ABSTRACT
Purpose:
To evaluate the outcomes of the first 30 cataract surgeries performed with a new disposable, injector-free, small-pupil expansion device.
Methods:
This consecutive case series included 30 eyes from 29 patients who underwent cataract surgery using a new disposable small-pupil expansion device called the Canabrava Ring (AJL Ophthalmic S.A, Spain). It is the first iris expansion ring produced with indents that do not align with each other in the superior and inferior regions, resulting in a small vertical length (0.4 mm) that minimizes the risk of endothelial contact. All eyes had poorly dilated pupils of less than 5 mm preoperatively. Fifteen eyes had significant infective or traumatic pathologies preoperatively. Vertical and horizontal pupil diameters were evaluated preoperatively, intraoperatively, and 1 month postoperatively.
Results:
The mean patient age was 64 ± 11.8 (standard deviation) years. The Canabrava Ring remained engaged throughout all surgeries, except one. All pupils were intraoperatively expanded to a diameter of 6.3 mm. Although preexisting pathology on the innervation of the pupils, the mean pupil diameter returns to a close preoperative size after 1 month surgery. The mean pupil diameters postoperatively and preoperatively were 4.41 and 3.77 mm, respectively (p<0.05). Postoperative complications occurred in eight eyes (one toxoplasmosis reactivation, one retinal detachment, one posterior capsule rupture, one posterior capsule opacification, and four posterior synechiae). These complications occurred in eyes with preexisting traumatic or infective pathologies or synechiae.
Conclusion:
The Canabrava Ring is effective for expanding and maintaining expansion of small pupils in cataract surgery. The increase in postoperative pupil diameter is clinically diminutive and can most likely be attributed to preexisting pathologies affecting pupil innervation. Further large-scale studies are required to support the present findings.
Keywords:
Cataract extraction; Miosis; Pupils/physiology; Prostheses and implants; Tissue expansion/instrumentation
RESUMO
Objetivo:
Avaliar a estabilidade intraoperatória, segurança e eficácia dos 30 primeiros casos operados com um novo anel expansor de pupilas.
Métodos:
Série de casos de 30 olhos de 29 pacientes submetidos a cirurgia de catarata com Anel de Canabrava (AJL Oftalmic, SPAIN). Trata-se do primeiro anel expansor de íris produzido com indentações não alinhadas entre as regiões superiores e inferiores. Devido a isso, apresenta altura vertical de 0,4 mm, diminuindo os riscos de toque endotelial. O diâmetro pupilar dos pacientes era menor que 5 mm. Os diâmetros verticais e horizontais foram avaliados antes, durante e um mês após a cirurgia.
Resultados:
A idade média dos pacientes foi de 64 ± 11,8 (desvio padrão) anos. O anel permaneceu estável em todas as cirurgias, exceto uma. Todas as pupilas foram expandidas no intraoperatório para um diâmetro de 6,3 mm. Apesar de patologias pupilares pré-existentes, o diâmetro médio da pupila retornou a um tamanho próximo após 1 mês de cirurgia. Os tamanhos médios da pupila no pós-operatório e pré-operatório foram medidos em 4,41 e 3,77 mm, respectivamente (p<0,05). As complicações pós-operatórias ocorreram em 8 olhos: 1 reativação de toxoplasmose, 1 descolamento de retina, 1 ruptura de cápsula posterior, 1 opacificação da cápsula posterior, 4 sinéquias posteriores. Essas complicações ocorreram nos olhos com patologias traumáticas, infecciosas ou sinéquias pré-existentes.
Conclusão:
O Anel de Canabrava parece efetivo na expansão e manutenção de pupilas pequenas submetidas à cirurgia de catarata. O aumento do diâmetro da pupila pós-operatória é clinicamente pouco relevante e provavelmente pode ser atribuído à patologias pré-existentes que afetam as inervações pupilares. Outros estudos em larga escala são necessários para suportar os achados do estudo.
