as cyclosporin A, is reserved for cases with steroid-dependent allergic keratoconjunctivitis or when the use of corticosteroids is contraindicated, for example in patients with advanced glaucoma55 Cornish KS, Gregory ME, Ramaesh K. Systemic cyclosporin A in severe atopic keratoconjunctivitis. Eur J Ophthalmol. 2010;20(5):844-51.. In severe cases refractory to medical treatment, surgical treatment, including excision of the tarsal papillae, superficial keratectomy with or without amniotic membrane graft, and eyelid surgery, may be performed.
Topical tacrolimus (TCL) is a potent immunosuppressive drug that is widely used to prevent allograft rejection of transplanted organs, such as the liver and kidneys66 Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot. 1987;40(9):1249-55.. Its immunosuppressive effect is a result of the reduction in IL-2 production by T lymphocytes via a mechanism of action that is similar with that of cyclosporin. Moreover, previous studies reported that TCL as more potent and better tolerated than cyclosporine77 Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: review of randomised, double-blind clinical studies conducted in Japan. Clin Drug Investig. 2006;26(5):235-46.,88 Reinhard T, Reis A, Mayweg S, Oberhuber H, Mathis G, Sundmacher R. [Topical Fk506 in inflammatory corneal and conjunctival diseases. A pilot study]. Klin Monbl Augenheilkd 2002;219(3):125-31. German.. Moreover, topical TCL has been described to yield good results for AKC and other types of inflammatory diseases of the ocular surface99 Miyazaki D, Tominaga T, Kakimaru-Hasegawa A, Nagata Y, Hasegawa J, Inoue Y. Therapeutic effects of tacrolimus ointment for refractory ocular surface inflammatory diseases. Ophthalmology. 2008;115(6):988-992.e5.. The other therapeutic indications for which TCL gave satisfactory results were inflammatory diseases, such as Mooren's ulcer1010 Dhaliwal JS, Mason BF, Kaufman SC. Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008;27(4):488-93.,1111 Kymionis GD, Goldman D, Ide T, Yoo SH. Tacrolimus ointment 0.03% in the eye for treatment of giant papillary conjunctivitis. Cornea. 2008;27(2):228-9., high-risk penetrating keratoplasty1212 Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol.2010;10(5):478-85., giant papillary conjunctivitis1313 García DP, Alperte JI, Cristóbal JA, Mateo Orobia AJ, Muro EM, Valyi Z, et al. Topical tacrolimus ointment for treatment of intractable atopic keratoconjunctivitis: a case report and review of the literature. Cornea. 2011;30(4):462-5., AKC1414 Nivenius E, van der Ploeg I, Jung K, Chryssanthou E, van Hage M, Montan PG. Tacrolimus ointment vs steroid ointment for eyelid dermatitis in patients with atopic keratoconjunctivitis. Eye (Lond). 2007;21(7):968-75.
15 Taddio A, Cimaz R, Caputo R, de Libero C, Di Grande L, Simonini G, et al. Childhood chronic anterior uveitis associated with vernal keratoconjunctivitis (VKC): successful treatment with topical tacrolimus. Case series. Pediatr Rheumatol Online J. 2011;9:34.-1616 Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006; 90(4):461-4., VKC1717 Pucci N, Caputo R, Mori F, De Libero C, Di Grande L, Massai C, et al. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010;23(3):865-71., and anterior uveitis1818 Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 1987;40(9):1256-65.. There are two concentrations of TCL ointment approved by the Food and Drug Administration (FDA) for the treatment of atopic dermatitis; these are the 0.1% preparation for use in patients over 16 years old and the 0.03% preparation for use in children over the age of 2 years. These reported benefits and few side effects of TCL, as well as the few available data in children, compelled us to carry out this work. The objective of the present study was to evaluate the use of topical TCL for the treatment of severe allergic keratoconjunctivitis in children.
METHODS
This prospective study evaluated the use of topical TCL in pa tients with severe ocular allergies. Patients who were on topical antiallergic treatment for severe allergic keratoconjunctivitis and who had suffered recent progression of signs and symptoms after discontinuation of topical corticosteroid therapy were included in the study. Allergic keratoconjunctivitis was defined as the presence of recurrent ocular surface inflammation characterized by keratitis, gelatinous limbal infiltration, and/or giant papillae on the tarsal conjunctiva. Patients under 7 years of age, those with diseases lea ding to scarring of the conjunctiva, those with infectious corneal ulcer, and those with a history of herpetic keratitis were excluded from the study. Patients who have undergone surgical excision of giant papillae on the tarsal conjunctiva and those who have used systemic immunosuppressors or supratarsal injection of corticosteroids within the previous 6 months were also excluded.
