ABSTRACT
Purpose:
To determine the effect of panretinal photocoagulation on optic disk topographic parameters in non-glaucomatous patients with proliferative diabetic retinopathy.
Methods:
This was a prospective, single-center, observational study. Thirty-eight eyes of 26 patients with diabetes underwent panretinal photocoagulation for proliferative diabetic retinopathy. Stereoscopic disk photographs and optic nerve head parameters were evaluated using the Zeiss fundus camera and the confocal scanning laser ophthalmoscope (Heidelberg Retinal Tomograph), respectively, at baseline and 12 months after the completion of panretinal photocoagulation.
Results:
Thirty-eight eyes of 26 patients (15 female) with a mean age of 53.7 (range 26-74) years were recruited. No significant difference was found between the stereo photography determined mean horizontal and vertical cup-to-disk ratio before and after panretinal photocoagulation treatment (p=0.461 and 0.839, respectively). The global values of the optic nerve head parameters analyzed with the HRT3 showed no significant change from baseline to 12 months, including the disk area, cup area, rim area, cup volume, rim volume, cup-to-disk area ratio, linear cup-to-disk ratio, mean cup depth, maximum cup depth, cup shape measure, height variation contour, mean retinal nerve fiber layer thickness, and cross-sectional area.
Conclusion:
Our results suggest that panretinal photocoagulation does not cause morphological optic disk changes in patients with diabetic proliferative retinopathy after 1 year of follow-up.
Keywords:
Light coagulation; Optic disk; Microscopy, confocal; Diabetic retinopathy; Scanning laser polarimetry
RESUMO
Objetivo:
Determinar o efeito da panfotocoagulação retiniana nos parâmetros topográficos do disco óptico em pacientes não glaucomatosos com retinopatia diabética proliferativa.
Métodos:
Este é um estudo observacional prospectivo e unicêntrico. Trinta e oito olhos de 26 pacientes diabéticos foram submetidos à panfotocoagulação retiniana para retinopatia diabética proliferativa. As estereofotografias e os parâmetros do disco óptico foram avaliados usando o retinógrafo Visucam da Zeiss e o oftalmoscópio confocal de varredura a laser (Heidelberg Retinal Tomograph), respectivamente, no início e 12 meses após a conclusão da panfotocoagulação.
Resultados:
Trinta e oito olhos de 26 pacientes (15 mulheres) com média de idade de 53,7 anos (intervalo de 26-74) foram recrutados. Nenhuma diferença significativa foi encontrada entre a média horizontal e vertical para relação escavação/disco óptico determinadas pelas estereofotografias antes e após o tratamento com panfotocoagulação retiniana (p=0,461 e 0,839, respectivamente). Os valores globais dos parâmetros do disco óptico analisados com a tomografia de varredura a laser não mostraram nenhuma mudança significativa entre o início até os 12 meses, incluindo disk area, cup area, rim area, cup volume, rim volume, C/D area ratio, linear C/D ratio, mean cup depth, maximum cup depth, cup shape measure, height variation contour, mean retinal nerve fiber layer thickness e cross-sectional area.
Conclusão:
Nossos resultados sugerem que a panfotocoagulação retiniana não causa alterações morfológicas no disco óptico em pacientes com retinopatia diabética proliferativa após um ano de seguimento.
Descritores:
Fotocoagulação; Disco óptico; Microscopia confocal; Retinopatia diabética; Polarimetria de varredura por laser
INTRODUCTION
Diabetic retinopathy (DR) is the most common ocular complication of diabetes mellitus (DM) and is one of the leading causes of blindness in developed countries(11 Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503.). It is also known to be an important risk factor for chronic open-angle glaucoma, and both diseases often coexist.
In cases of proliferative diabetic retinopathy (PDR), panretinal photocoagulation (PRP) is the first-line treatment. Although PRP reduces the risk of severe vision loss(22 Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583-600.), it laser energy has been shown to cause destruction to all layers of the retina, including the ganglion cells and the retinal nerve fiber layer (RNFL), and therefore generate visual field defects similar to those observed in glaucomatous damage(33 Diabetic Retinopathy Clinical Research Network, Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, Browning DJ, Elman MJ, Glassman AR, Gross JG, Kollman C, Wells JA 3rd. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127(2):132-40. 4. Comment in: Arch Ophthalmol. 2010;128(2):262; author reply 262.). In such cases, visual field testing can be less helpful to evaluate glaucomatous damage in patients with PDR treated with PRP.