Descritores:
Extração de catarata; Miose; Pupila/fisiologia; Próteses e implantes; Expansão de tecido/instrumentação
INTRODUCTION
Safe and effective cataract surgery is dependent on clear intraoperative visualization of the operative field. The use of this procedure for eyes with small pupils has been shown to be associated with an increased risk of iris bleeding, prolapse, and chafing that can lead to significant postoperative inflammation(11 Malyugin B. Complications of small-pupil cataract surgery: The use of minimally invasive approaches can help minimize risks and optimize outcomes. Cataract Refr Surg Today Europe. 2013;26-30. Available from: http://crstodayeurope.com/wp-content/themes/crste/assets/downloads/0913CRSTEuro_bf3_Malyugin.pdf
http://crstodayeurope.com/wp-content/the...
). Topical mydriatic eye drops produce sufficient pupil dilation in the majority of patients; however, the treatment of those who fail to respond to mydriatic eye drops can pose a challenge for cataract surgeons(22 Hovanesian JA, Sheppard JD, Trattler WB, Gayton JL, Malhotra RP, Schaaf DT, et al. Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain: Integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg. 2015;41(10):2060-8.). The approaches for overcoming such a challenge include iris sphincterotomy and physical stretching of the pupil using hooks, such as the Lester Hook (Katena Products Inc., Denville, NJ), or the 3-pronged Beehler Pupil Dilator (Moria, Antony, France)(33 Gogate P, Wood M. Recognizing 'high-risk' eyes before cataract surgery. Community Eye Health. 2008;21(65):12-4.). Although these devices adequately expand the pupil, there can be problems in cases where the stretched pupil refuses to remain dilated. In the case of iris retraction hooks, at least four new clear corneal incisions are necessary, resulting in more frequent iris sphincter trauma. Considering the increased invasiveness and risk associated with these modalities, a ring is the preferred expansion device(44 Agarwal A, Malyugin B, Kumar DA, Jacob S, Agarwal A, Laks L. Modified Malyugin Ring iris expansion technique in small-pupil cataract surgery with posterior capsule defect. J Cataract Refract Surg. 2008;34(5):724-6.).
The following four expansion rings are currently available in the US: 5S Pupil Ring (Morcher GmbH; Stuttgart, Germany), Perfect Pupil (Milvella Inc. Eden Prairie, MN), Malyugin Ring (MST, Redmond, WA), and Graether Pupil Expander (Eagle Vision Inc., Memphis, TN). The 5S Pupil Ring and Perfect Pupil are both disposable rings made from polymethylmethacrylate (PMMA) and polyurethane, respectively. These are rigid materials that have been shown to be associated with traumatic contact with the endothelium during intraoperative manipulation in the anterior chamber when the rings are large in size(55 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.). Both the rings require a reusable metal injector for insertion. The Graether Pupil Expander also requires a disposable injector. However, surgeons have reported that this ring is difficult to insert and handle when compared with competing rings(55 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.). The Malyugin Ring is made of 5-0 polypropylene, and it requires a specific injector. It has been reported to behave unpredictably during recovery back to the injector(66 Rauen M, Oetting T. Partial retraction of Malyugin pupil expansion device to improve safety during ring removal. J Cataract Refract Surg. 2010;36(3):522-3.) and to have a low density associated with an increased risk of ring deformation during surgery and endothelial contact on detachment from the iris(55 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.,66 Rauen M, Oetting T. Partial retraction of Malyugin pupil expansion device to improve safety during ring removal. J Cataract Refract Surg. 2010;36(3):522-3.).
The Canabrava Ring (CR; AJL Ophthalmic S.A, Spain) was developed to capitalize on the strengths of the four existing rings while avoiding their disadvantages. As such, this ring is disposable, can be used without an injector (resulting in lower cost), has a smaller vertical length (0.4 mm) than that of the 5S Pupil Ring (minimizing the risk of endothelial contact), and is easy to manipulate in the eye. Additionally, the CR requires only the original phaco incision when compared with the four incisions required for iris retractor hooks. Furthermore, the ring is made from the highly resistant material PMMA.
The purpose of this study was to evaluate the intraoperative stability, safety, and overall efficacy of the new CR when used to expand the pupils of high-risk eyes during cataract surgery. High-risk eyes were defined as eyes with coexisting infective or traumatic pathologies and small preoperative pupils.
