Abstracts
BACKGROUND: N2-mercaptopropionylglycine is a powerful super oxide synthesis inhibitor and has been tested as a preventive agent of metabolic and structural hepatic damage in the ischemia/reperfusion process. AIM: To analyze some effects of N2-mercaptopropionylglycine administration to animals of two species submitted to normothermic liver ischemia/reperfusion. MATERIAL AND METHODS: Twenty-two rats and 22 dogs were divided into four groups: group I: rats that received intravenous saline 0.9%; group II: rats that received 100 mg/kg of N2-mercaptopropionylglycine; group III: dogs that received saline intravenous 0.9% and group IV: dogs that received 100 mg/kg N2-mercaptopropionylglycine. RESULTS: Ten minutes after the saline or drug administration, each group was submitted to left lobe liver ischemia for 25 minutes followed by reperfusion. Biochemical studies 24 hours after reperfusion revealed a significantly lower elevation of transaminases in animals of groups II (AST = 271 ± 182; ALT = 261 ± 161 ) and IV (AST = 101 ± 45; ALT = 123 ± 89) when compared to the controls group: I (AST = 2144 ± 966; ALT = 1869 ± 1040 00) and III (AST = 182 ± 76.51; ALT = 277 ± 219), respectively. Histology study demonstrated a significantly minor aggression to animals of groups II and IV when compared to groups I and III, respectively. CONCLUSION: These results suggest a significant release of free radicals of oxygen in the process and that N2-mercaptopropionylglycine may have a significant protective effect on liver parenchyma when submitted to ischemia/reperfusion.
Mercaptopropionylglicyne; Ischemia; Reperfusion; Liver diseases; Rats; Dogs
RACIONAL: O medicamento N2-mercaptopropionilglicina é um potente inibidor da síntese de radicais superóxidos e foi testado como agente preventivo de lesão metabólica e estrutural do parênquima hepático, no processo de isquemia/reperfusão. OBJETIVOS: Analisar alguns efeitos da administração do N2-mercaptopropionilglicina a animais de duas espécies submetidas a isquemia/reperfusão normotécnica do fígado. MATERIAL E MÉTODOS: Vinte e dois ratos e 22 cães foram divididos em quatro grupos: grupo I: ratos que receberam solução salina a 0,95%; grupo II: ratos que receberam 100 mg/kg de N2-mercaptopropionilglicina; grupo III: cães que receberam salina a 0,9%; grupo IV: cães que receberam 100 mg/kg de N2-mercaptopropionilglicina. Cada um dos grupos de animais foi, após 10 minutos da infusão tanto de salina, como de N2-mercaptopropionilglicina, submetidos a isquemia dos respectivos lobos esquerdos por um período de 25 minutos, seguida de reperfusão. RESULTADOS: Estudos bioquímicos, 24 horas após a reperfusão revelaram menor e significativa elevação das transaminases nos animais do grupo I (AST = 271 ± 182; ALT = 261 ± 161) e grupo IV (AST = 101 ± 45; ALT = 123 ± 89), quando comparados com os controles: grupo I (AST = 2144 ± 966; ALT = 1869 ± 1040) e grupo III (AST = 182 ± 76; ALT = 277 ± 219), respectivamente e todos em UI/dL. O estudo histológico demonstrou agressão significativamente menor nos animais dos grupos experimentais II e IV, quando comparados aos grupo I e grupo III, respectivamente. CONCLUSÃO: Estes resultados sugerem liberação de radicais livres de oxigênio real e significativa no processo e que o N2-mercaptopropionilglicina pode ter efeito protetor apreciável no parênquima hepático, quando submetido a isquemia e posterior reperfusão.
Mercaptopropionilglicina; Isquemia; Reperfusão; Hepatopatias; Ratos; Cães
GASTROENTEROLOGIA EXPERIMENTAL EXPERIMENTAL GASTROENTEROLOGY
Protective effect of N2-mercaptopropionylglycine on rats and dogs liver during ischemia/reperfusion process
Efeito protetor do N2-mercaptopropionilglicina em ratos e cães submetidos a isquemia/reperfusão normotécnica do fígado
Emilio Elias Abdo; José Eduardo Monteiro Cunha; Pérsio Deluca; Ana Maria Mendonça Coelho; Telesforo Bacchella; Marcel Cerqueira César Machado
Department of Gastroenterology, Surgical Division, São Paulo University Medical School, São Paulo, SP, Brazil
Correspondence Address for correspondence Dr. Emilio Elias Abdo Rua Haddock Lobo, 281 ap. 101 01414-001 São Paulo, SP, Brazil
ABSTRACT
BACKGROUND: N2-mercaptopropionylglycine is a powerful super oxide synthesis inhibitor and has been tested as a preventive agent of metabolic and structural hepatic damage in the ischemia/reperfusion process.
AIM: To analyze some effects of N2-mercaptopropionylglycine administration to animals of two species submitted to normothermic liver ischemia/reperfusion.