Pathological cupping of the optic disk is often associated with glaucoma, and it has been described in several other optic neuropathies, but rarely in retinal diseases(44 Zhang YX, Huang HB, Wei SH. Clinical characteristics of nonglaucomatous optic disc cupping. Exp Ther Med. 2014;7(4):995-9.). It DR has been suggested to enhance the apoptosis of retinal ganglion cells, causing neurodegenerative changes in the retina(55 Abu El-Asrar AM, Nawaz MI, Mohammad G, Siddiquei MM, Alam K, Mousa A, et al. Expression of bioactive lysophospholipids and processing enzymes in the vitreous from patients with proliferative diabetic retinopathy. Lipids Health Dis. 2014;13:187.). With the development of clinically available imaging technology, recent studies have shown that patients with diabetes, with or without DR, have thinner RNFL thickness than the normal population(66 Peng PH, Lin HS, Lin S. Nerve fibre layer thinning in patients with preclinical retinopathy. Can J Ophthalmol. 2009;44(4):417-22.
7 Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219(6):379-85.-88 Takahashi H, Goto T, Shoji T, Tanito M, Park M, Chihara E. Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006;142(1):88-94.). Moreover, it has been suggested that ganglion cell loss secondary to PRP and ascending RNFL atrophy could change the appearance and topography of the optic disk(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.). Therefore, evaluating the optic disk cupping and possible glaucomatous damage in patients with DR can be difficult, especially after PRP treatment.
In this scenario, confocal scanning laser ophthalmoscopy (CSLO), with a three-dimensional topographic analysis, can be a valuable diagnostic tool to assess the topographic changes of the optic disk, and obtain objective measurements of the rim area and cup parameters. Previous studies have shown conflicting results in both RNFL and optic disk topographic measurements after PRP treatment in patients with diabetes(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.
10 Lim MC, Tanimoto SA, Furlani BA, Lum B, Pinto LM, Eliason D, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol. 2009;127(7):857-62.-1111 Singh H, Garg S, Sharma R, Venkatesh P, Saxena R, Dada T. Evaluation of the effect of pan retinal photocoagulation on optic nerve head parameters using HRT3. J Glaucoma. 2014;23(7):467-70.). The purpose of this study was to prospectively determine the effect of PRP on CSLO optic disk parameters and stereo photographic analysis in patients with non-glaucomatous PDR.
METHODS
This prospective observational cohort study enrolled patients from the retina service of the Ophthalmology Division of the University of São Paulo Medical School. The study protocol was approved by the local ethics committee and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects.
The inclusion criteria for the study were a diagnosis of PDR (due to type 1 or 2 DM), intraocular pressure <18 mmHg, non-glaucomatous optic disk characteristics at fundus examination, a vertical cup-to-disk (C/D) ratio <0.7, and the absence of media opacities. Subjects were excluded from this study if they had a previous diagnosis of glaucoma or a family history of glaucoma, or any coexisting neuro-ophthalmic disease, uveitis, retinal artery or vein occlusion, optic disk neovascularization, diabetic macular edema (DME), corneal opacity, or previous laser photocoagulation treatment.
All participants underwent a complete ophthalmological examination at baseline, including best-corrected visual acuity with Snellen charts, Goldmann applanation tonometry, slit-lamp biomicroscopy of anterior and fundus segment using a 78D lens (Volk, Mentor, OH, USA), and indirect binocular ophthalmoscopy. Stereoscopic disk photographs and Heidelberg Retinal Tomograph (HRT3; Heidelberg Retinal Tomography, Heidelberg Engineering, Heidelberg, Germany) images were obtained at baseline and 12 months after the completion of PRP.