METHODS
Enrollment
This consecutive case series included 30 eyes from 29 patients (14 female and 15 male patients) who had undergone cataract surgery. Both patient enrollment and treatment occurred at Clínica de Olhos Santa Casa de Misericórdia de Belo Horizonte in Minas Gerais, Brazil. Treatment was performed by a single surgeon (SC), and approval was obtained from the local ethics committee before study commencement. All procedures were carried out in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all patients before participation in this study.
The study size was determined according to patient presentation at the study clinic for cataract surgery during the study period. All presenting patients who met the inclusion criteria and provided consent for participation were included in the final study. The inclusion criteria were as follows: clinical indication for cataract surgery, age at least 18 years, pupil diameter less than 5 mm after dilation with phenylephrine and tropicamide, and lens with any degree of opacity. The exclusion criteria were as follows: inability to perceive light during visual acuity testing, pupils with a diameter greater than 5 mm, and clear lenses.
Preoperatively, all patients underwent fundoscopy after instillation of mydriatic eye drops. Both vertical and horizontal pupil diameters were measured with a slit lamp. Multiple horizontal and vertical pupil diameters were measured per eye, and the average was used to compare preoperative and postoperative pupil size. Biomicroscopy was performed preoperatively, using a slit lamp. Intraocular pressure (IOP) was assessed with Goldmann applanation tonometry. Best-corrected visual acuity (BCVA; Snellen decimal) and presence of posterior synechiae were assessed preoperatively. In specific cases, imaging was performed using optical coherence tomography and ultrasonography.
Complications and adverse events, including chamber instability, iris lesions, corneal lesions, and other atypical intraoperative occurrences, were assessed intraoperatively.
Surgical technique