The use of a placebo control group in this trial was not allowed due to ethical considerations because it would have required some patients to receive nonuseful treatment. This design may have led to some bias in the estimation of treatment effect.
This study was undertaken at the Cornea and External Eye Disea se Clinic, Department of Ophthalmology, Federal University of São Paulo, Brazil from January 2009 to July 2011. It was approved by the institute's internal review board under reference #1912-10. All individuals who participated in the study and/or their legal guardians gave a written informed consent for the study.
The treatment protocol comprised application on both eyes of 0.03% TCL ointment (Ophthalmos, São Paulo, Brazil) twice daily for at least 1 year, olopatadine hydrochloride 0.2% (Patanol S®) once daily, and an ocular lubricant containing polyethylene glycol (Oftane®) four times daily. All patients had been using olopatadine for at least 1 month before the treatment regimen was started. Topical corticosteroid therapy was discontinued but was resumed for a few days and with fast tapering when exacerbations occurred and when the above-mentioned drugs were unable to control the allergic process.
At each visit, all patients underwent detailed ophthalmologic eva luation, including visual acuity, intraocular pressure measurement, and slit-lamp biomicroscopic examination. Dilated fundus examination was performed on the first visit and was repeated as needed. The eyes were photographed for later comparison following TCL use.
Biomicroscopy included evaluation of the following items, in accor dance with the score system shown in chart 1: papillary reaction, gelatinous limbal infiltrates, and corneal epitheliopathy. A score ranging from 0 to 9, with 9 being the highest and 0 being the lowest, was assigned to each eye prior to and following treatment to assess for any improvement in the patient's clinical status (Figure 1). Classification was made in accordance with chart 1.
Classification of the signs observed at biomicroscopy. A score of 0-9 was assigned to each eye
Clinical scores before and after tacrolimus ointment treatment for patients with allergic keratoconjunctivitis (n=66 eyes), mean follow-up duration was 13 months (range, 12-29 months).
The patients were followed up at scheduled visits on days 1 and 15 then monthly thereafter, except in cases that developed exacerbations occurred, in which case the patients were evaluated weekly. The final evaluation took place on July 2011.
Data were presented as frequencies and proportions or means and standard deviations. Wilcoxon test was used to compare the symptoms in the right and left eyes prior to and following TCL use. P-values <0.05 were considered statistically significant. The STATA software program, version 10 (College Station, Texas, USA), was used throughout the entire statistical analyzes. Data from each eye were analyzed separately to avoid the problem of rejecting useful data. Otherwise, the use of an overall summary of ocular findings for an individual may result in loss of relevant information and lead to less power and less precise estimates of effect, similar to when only one eye per individual is used.
RESULTS
The study included 33 patients, with a mean age of 12 ± 3.97 years (range, 7-16 years): 23 (69.7%) were men and 10 (30.3%) were women. The mean duration of keratoconjunctivitis symptoms was 5.8 ± 2.97 years (range, 1-15 years). VKC was diagnosed in 25 patients (75.76%), whereas AKC was seen in 8 patients (24.24%). Of the 25 patients with VKC, 5 (20%) had the limbal form, 9 (36%) had the palpebral form, and 11 (44%) had the mixed form. The associated diseases present prior to starting TCL treatment were rhinitis in 24 patients (72.73%), asthma in 14 (42.42%), dermatitis in 13 (39.39%), glaucoma in 6 (18.18%), and keratoconus in 4 (12.12%).
The mean follow-up duration was 13 months (range, 12-29 months). Of the 33 patients, 20 cases (60.61%) had successfully controlled disease and cessation of ocular allergy symptoms following TCL use, without recurrence of acute crisis (Figure 2). Recurrence was observed once in six patients (18.18%), twice in four patients (12.12%), and thrice in three patients (9.09%). As previously mentioned, short-course topical corticosteroids were administered as needed for some of these cases.
Pretreatment (A and B) and post treatment (C and D) with topical tacrolimus in a patient with vernal keratoconjunctivitis. Note the improvement in the papillae and keratitis after the treatment (C and D).
Four patients discontinued TCL because they developed irritation and burning sensation in the eyes. In one case, TCL was discontinued due to the appearance of ocular herpes (dendritic epithelial keratitis) following initiation of treatment; this patient was treated with topical antiviral medication for 10 days until complete resolution of the clinical condition. These five patients were excluded from the statistical analyzes.