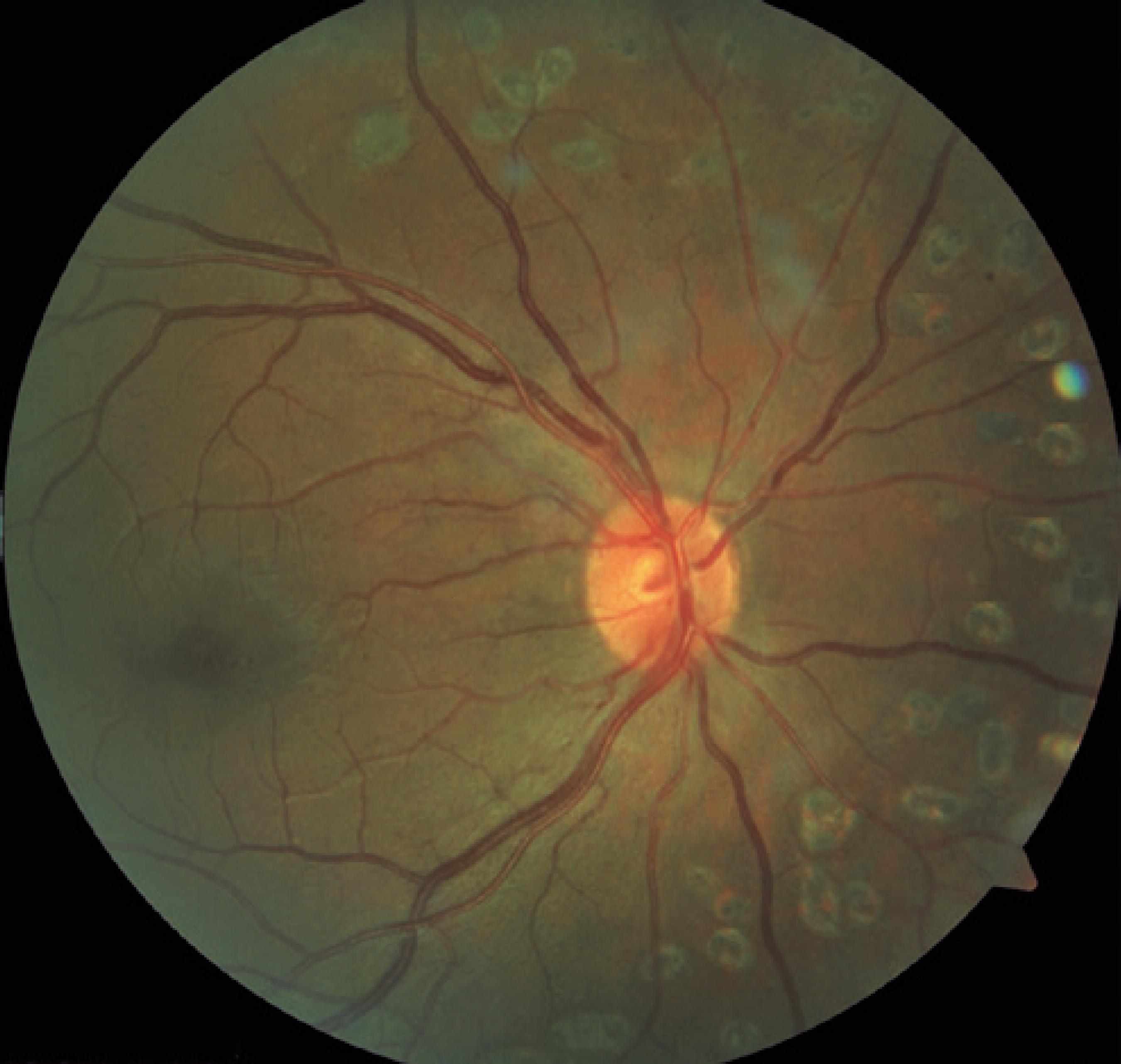
PRP treatment was carried out with a single spot green laser (Purepoint® laser system, 532-nm wavelength, Alcon, Fort Worth, TX, USA). All patients received at least 1,500 peripheral laser photocoagulation burns. The laser parameters used were a spot size of 250 mm, a pulse duration of 0.2 s, and enough power to cause grayish white burns following the Early Treatment Diabetic Retinopathy Study (ETDRS) guidelines. PRP was carried out in three sessions, with each session 1 week apart. Patients were examined after 6 weeks to evaluate the effect of laser treatment and the need for further laser sessions. Figure 1 exemplifies the pattern of laser photocoagulation applied in the study and the distance between the photocoagulation burns and the optic disk margins.
Retinography of the posterior pole exemplifying the type of laser photocoagulation pattern used in the study.
All stereoscopic disk photographs were taken with a Zeiss fundus camera (Carl Zeiss, Inc., Thornwood, NY, USA) and were centered on the optic disk. The horizontal and vertical diameters of the C/D ratio were determined for each stereo pair by an experienced glaucoma specialist. In order to assure a blinded analysis, all image files received a numeric code. Any laser burn observed in the picture was cropped out to ensure that the glaucoma specialist was blinded to the patient name and also the pre- or post-PRP status. A stereo viewer was used to enhance details.