All cataract surgeries were performed under peribulbar anesthetic block by the same surgeon (SC), and a standard protocol was followed. Pupillary dilation was initially attempted using one drop of phenylephrine 10%, one drop of tropicamide 1%, and viscodilation. Intracameral adrenaline was not used, as no study to date has shown intracameral mydriatics to be more potent than topical modalities. A total pupil diameter of less than 5 mm was achieved in all eyes, and the CR was used to expand all pupils throughout surgery.
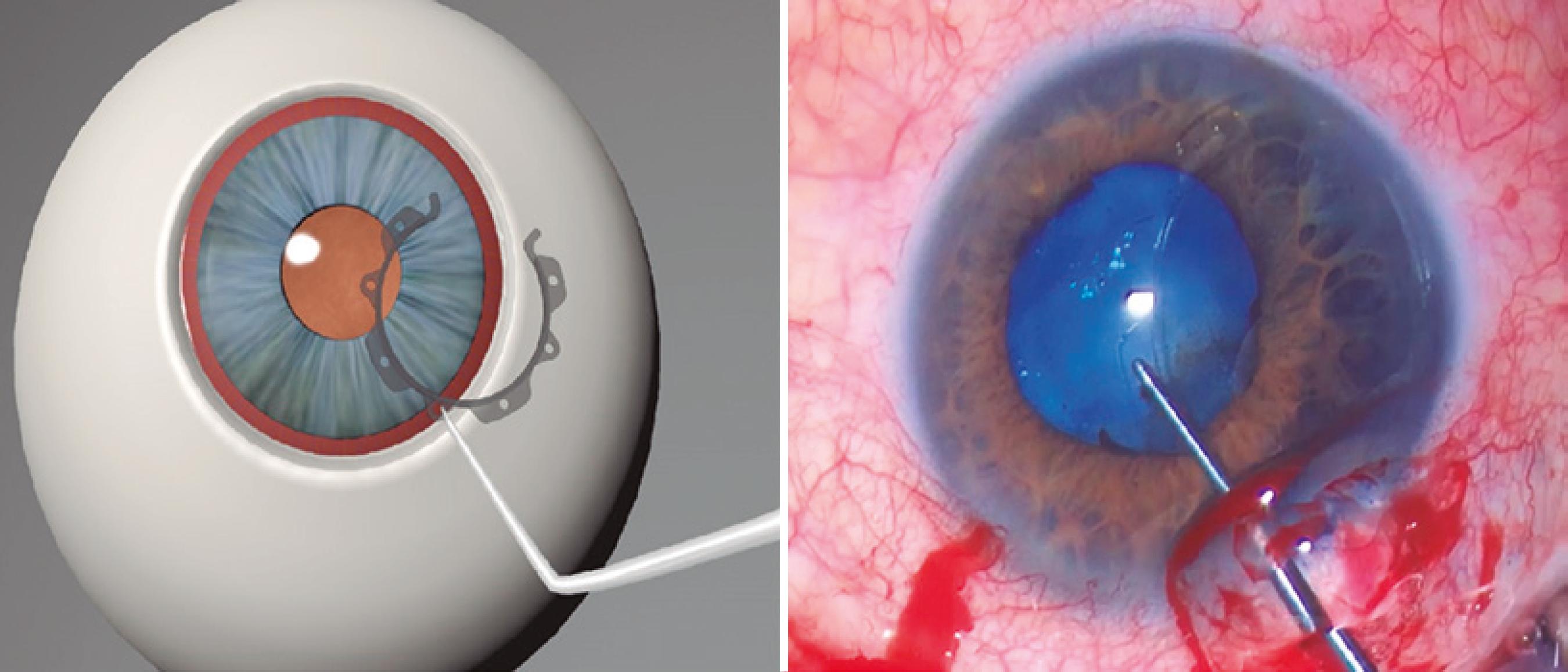
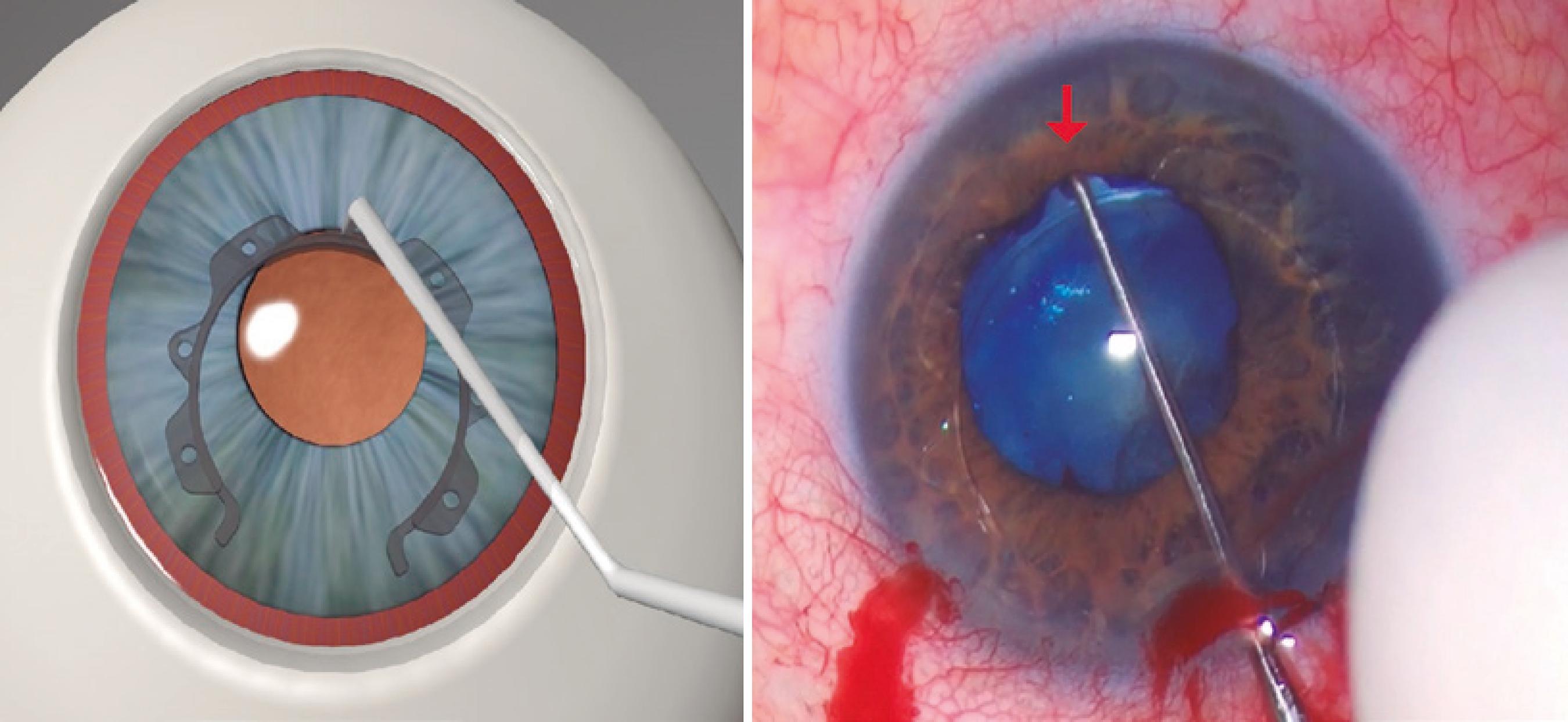
The ring was inserted through a 2.2 mm corneal incision. External calipers were used to ensure a consistent intraoperative pupil diameter of 6.3 mm with the CR, which is the optimal pupil diameter achievable with the CR (Figure 1). McPherson Forceps (Odous Instruments, Belo Horizonte, Brazil) were used to insert the first half of the ring into the eye, and then, the other half was rotated into the eye using a Sinskey Hook (Odous Instruments, Belo Horizonte, Brazil) (Figure 2). The small central inferior indent was then inserted beneath the iris (Figure 3). This process was repeated for each inferior indent (Figure 4 A and B). The insertion of the CR was completed by docking its hooks below the iris via connection of the Sinskey Hook to the superior indents (Figure 5 A and B). Phacoemulsification was then performed with the Laureate or Infiniti Vision System (Alcon, Fort Worth, TX) using the phaco-chop technique. Cataract surgery was completed along with intraocular lens implantation. To remove the ring, a Sinskey Hook was connected to the central upper indent. The ring was then moved to the incision side and rotated to the anterior chamber, with positioning of the open part of the ring in close proximity to the incision. McPherson Forceps were used to grasp the large upper indent of the ring and rotate it out of the eye. The removal technique has been demonstrated in figure 6.
The Canabrava Ring (CR). (1) open CR side; (2) horizontal internal diameter (6.3 mm); (3) engaged hook; (4) large superior indent; (5) small inferior indent; (6) hole.
Insertion of the second half of the Canabrava Ring into the eye with the help of a Sinskey Hook
A and B) Insertion of the Canabrava Ring is completed by docking its hooks below the iris through connection of the Sinskey Hook to the superior indents and use of a rocking motion for each attachment.