Following TCL use, there were no reports of intraocular inflammation, glaucoma, increase of ocular pressure, and cataract.
DISCUSSION
The treatment of severe allergic keratoconjunctivitis involves cor ticosteroid eye drops in addition to topical antiallergic drugs and artificial tears. However, due to the possible side effects such as cataract and glaucoma, corticosteroid-sparing drugs are frequently needed44 Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004;18(4):345-51.. Topical immunomodulators, such as TCL and cyclosporine, are safe alternatives for long-term treatment of these patients, with side effects that are generally transient and without rebound effects following discontinuation of the drug1313 García DP, Alperte JI, Cristóbal JA, Mateo Orobia AJ, Muro EM, Valyi Z, et al. Topical tacrolimus ointment for treatment of intractable atopic keratoconjunctivitis: a case report and review of the literature. Cornea. 2011;30(4):462-5..
The effect of topical cyclosporine depends on its concentration, which ranges from 0.05% to 2%; the higher the concentration, the poorer the tolerability and compliance with the treatment, particularly in young patients1919 Hingorani M, Moodaley L, Calder VL, Buckley RJ, Lightman S. A randomized, placebo-controlled trial of topical cyclosporin A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998;105(9):1715-20.
20 Tesse R, Spadavecchia L, Fanelli P, Rizzo G, Procoli U, Brunetti L, et al. Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol. 2010;21(2 Pt 1):330-5.-2121 Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13(3):308-14.. Daniell et al. showed that topical 0.05% cyclosporin failed to control VKC and AKC in steroid-dependent patients1616 Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006; 90(4):461-4.. In another study conducted on patients with VKC, topical cyclosporin at 1% and 2% was proven to be effective for a long-term control of the allergy1717 Pucci N, Caputo R, Mori F, De Libero C, Di Grande L, Massai C, et al. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010;23(3):865-71.. A systematic review suggested that topical cyclosporin may provide clinical and symptomatic relief from AKC and may help reduce topical steroid use in patients with steroid-dependent AKC2222 González-López JJ, López-Alcalde J, Morcillo Lais R, Fernández Buenaga R, Rebolleda Fernández G. Topical cyclosporine for atopic keratoconjunctivitis. Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009078..
In the present study, TCL was chosen for the treatment of severe allergic keratoconjunctivitis because of the poor tolerability to cyclosporine,ambiguous results, and of little experience with TCL at the beginning of the clinical trial. Following the use of topical TCL 0.03% for severe cases of allergic keratoconjunctivitis in children, there were significant improvements in clinical signs of corneal epitheliopathy, limbal involvement, and papillary reaction; this reduced the need for topical corticoids and the associated side effects.
Labcharoenwongs et al. published a clinical trial comparing topical TCL and cyclosporine for VKC; twice daily use of TCL ophthalmic ointment tended to be more effective than cyclosporine in improving the ocular signs2323 Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eyedrops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012;30(3):177-84.. Systemic TCL, which is used to prevent organ rejection in individuals subjected to heart, lung, liver, kidney, pancreas, bowel, or bone marrow transplant, was reported to be 10-100 times more powerful than cyclosporine1313 García DP, Alperte JI, Cristóbal JA, Mateo Orobia AJ, Muro EM, Valyi Z, et al. Topical tacrolimus ointment for treatment of intractable atopic keratoconjunctivitis: a case report and review of the literature. Cornea. 2011;30(4):462-5.. In an in vitro study, the immunosuppressive effect of TCL was found to be up to 100 times more potent than that of cyclosporine1818 Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 1987;40(9):1256-65.. Topical TCL ointment and pimecrolimus cream have been commercially available for more than a decade and are the first and only drugs approved for chronic treatment of atopic dermatitis in pediatric patients because these are not associated with skin barrier compromise or increased percutaneous absorption. TCL at concentrations of 0.1% and 0.03% is currently available on the market for use in dermatology, with ex cellent results in atopic dermatitis2424 Cheer SM, Plosker GL. Tacrolimus ointment. A review of its therapeutic potential as a topical therapy in atopic dermatitis. Am J Clin Dermatol. 2001;2(6):389-406..