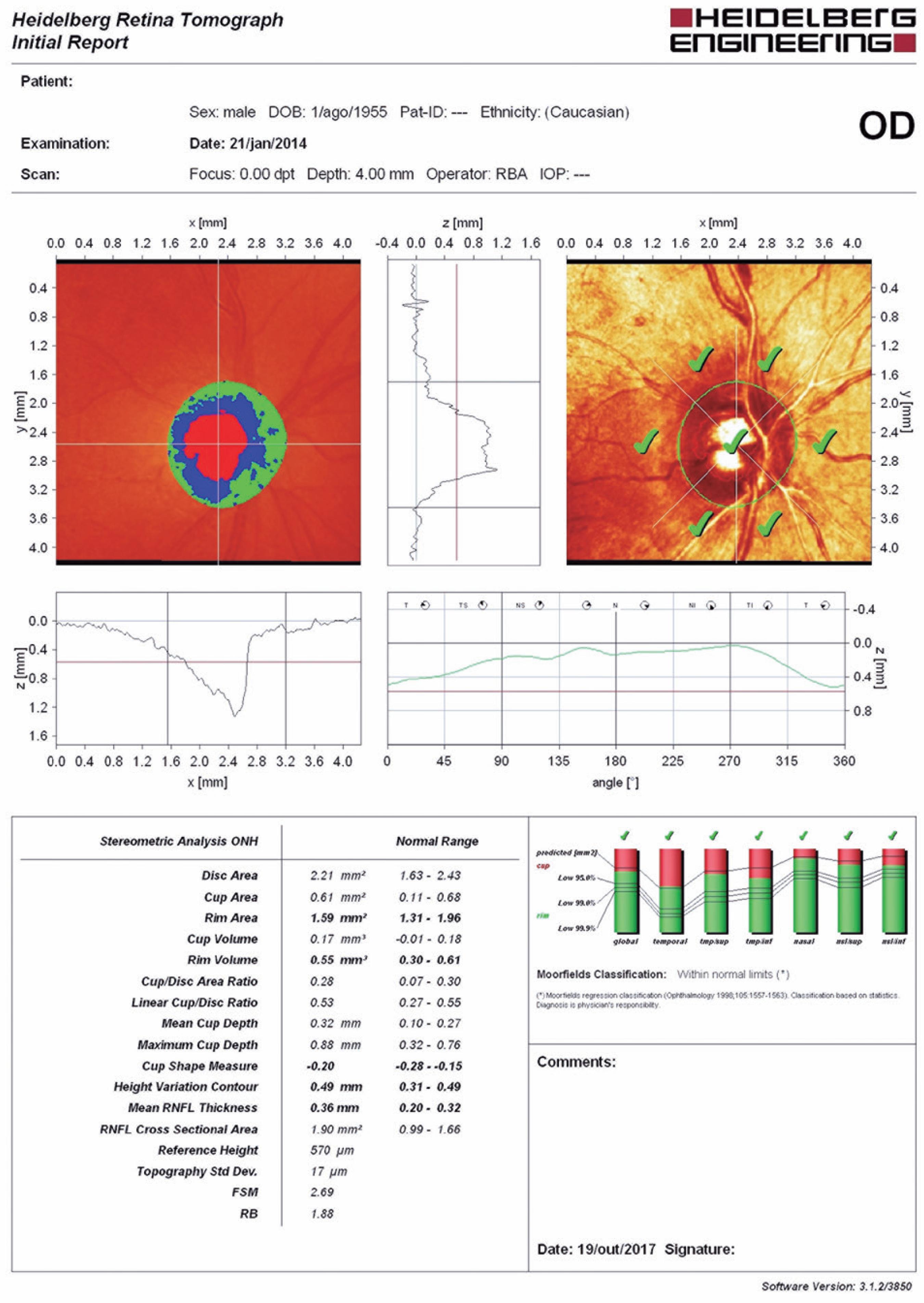
Optic nerve head (ONH) topography was analyzed with the HRT3 using confocal scanning parameters. A 15-degree angle view was used under the same intensity of dim room light. After generating a mean topographic image, contour lines were drawn by the same experienced technician. Subjects with standard deviations less than 30 mm were included in the study. The reference plane was automatically set at the standard value of 50 mm below the contour line at the temporal sector of the disk margin between 350° and 356°. Figure 2 is an example of a printout obtained from an eye using the HRT3, before laser treatment.