The Canabrava Ring
Figure 1 shows the CR, which is a PMMA ring with a semi-arch opening of 60°, internal diameter of 6.3 mm, and vertical length of 0.4 mm. Its technology differs from that of competing pupil expansion devices, because the parts that attach to the iris (indents) are arranged in an alternating fashion. There are seven indents (0.9 mm horizontal length), which are positioned on the ring in an alternating manner (one facing upwards and the next facing downwards). These alternating attachments are horizontally aligned and not vertically aligned. They are specifically spaced from each other to create sufficient room when arranged in the iris. There are two small hooks at each end, which attach to the iris. Each indent has a 0.28-mm-wide orifice for intraocular device manipulation with a Sinskey Hook. The CR is the first pupil ring to have indents that do not align superiorly and inferiorly with each other, resulting in a reduced vertical ring length. This ring attaches to the iris in a corrugated manner between the superior and inferior indents, thus allowing for superior fixation to the pupil border using the narrowest width possible for a PMMA ring. The ring’s compact layout minimizes its thickness, ensuring that it easily enters the ocular globe. This makes implantation easier as the iris connects to the ring like a wave.
Postoperative assessments
Follow-ups were carried out at postoperative days 1, 4, and 18 and month 1, and the pupil diameter and BCVA were assessed.
Outcome measures
The primary outcome measures were intraoperative and postoperative pupil diameter and intraoperative ring stability. Intraoperative and postoperative adverse events were assessed as secondary outcomes. The stability of the ring was defined as maintenance of pupillary dilation and not ring disengage from the iris throughout phacoemulsification.
Statistical analysis
The Student’s t-test was used to compare preoperative and postoperative pupil diameters and BCVA values. Statistical analyses were performed using Statistica Version 12 (StatSoft, Tulsa). A p-value <0.05 was considered statistically significant. Pupil size was measured as vertical and horizontal diameters preoperatively and postoperatively.