A study conducted on patients treated with topical TCL evalua ted cytology samples of the conjunctiva and found a statistically significant reduction in the number of eosinophils, neutrophils, and squamous metaplasia; no cases of cellular atypia were found2525 Virtanen HM, Reitamo S, Kari M, Kari O. Effect of 0.03% tacrolimus ointment on conjunctival cytology in patients with severe atopic blepharoconjunctivitis: a retrospective study. Acta Ophthalmol Scand. 2006;84(5):693-5.. According to Attas-Fox et al., the blood level of TCL following topical use is negligible2626 Attas-Fox L, Barkana Y, Iskhakov V, Rayvich S, Gerber Y, Morad Y, et al. Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res. 2008;33(7):545-9.. Ohashi et al. reported that the use of topical TCL 0.1% for the treatment of allergic conjunctivitis was effective in improving the symptoms, in decreasing the size of the giant papillae on the tarsal conjunctiva, and in addressing corneal involvement2727 Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26(2):165-74.. Tzu et al. evaluated the long-term effectiveness of the combination of topical cyclosporine drops and TCL ointment in the treatment of steroid-dependent AKC; there was improvement of the symptoms and signs, and the number of flare-up episodes requiring topical steroids was low2828 Tzu JH, Utine CA, Stern ME, Akpek EK. Topical calcineurin inhibitors in the treatment of steroid-dependent atopic keratoconjunctivitis. Cornea. 2012;31(6):649-54.25..
The side effects of ocular use of TCL are generally tolerable, and only a few have reported the need to discontinue treatment99 Miyazaki D, Tominaga T, Kakimaru-Hasegawa A, Nagata Y, Hasegawa J, Inoue Y. Therapeutic effects of tacrolimus ointment for refractory ocular surface inflammatory diseases. Ophthalmology. 2008;115(6):988-992.e5.. There have been no reports on increase in intraocular pressure associated with the use of topical TCL; therefore, this drug appears to be a good option for patients with glaucoma or history of increased intraocular pressure following the use of topical corticosteroids, as well as for those with cataracts induced by corticosteroids2929 Kymionis GD, Tsilimbaris MK, Iliaki OE, Christodoulakis E, Siganos CS, Pallikaris IG. Treatment of atopic eyelid disease using topical tacrolimus following corticosteroid discontinuation in a patient with open-angle glaucoma. Cornea. 2004;23(8):828-30.. Only 4 of the 33 patients in this study were unable to continue the use of TCL due to irritation and burning in their eyes. There are no data in literature describing the cause of TCL intolerance, as has been reported for cyclosporine1919 Hingorani M, Moodaley L, Calder VL, Buckley RJ, Lightman S. A randomized, placebo-controlled trial of topical cyclosporin A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998;105(9):1715-20..
In patients given this immunosuppressive therapy with TCL, the risk for potential adverse effects, including reactivation of herpes and malignancy, should be taken into consideration. Development of cutaneous herpes simplex after application of topical TCL for atopic dermatitis was reported in one study3030 Soter NA, Fleischer AB Jr, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(1 Suppl):S39-46.. In the present study, only one patient had an episode of ocular herpes after initiation TCL treatment. Although the use of topical calcineurin inhibitors, such as TCL, has been reported to have a theoretical risk for malignancy, including lymphoma, analyzes of epidemiologic and clinical data failed to demonstrate a causal relationship between TCI use and malignancy or lymphoma risk. Despite this potential risk, these drugs are considered safe and remain to be the only approved long-term treatment medication for children 2 years and older with atopic dermatitis3131 Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013; 14(3):163-78..
The present study suggested that topical TCL was effective and rendered satisfactory results, specifically significant improvements in the clinical signs of allergic keratoconjuctivitis, in children; it might be a new option for severe and challenging cases of ocular allergy. A longer follow-up time is required to evaluate the long-term reproducibility of these results. The absence of a control group for comparison with patients who received standard treatment for ocular allergy and the difference in treatment duration among patients may have led to some bias in the estimation of treatment effect.
-
Funding: No specific financial support was available for this study.
-
Approved by the following research ethics committee: Universidade Federaal de São Paulo (# 1912-10)
REFERENCES
-
1Barney NP. Vernal and atopic keratoconjunctivitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. Fundamentals, diagnosis and management. 2nd ed. Philadelphia (PA): Elsevier Mosby; 2005. p. 667-73.
-
2Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153 Suppl 1:17-21.
-
3Singh S, Pal V, Dhull CS. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J Ophthalmol. 2001;49(4):241-5.
-
4Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye (Lond). 2004;18(4):345-51.
-
5Cornish KS, Gregory ME, Ramaesh K. Systemic cyclosporin A in severe atopic keratoconjunctivitis. Eur J Ophthalmol. 2010;20(5):844-51.
-
6Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot. 1987;40(9):1249-55.