Example of a printout obtained from an eye using the Heidelberg Retinal Tomograph 3, before laser treatment.
The following topographic parameters calculated by the HRT3 hardware were evaluated at baseline and 12 months after PRP completion: disk area (mm2), cup area (mm2), rim area (mm2), cup volume (mm3), rim volume (mm3), C/D area ratio, linear C/D ratio, mean cup depth (mm), maximum cup depth (mm), cup shape measure, height variation contour (mm), mean RNFL thickness (mm), and RNFL cross-sectional area (mm2). These variables were used for the topographic global analysis.
Statistical analyses were performed using a commercially available computer software package (SPSS, ver. 23.0; SPSS, Chicago, IL, USA). Normality was tested using the one-sample Kolmogorov-Smirnov test. The Wilcoxon signed-rank test was used to compare the stereoscopic pre- and post-PRP C/D ratios (horizontal and vertical) and HRT parameters. The statistically significant level was accepted as p<0.05.
RESULTS
This study included a total of 42 eyes of 30 patients. Four eyes of four patients were excluded during the follow-up visits (one patient developed preretinal membranes causing tractional detachment; another eye developed vitreous hemorrhage and both patients were submitted to pars plana vitrectomy; two eyes developed macular edema and were treated with intravitreal anti-vascular endothelial growth factor [VEGF] injections). Thirty-eight eyes of 26 individuals (15 female, 11 male) completed the 1-year follow-up. The mean age was 53.7 years, ranging from 26 to 74 years. Stereoscopic disk photographs resulted in a mean (± standard deviation) pretreatment horizontal C/D ratio of 0.316 ± 0.065 and a post-treatment horizontal C/D ratio of 0.303 ± 0.065. The difference between these means (-0.013) was not statistically significant (p=0.461). Similarly, the difference between the mean vertical C/D ratio in the pretreatment (0.363) and post-treatment (0.366) visits was also not statistically significant (p=0.839). These data are summarized in table 1.
The global values of the ONH parameters obtained from the HRT3 examination are presented in table 2. There was no statistically significant difference between the baseline and the 12-month HRT parameters. The cup area showed a non-significant (p=0.510) increase from the baseline (0.366 ± 0.216 mm2) to 12 months (0.376 ± 0.239 mm2). Similarly, there was an increase in the mean values of the maximum cup depth from the baseline (0.600 ± 0.244 mm) to the 12-month visit (0.636 ± 0.232 mm), without statistical significance (p=0.135). Rim area parameters, moreover, showed smaller values in the 12-month evaluation (1.638 ± 0.349 mm2) than at the baseline (1.649 ± 0.339 mm2) measurement, although this difference was also not statistically significant (p=0.451). The ratio between the cup area and disk area also showed a non-significant increase between examinations, from 0.178 ± 0.098 to 0.183 ± 0.108, with a p-value of 0.477 (Table 2).
DISCUSSION
PRP decreases the risk of severe visual loss in patients with PDR and, despite the evidence regarding the efficacy of antiangiogenic drugs, it is still the standard of care for the management of proliferative disease, according to the American Academy of Ophthalmology’s Preferred Practice Pattern for Diabetic Retinopathy(1212 American Academy of Ophthalmology and Preferred Practice Pattern. Diabetic Retinopathy [Internet]. San Francisco, CA: American Academy of Ophthalmology; 2017. [cited 2017 jun 21]. Available from: https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2017
https://www.aao.org/preferred-practice-p...
), which is based on the Diabetic Retinopathy Study (DRS) and the ETDRS (level 1 evidence). While most visual complications in patients with PDR are related to retinal damage, it is not uncommon for patients to have glaucoma-associated visual loss, either because of disease unrelated to diabetes or because of intraocular pressure elevation from PRP or other treatment modalities, such as corticosteroid injections. Therefore, while the causal relationship between DM and open-angle glaucoma in many cases remains unclear(1313 Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP; The Los Angeles Latino Eye Study Group. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study Group. Ophthalmology. 2008;115(2):227-32.), the diagnosis of chronic open-angle glaucoma in patients who have PDR can be challenging, particularly when submitted to PRP, as this type of treatment can produce visual field changes that may mimic glaucomatous field loss.