RESULTS
This study included 30 eyes from 29 patients (14 female and 15 male patients) with a mean age of 64 ± 11.8 years (range, 35-85 years). Of the 30 eyes, 15 eyes had significant infective or traumatic pathologies (five had toxoplasmosis, one had corneal trauma, three had retinal detachment, three had uveitis, two had Harada’s disease, and one had pseudoexfoliation glaucoma) (Table 1).
The ring was successfully inserted through a 2.2 mm incision in all cases. All pupils were intraoperatively expanded to a diameter of 6.3 mm. The ring remained stable and engaged throughout all surgeries, except one, where the pupil expanded more than a circumference of 6.3 mm intraoperatively, causing the ring to disengage. The device was easily removed after use in all cases. Although preexisting pathology on the innervation of the pupils, the mean pupil diameter returned to a close preoperative size 1 month after surgery.
The mean pupil diameters postoperatively and preoperatively were 4.41 and 3.77 mm, respectively (p<0.05). Figure 7 shows a comparison of the pupil circumference.
Preoperative and postoperative BCVA values were measured in 29 of the 30 eyes. Owing to significant preoperative pathology, only 16 eyes had a preoperative BCVA that could be quantified using Snellen decimal and 24 eyes had a postoperative BCVA that could be quantified. The mean preoperative BCVA was 0.36, and it significantly improved to 0.59 at 1 month postoperatively (p=0.00001).
Intraoperative complications were noted in three eyes (one capsulorhexis tear because of existing capsular bag fragility and two small iris bleeds associated with contact between the ring’s small end hook and the base of the iris during removal).
Postoperative complications were noted in eight eyes (one toxoplasmosis reactivation, one retinal detachment, one posterior capsule rupture, one posterior capsule opacification, and four posterior synechiae). All complications occurred in eyes with preexisting postoperative disease and/or preoperative synechiae, suggesting that the complications were not caused by the ring.
DISCUSSION
Although approaches involving mechanical pupil stretching and iris cutting are effective for stretching pupils resistant to dilation with mydriatic agents, these strategies can lead to permanent pupil sphincter damage and secondary complications, including hyphema and photophobia(77 Hashemi H, Seyedian MA, Mohammadpour M. Small pupil and cataract surgery. Curr Opin Ophthalmol. 2015;26(1):3-9.). Pupil dilating rings allow for continuous and adequate pupil dilation, with minimal risk of iris lesions. It has been shown that existing rings, namely, the 5S Pupil Ring, Perfect Pupil, Malyugin Ring, and Graether Pupil Expander, work well but require an injector (increasing cost) and carry the risk of endothelial contact during insertion, anterior chamber manipulation, and removal(55 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.).
The CR is an injector-free pupil expander, and thus, it reduces cost. Its PMMA composition allows good stability and predictable movement during insertion and anterior chamber manipulation. On the basis of the lead author’s experience with the ring, it is believed that the ring can be inserted through an incision as small as 1.4 mm, which makes it suitable for use in microincision cataract surgery(88 Cavallini GM, Verdina T, Forlini M, Volante V, De Maria M, Torlai G, et al. Long-term follow-up for bimanual microincision cataract surgery: comparison of results obtained by surgeons in training and experienced surgeons. Clin Ophthalmol. 2016 26;10:979-87.). One disadvantage of the PMMA material is its transparency, which may increase difficulty with regard to the identification of the correct insertion side of the ring and the subsequent visualization of the ring in the anterior chamber, when compared with its competitors.
Competing pupil expansion rings have been typically designed with double hooks (superior and inferior), and they have a sulcus that attaches to the iris, causing the superior and inferior projections to align and create a central groove in which the sphincter docks. With this layout, the total vertical length is 0.9 mm for the 5S Pupil Ring and 0.7-0.9 mm for the Malyugin Ring, when docked(99 Bhattacharjee S. Pupil-expansion ring implantation through a 0.9 mm incision. J Cataract Refract Surg. 2014;40(7):1061-7.). The exception is the Bhattacharjee Ring, which does not have hooks. It instead has four to six mono-nylon regions that form a ring with a lower vertical length of 0.1 mm(99 Bhattacharjee S. Pupil-expansion ring implantation through a 0.9 mm incision. J Cataract Refract Surg. 2014;40(7):1061-7.). However, the small size of these nylon regions can make ring placement in pupils with a small diameter cumbersome.