-
7Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: review of randomised, double-blind clinical studies conducted in Japan. Clin Drug Investig. 2006;26(5):235-46.
-
8Reinhard T, Reis A, Mayweg S, Oberhuber H, Mathis G, Sundmacher R. [Topical Fk506 in inflammatory corneal and conjunctival diseases. A pilot study]. Klin Monbl Augenheilkd 2002;219(3):125-31. German.
-
9Miyazaki D, Tominaga T, Kakimaru-Hasegawa A, Nagata Y, Hasegawa J, Inoue Y. Therapeutic effects of tacrolimus ointment for refractory ocular surface inflammatory diseases. Ophthalmology. 2008;115(6):988-992.e5.
-
10Dhaliwal JS, Mason BF, Kaufman SC. Long-term use of topical tacrolimus (FK506) in high-risk penetrating keratoplasty. Cornea. 2008;27(4):488-93.
-
11Kymionis GD, Goldman D, Ide T, Yoo SH. Tacrolimus ointment 0.03% in the eye for treatment of giant papillary conjunctivitis. Cornea. 2008;27(2):228-9.
-
12Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol.2010;10(5):478-85.
-
13García DP, Alperte JI, Cristóbal JA, Mateo Orobia AJ, Muro EM, Valyi Z, et al. Topical tacrolimus ointment for treatment of intractable atopic keratoconjunctivitis: a case report and review of the literature. Cornea. 2011;30(4):462-5.
-
14Nivenius E, van der Ploeg I, Jung K, Chryssanthou E, van Hage M, Montan PG. Tacrolimus ointment vs steroid ointment for eyelid dermatitis in patients with atopic keratoconjunctivitis. Eye (Lond). 2007;21(7):968-75.
-
15Taddio A, Cimaz R, Caputo R, de Libero C, Di Grande L, Simonini G, et al. Childhood chronic anterior uveitis associated with vernal keratoconjunctivitis (VKC): successful treatment with topical tacrolimus. Case series. Pediatr Rheumatol Online J. 2011;9:34.
-
16Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006; 90(4):461-4.
-
17Pucci N, Caputo R, Mori F, De Libero C, Di Grande L, Massai C, et al. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010;23(3):865-71.
-
18Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 1987;40(9):1256-65.
-
19Hingorani M, Moodaley L, Calder VL, Buckley RJ, Lightman S. A randomized, placebo-controlled trial of topical cyclosporin A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998;105(9):1715-20.
-
20Tesse R, Spadavecchia L, Fanelli P, Rizzo G, Procoli U, Brunetti L, et al. Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol. 2010;21(2 Pt 1):330-5.
-
21Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13(3):308-14.
-
22González-López JJ, López-Alcalde J, Morcillo Lais R, Fernández Buenaga R, Rebolleda Fernández G. Topical cyclosporine for atopic keratoconjunctivitis. Cochrane Database Syst Rev. 2012 Sep 12;(9):CD009078.
-
23Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, Kosrirukvongs P, Saengin P, Vichyanond P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eyedrops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012;30(3):177-84.
-
24Cheer SM, Plosker GL. Tacrolimus ointment. A review of its therapeutic potential as a topical therapy in atopic dermatitis. Am J Clin Dermatol. 2001;2(6):389-406.
-
25Virtanen HM, Reitamo S, Kari M, Kari O. Effect of 0.03% tacrolimus ointment on conjunctival cytology in patients with severe atopic blepharoconjunctivitis: a retrospective study. Acta Ophthalmol Scand. 2006;84(5):693-5.
-
26Attas-Fox L, Barkana Y, Iskhakov V, Rayvich S, Gerber Y, Morad Y, et al. Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res. 2008;33(7):545-9.
-
27Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26(2):165-74.
-
28Tzu JH, Utine CA, Stern ME, Akpek EK. Topical calcineurin inhibitors in the treatment of steroid-dependent atopic keratoconjunctivitis. Cornea. 2012;31(6):649-54.25.
-
29Kymionis GD, Tsilimbaris MK, Iliaki OE, Christodoulakis E, Siganos CS, Pallikaris IG. Treatment of atopic eyelid disease using topical tacrolimus following corticosteroid discontinuation in a patient with open-angle glaucoma. Cornea. 2004;23(8):828-30.
-
30Soter NA, Fleischer AB Jr, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(1 Suppl):S39-46.
-
31Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013; 14(3):163-78.
Publication Dates
-
Publication in this collection
Jul-Aug 2017
History
-
Received
01 Aug 2016 -
Accepted
22 Feb 2017