Ascending optic atrophy may assume several characteristic forms that can be distinguished both histopathologically and ophthalmoscopically. It occurs when a primary lesion involves the retina or optic nerve, producing an atrophic process that proceeds toward the brain. In experimental models(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.), the distal aspect of a damaged axon was shown to degenerate 2-4 weeks after injury(1414 Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86(10):1803-30.). In the pattern of atrophy and excavation of the ONH associated with glaucomatous damage, the loss of ganglion cell axonal fibers anterior to the lamina cribrosa gives rise to an increased C/D ratio(1414 Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86(10):1803-30.). In general, this characteristic pattern of glaucomatous disk cupping is seen in association with elevated intraocular pressure(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.) but it has also been reported in a number of other conditions(44 Zhang YX, Huang HB, Wei SH. Clinical characteristics of nonglaucomatous optic disc cupping. Exp Ther Med. 2014;7(4):995-9.). In patients with diabetes submitted to PRP, the laser could damage photoreceptors that would secondarily affect ganglion cells, possibly producing increased cupping of the optical disk. Therefore, laser treatment could potentially induce ascending optic atrophy and mimic glaucomatous conditions. The 1-year interval between stereo photographs in our study was chosen because ample time would have elapsed to allow a possible atrophy to occur. On the other hand, an even longer interval would potentially introduce an aging change bias that could interfere with the interpretation of the C/D ratio.
Even in cases without DR, diabetes can cause alterations in RNFL and hence, consequently affect the ONH morphology. Recently, clinically available devices measuring the RNFL thickness have shown that patients with diabetes without background retinopathy and those with non-PDR have a thinner RNFL than healthy subjects(66 Peng PH, Lin HS, Lin S. Nerve fibre layer thinning in patients with preclinical retinopathy. Can J Ophthalmol. 2009;44(4):417-22.
7 Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219(6):379-85.-88 Takahashi H, Goto T, Shoji T, Tanito M, Park M, Chihara E. Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006;142(1):88-94.,1515 Ozdek S, Lonneville YH, Onol M, Yetjub Um Gasabreusigky BB. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye (Lond). 2002;16(6):761-5.,1616 Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fiber layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86(7):725-8. Comment in: Br J Ophthalmol. 2002;86(7):709.). This finding might be explained by the death of retinal ganglion cells, which occurs early in diabetic eyes due to enhanced apoptosis-promoting factors(1717 Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45(8):2760-6.). Amano et al.(1818 Amano S, Kaji Y, Oshika T, Oka T, Machinami R, Nagai R, Horiuchi S. Advanced glycation end products in human optic nerve head. Br J Ophthalmol. 2001;85(1):52-5.) detected the presence of abnormal looking ONH in patients with diabetes and proposed that glycation end products might directly damage the optic nerve. However, Königsreuther and Jonas(1919 Königsreuther KA, Jonas JB. Optic disc morphology in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 1995;233(4):200-4.) analyzed the appearance of the optic disk in patients with and without diabetes using color photographs and found no difference between both these groups. Similar results were obtained by Tekeli et al.(2020 Tekeli O, Turaçli ME, Atmaca LS, Elhan AH. Evaluation of the optic nerve head with the Heidelberg Retinal Tomograph in diabetes mellitus. Ophthalmologica. 2008;222(3):168-72.) and Lim et al.(1010 Lim MC, Tanimoto SA, Furlani BA, Lum B, Pinto LM, Eliason D, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol. 2009;127(7):857-62.) using different imaging techniques.
Cross-sectional studies have indicated that PRP may cause morphologic changes in the ONH. Lim et al.(1010 Lim MC, Tanimoto SA, Furlani BA, Lum B, Pinto LM, Eliason D, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol. 2009;127(7):857-62.) compared diabetic eyes submitted or not to PRP using optical coherence tomography imaging. Optic nerves in eyes treated with PRP were more likely to be graded as abnormal, but the authors did not perform longitudinal comparisons and therefore disease severity could not be ruled out as a causative factor. Cancaya et al.(2121 Cankaya AB, Ozdamar Y, Ozalp S, Ozkan SS. Impact of panretinal photocoagulation on optic nerve head parameters. Ophthalmologica. 2011;225(4):193-9.) found similar results using CSLO technology.