The alternating indents of the CR result in a smaller vertical length of 0.4 mm that, in the experience of the lead author, permits easy insertion through the cornea and intraocular manipulation. The low density of the ring’s PMMA material (1.185 g/cm3) prevents it from moving in the anterior chamber if decoupling from the iris occurs when only balanced salt solution is present in the chamber. This greatly reduces the risk of endothelial contact. The PMMA material may make the CR a more appropriate ring for patients with fibrosis, owing to the increased resilience of a dilated CR. The CR can be used in patients with eyes that cannot be easily treated, such as patients with sectorial traumatic iridectomy and coloboma, as it has a 60° opening (Figure 1). In contrast, the Malyugin Ring is only suitable for use in patients with intact irises, as it requires four points of engagement within the iris(55 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.). A comparison between the two pupil expansion devices is presented in figure 8. Additional studies specifically assessing the efficacy of the CR in iridectomy and coloboma are needed to investigate the outcomes that can be achieved with this ring in eyes with sectorial trauma.
Comparison of the Canabrava Ring and a traditional device (the Malyugin Ring) in patient with coloboma.
Among all the existing pupil expansion rings in the market, the 5S Pupil Ring is the closest competitor of the CR. The CR has no sulcus to engage the iris, has a small vertical length of 0.4 mm, has alternating indents with large superior indents to provide support above the iris, has no aligned indents in the vertical plane, has smaller inferior indents that connect the iris to the ring like a wave, and has two hooks at the end of its opening that increase intraoperative ring stability by engaging above the iris. On the other hand, the 5S Pupil Ring has a sulcus (for engaging the iris), has a larger vertical length of 0.9 mm, has superior and inferior indents of the same size, has vertically aligned indents, and has no hook at the end of the ring. Other than the PMMA material and the semi-circular structure, the CR differs significantly from the 5S Pupil Ring. The CR’s positioning of outward facing indents in a sequential manner results in a lower profile within the eye than that with the 5S Pupil Ring.
In the current study, only one case of ring disengagement occurred among 30 cases. On the basis of experience, it is believed that this is a rare event only occurring in the unlikely case of spontaneous intraoperative small-pupil dilation. A small, but significant, 0.64 mm increase in the pupil diameter postoperatively (from 3.77 mm preoperatively) was observed in the study group. However, considering that half of the eyes had preexisting pathologies involving inflammation, which is known to affect pupil innervation, it is likely that the pathologies hindered the ability of the pupils to return to their preoperative size, and this could occur regardless of the expansion device used(1010 Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye (2007) 21:746-51,1111 Guzman-Aranguez A, Gasull X, Diebold Y, Pintor J. Purinergic receptors in ocular inflammation. Mediators Inflamm. 2014;2014: 320906. Epub 2014 Jul 14.).
A previous study that used the Perfect Pupil device to intraoperatively expand 30 small pupils (not dilating beyond a diameter of 4.0 mm) during cataract surgery revealed a 1.1 mm increase in pupil diameter postoperatively compared with preoperatively. This increase in diameter was nearly twice the increase seen in the current study, suggesting that the CR may be better at restoring pupil size postoperatively than the Perfect Pupil device(1313 Yu A-Y, Guo H, Wang Q-M, Bao F-J, Huang J-H. Pupil dilation with intracameral epinephrine hydrochloride during phacoemulsification and intraocular lens implantation. J Ophthalmol. 2016; 2016:4917659. doi:10.1155/2016/4917659.
https://doi.org/10.1155/2016/4917659...
).
In the current study, intraoperative complications included two small iris bleeds. The bleeds occurred because the ring’s small end hook touched the base of the iris during ring implantation. This finding is similar to that in the study by Chang(55 Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.) on the efficacy of the Malyugin Ring in 30 eyes with small pupils. In that study, microbleeding occurred as an intraoperative complication in one patient. This previous study also revealed the risk of postoperative complications with surgery in eyes having pathologies. Indeed, sphincter tears, transillumination defects, and raised IOP occurred in more than one-third of the eyes in this previous study. Similarly, in the present study, around one-third of the eyes showed postoperative complications.
The present study has some limitations. The study had a small sample size and included eyes with multiple pathologies. Although the findings provide good evidence of the stability and efficacy of the CR during cataract surgery, larger studies are needed to provide greater evidence. By including eyes with multiple pathologies, this study demonstrated that the CR is an effective pupil expansion device in eyes with small pupils and multiple pathologies, including pupil synechiae. It is suspected that the presence of such pathologies led to the complications seen in the current participant group. A further study involving disease-free eyes is needed to confirm that the CR is not associated with complications.
The CR appears to be effective at expanding and maintaining expansion of small pupils in cataract surgery, without a risk of disengagement. Long pupil expansion rings have a high risk of endothelial contact and can be difficult to manipulate intraocularly. Therefore, the CR, which is reasonably thin, is a new alternative for small, challenging pupils, particularly in cases of colobomas and traumatic iris injuries.
-
Funding: No specific financial support was available for this study.
-
Approved by the following research ethics committee: Santa Casa de Misericórdia de Belo Horizonte (#787.530/2014).
-
Clinical trial registration number: NCT03206983
REFERENCES
-
1Malyugin B. Complications of small-pupil cataract surgery: The use of minimally invasive approaches can help minimize risks and optimize outcomes. Cataract Refr Surg Today Europe. 2013;26-30. Available from: http://crstodayeurope.com/wp-content/themes/crste/assets/downloads/0913CRSTEuro_bf3_Malyugin.pdf
» http://crstodayeurope.com/wp-content/themes/crste/assets/downloads/0913CRSTEuro_bf3_Malyugin.pdf -
2Hovanesian JA, Sheppard JD, Trattler WB, Gayton JL, Malhotra RP, Schaaf DT, et al. Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain: Integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg. 2015;41(10):2060-8.
-
3Gogate P, Wood M. Recognizing 'high-risk' eyes before cataract surgery. Community Eye Health. 2008;21(65):12-4.
-
4Agarwal A, Malyugin B, Kumar DA, Jacob S, Agarwal A, Laks L. Modified Malyugin Ring iris expansion technique in small-pupil cataract surgery with posterior capsule defect. J Cataract Refract Surg. 2008;34(5):724-6.
-
5Chang DF. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. Cataract Refract Surg. 2008;34(5):835-41.
-
6Rauen M, Oetting T. Partial retraction of Malyugin pupil expansion device to improve safety during ring removal. J Cataract Refract Surg. 2010;36(3):522-3.
-
7Hashemi H, Seyedian MA, Mohammadpour M. Small pupil and cataract surgery. Curr Opin Ophthalmol. 2015;26(1):3-9.
-
8Cavallini GM, Verdina T, Forlini M, Volante V, De Maria M, Torlai G, et al. Long-term follow-up for bimanual microincision cataract surgery: comparison of results obtained by surgeons in training and experienced surgeons. Clin Ophthalmol. 2016 26;10:979-87.
-
9Bhattacharjee S. Pupil-expansion ring implantation through a 0.9 mm incision. J Cataract Refract Surg. 2014;40(7):1061-7.
-
10Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye (2007) 21:746-51
-
11Guzman-Aranguez A, Gasull X, Diebold Y, Pintor J. Purinergic receptors in ocular inflammation. Mediators Inflamm. 2014;2014: 320906. Epub 2014 Jul 14.
-
12Kershner RM. Management of the small pupil for clear corneal cataract surgery. J Cataract Refract Surg. 2002;28(10):1826-31.
-
13Yu A-Y, Guo H, Wang Q-M, Bao F-J, Huang J-H. Pupil dilation with intracameral epinephrine hydrochloride during phacoemulsification and intraocular lens implantation. J Ophthalmol. 2016; 2016:4917659. doi:10.1155/2016/4917659.
» https://doi.org/10.1155/2016/4917659
Publication Dates
-
Publication in this collection
May-Jun 2018
History
-
Received
17 July 2017 -
Accepted
15 Nov 2017