We could not identify topographic ONH changes using either CSLO or stereoscopic analysis after a 1-year follow-up of PRP for PDR. However, other longitudinal prospective studies comparing pre- and post-PRP ONH aspects have already been performed, with conflicting results. Using stereoscopic disk photographs, Johns et al.(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.) observed that the C/D ratio showed no significant change 1 year after PRP, but the authors reported increased optic disk pallor in the study period. On the other hand, Singh et al.(1111 Singh H, Garg S, Sharma R, Venkatesh P, Saxena R, Dada T. Evaluation of the effect of pan retinal photocoagulation on optic nerve head parameters using HRT3. J Glaucoma. 2014;23(7):467-70.) found significant changes in CSLO parameters 6 months after PRP treatment. The results of our investigation are in conflict with this last study, since we have used the same technology to analyze the ONH morphology changes. One hypothesis to explain these inconsistent findings is that different laser delivery systems may alter the amount of ascending optic atrophy observed. While we used a spot size of 250 mm in our study, Singh et al. used a spot size of 300 mm. A larger spot size may be related to greater inner retinal damage and therefore more ascending optic atrophy. This possible explanation is confirmed by the findings of Lee et al.(2222 Lee DE, Lee JH, Lim HW, Kang MH, Cho HY, Seong M. The effect of pattern scan laser photocoagulation on peripapillary retinal nerve fiber layer thickness and optic nerve morphology in diabetic retinopathy. Korean J Ophthalmol. 2014;28(5):408-16.), which suggest that PASCAL photocoagulation, a new form of multispot laser treatment for DR, may not cause changes in optic disk morphology due to its shorter pulse duration (and consequently more restricted retinal damage) than conventional single spot PRP. Additionally, Kim and Cho(2323 Kim HY, Cho HK. Peripapillary retinal nerve fiber layer thickness change after panretinal photocoagulation in patients with diabetic retinopathy. Korean J Ophthalmol. 2009;23(1):23-6.) measured RNFL thickness before and 6 months after PRP and concluded that laser photocoagulation of a moderate degree does not damage the RNFL.
A limitation of our investigation is its relatively small sample size, although the number of patients recruited was similar to other prospective interventional studies(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.
10 Lim MC, Tanimoto SA, Furlani BA, Lum B, Pinto LM, Eliason D, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol. 2009;127(7):857-62.-1111 Singh H, Garg S, Sharma R, Venkatesh P, Saxena R, Dada T. Evaluation of the effect of pan retinal photocoagulation on optic nerve head parameters using HRT3. J Glaucoma. 2014;23(7):467-70.). Moreover, we adopted rigid inclusion criteria that excluded patients with optic disk neovascularization and/or baseline DME. The presence of disk neovascularization does not allow an adequate analysis of ONH parameters by CSLO, because fibrovascular tissue can obscure the contour of the optic cup(99 Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.). Moreover, DME is frequently present when PDR is detected in patients with diabetes, and often requires additional treatment with intravitreal anti-VEGF injections, which could somehow interfere with the parameters analyzed in our study. In order to allow proper HRT documentation of the optic disc and to avoid the bias of anti-VEGF injections, only patients with PDR without disc neovascularization and without macular edema were eligible for this particular study. We know from the DRS that only 40% of PDR cases do not present with disc neovascularization(22 Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583-600.).
The recently published protocol S(2424 Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL 3rd, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-48.) from the DCRC network, a multicentric and randomized clinical trial that compared PRP or anti-VEGF for PDR cases, reported that around 22% of the participants with PDR had DME at baseline. Therefore, most PDR cases presented at our service were not eligible for this study. The results of protocol S(2424 Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL 3rd, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-48.) showed that both therapies produced similar results in preventing complications of DR and visual acuity after 5 years of follow-up, but the group that received anti-VEGF intravitreous injections had lower rates of developing vision-impairing DME, and less visual field loss when compared to patients receiving PRP. However, the costs and frequency of visits are significantly higher and low adherence to treatment is an issue because it requires more constant follow-up.
In clinical practice, it is common to see many patients who were treated with PRP in the past with severe optic disc atrophy, detected several years after treatment. However, such damage can often be attributed to the higher intensity of the lasers used in the past. In contrast, data from our study show that after performing photocoagulation with moderate intensity power, PRP did not cause significant changes in the ONH morphology of patients with PDR after 1 year of follow-up.
-
Funding: This study was supported by a grant from the Brazilian CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
-
Approved by the following research ethics committee: Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (# 741.731).
REFERENCES
-
1Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503.
-
2Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583-600.
-
3Diabetic Retinopathy Clinical Research Network, Brucker AJ, Qin H, Antoszyk AN, Beck RW, Bressler NM, Browning DJ, Elman MJ, Glassman AR, Gross JG, Kollman C, Wells JA 3rd. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127(2):132-40. 4. Comment in: Arch Ophthalmol. 2010;128(2):262; author reply 262.
-
4Zhang YX, Huang HB, Wei SH. Clinical characteristics of nonglaucomatous optic disc cupping. Exp Ther Med. 2014;7(4):995-9.
-
5Abu El-Asrar AM, Nawaz MI, Mohammad G, Siddiquei MM, Alam K, Mousa A, et al. Expression of bioactive lysophospholipids and processing enzymes in the vitreous from patients with proliferative diabetic retinopathy. Lipids Health Dis. 2014;13:187.
-
6Peng PH, Lin HS, Lin S. Nerve fibre layer thinning in patients with preclinical retinopathy. Can J Ophthalmol. 2009;44(4):417-22.
-
7Sugimoto M, Sasoh M, Ido M, Wakitani Y, Takahashi C, Uji Y. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219(6):379-85.
-
8Takahashi H, Goto T, Shoji T, Tanito M, Park M, Chihara E. Diabetes-associated retinal nerve fiber damage evaluated with scanning laser polarimetry. Am J Ophthalmol. 2006;142(1):88-94.
-
9Johns KJ, Leonard-Martin T, Feman SS. The effect of panretinal photocoagulation on optic nerve cupping. Ophthalmology. 1989; 96(2):211-6.
-
10Lim MC, Tanimoto SA, Furlani BA, Lum B, Pinto LM, Eliason D, et al. Effect of diabetic retinopathy and panretinal photocoagulation on retinal nerve fiber layer and optic nerve appearance. Arch Ophthalmol. 2009;127(7):857-62.
-
11Singh H, Garg S, Sharma R, Venkatesh P, Saxena R, Dada T. Evaluation of the effect of pan retinal photocoagulation on optic nerve head parameters using HRT3. J Glaucoma. 2014;23(7):467-70.
-
12American Academy of Ophthalmology and Preferred Practice Pattern. Diabetic Retinopathy [Internet]. San Francisco, CA: American Academy of Ophthalmology; 2017. [cited 2017 jun 21]. Available from: https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2017
-
13Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP; The Los Angeles Latino Eye Study Group. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study Group. Ophthalmology. 2008;115(2):227-32.
-
14Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86(10):1803-30.
-
15Ozdek S, Lonneville YH, Onol M, Yetjub Um Gasabreusigky BB. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye (Lond). 2002;16(6):761-5.
-
16Lopes de Faria JM, Russ H, Costa VP. Retinal nerve fiber layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol. 2002;86(7):725-8. Comment in: Br J Ophthalmol. 2002;86(7):709.
-
17Abu-El-Asrar AM, Dralands L, Missotten L, Al-Jadaan IA, Geboes K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004;45(8):2760-6.
-
18Amano S, Kaji Y, Oshika T, Oka T, Machinami R, Nagai R, Horiuchi S. Advanced glycation end products in human optic nerve head. Br J Ophthalmol. 2001;85(1):52-5.
-
19Königsreuther KA, Jonas JB. Optic disc morphology in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 1995;233(4):200-4.
-
20Tekeli O, Turaçli ME, Atmaca LS, Elhan AH. Evaluation of the optic nerve head with the Heidelberg Retinal Tomograph in diabetes mellitus. Ophthalmologica. 2008;222(3):168-72.
-
21Cankaya AB, Ozdamar Y, Ozalp S, Ozkan SS. Impact of panretinal photocoagulation on optic nerve head parameters. Ophthalmologica. 2011;225(4):193-9.
-
22Lee DE, Lee JH, Lim HW, Kang MH, Cho HY, Seong M. The effect of pattern scan laser photocoagulation on peripapillary retinal nerve fiber layer thickness and optic nerve morphology in diabetic retinopathy. Korean J Ophthalmol. 2014;28(5):408-16.
-
23Kim HY, Cho HK. Peripapillary retinal nerve fiber layer thickness change after panretinal photocoagulation in patients with diabetic retinopathy. Korean J Ophthalmol. 2009;23(1):23-6.
-
24Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, Bressler NM, Elman MJ, Ferris FL 3rd, Gardner TW, Jampol LM, Martin DF, Melia M, Stockdale CR, Beck RW; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-48.
Publication Dates
-
Publication in this collection
29 Apr 2019 -
Date of issue
Jul-Aug 2019
History
-
Received
30 July 2018 -
Accepted
24 Nov 2018