MATERIAL AND METHODS: Twenty-two rats and 22 dogs were divided into four groups: group I: rats that received intravenous saline 0.9%; group II: rats that received 100 mg/kg of N2-mercaptopropionylglycine; group III: dogs that received saline intravenous 0.9% and group IV: dogs that received 100 mg/kg N2-mercaptopropionylglycine.
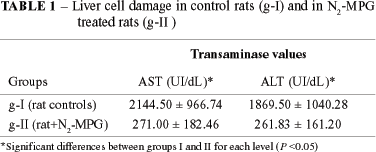
RESULTS: Ten minutes after the saline or drug administration, each group was submitted to left lobe liver ischemia for 25 minutes followed by reperfusion. Biochemical studies 24 hours after reperfusion revealed a significantly lower elevation of transaminases in animals of groups II (AST = 271 ± 182; ALT = 261 ± 161 ) and IV (AST = 101 ± 45; ALT = 123 ± 89) when compared to the controls group: I (AST = 2144 ± 966; ALT = 1869 ± 1040 00) and III (AST = 182 ± 76.51; ALT = 277 ± 219), respectively. Histology study demonstrated a significantly minor aggression to animals of groups II and IV when compared to groups I and III, respectively.
CONCLUSION: These results suggest a significant release of free radicals of oxygen in the process and that N2-mercaptopropionylglycine may have a significant protective effect on liver parenchyma when submitted to ischemia/reperfusion.
Headings: Mercaptopropionylglicyne. Ischemia. Reperfusion. Liver diseases. Rats. Dogs.
RESUMO
RACIONAL: O medicamento N2-mercaptopropionilglicina é um potente inibidor da síntese de radicais superóxidos e foi testado como agente preventivo de lesão metabólica e estrutural do parênquima hepático, no processo de isquemia/reperfusão.
OBJETIVOS: Analisar alguns efeitos da administração do N2-mercaptopropionilglicina a animais de duas espécies submetidas a isquemia/reperfusão normotécnica do fígado.
MATERIAL E MÉTODOS: Vinte e dois ratos e 22 cães foram divididos em quatro grupos: grupo I: ratos que receberam solução salina a 0,95%; grupo II: ratos que receberam 100 mg/kg de N2-mercaptopropionilglicina; grupo III: cães que receberam salina a 0,9%; grupo IV: cães que receberam 100 mg/kg de N2-mercaptopropionilglicina. Cada um dos grupos de animais foi, após 10 minutos da infusão tanto de salina, como de N2-mercaptopropionilglicina, submetidos a isquemia dos respectivos lobos esquerdos por um período de 25 minutos, seguida de reperfusão.
RESULTADOS: Estudos bioquímicos, 24 horas após a reperfusão revelaram menor e significativa elevação das transaminases nos animais do grupo I (AST = 271 ± 182; ALT = 261 ± 161) e grupo IV (AST = 101 ± 45; ALT = 123 ± 89), quando comparados com os controles: grupo I (AST = 2144 ± 966; ALT = 1869 ± 1040) e grupo III (AST = 182 ± 76; ALT = 277 ± 219), respectivamente e todos em UI/dL. O estudo histológico demonstrou agressão significativamente menor nos animais dos grupos experimentais II e IV, quando comparados aos grupo I e grupo III, respectivamente.
CONCLUSÃO: Estes resultados sugerem liberação de radicais livres de oxigênio real e significativa no processo e que o N2-mercaptopropionilglicina pode ter efeito protetor apreciável no parênquima hepático, quando submetido a isquemia e posterior reperfusão.
Descritores: Mercaptopropionilglicina. Isquemia. Reperfusão. Hepatopatias. Ratos. Cães.
INTRODUCTION
One of the major problems in emergency surgery and organ transplantation is organ recovery after acute tissue damage related to ischemia and reperfusion(2, 3). The morbidity and mortality secondary to these events have motivated the search for better organ preservation procedures(2, 3, 11).
Interruption of blood circulation determines biochemical and structural cell disintegration, that evolve to organelle disorganization with subsequent cellular death(18, 24). The oxidative-reduction activity, mainly mediated by newly formed highly reactive chemical radicals, is one of the processes responsible for that condition(5, 9, 25).
The main products formed by tissue hypo-oxygenation are produced by the degradation of adenosine-tri-phosphate (ATP) to hypoxanthine (Figure 1)(8).
Suppression of ion transportation through the cellular membranes leads to calcium increase in citosol, which activates proteases, such as phosphokinase, promoting excessive and prejudicial conversion of xanthine-dehydrogenase to xanthine-oxidase(15, 18, 19). After ischemic tissue reperfusion, high concentrations of xanthine-oxidase will use the incoming oxygen to produce hydro peroxide and subsequently liberate great amounts of aggressive free radicals(18). The dissociation of oxygenated water by metallic ions such as iron and copper, stimulates the production of free radical hydroxyls and is highly harmful to the reperfused tissue causing rupture of cellular and sub-cellular structures(2, 5).
The role of oxygen free radicals in liver ischemia/reperfusion damage was demonstrated by the occurrence of liver lipoperoxidation when oxygen returns to the hepatic vascular system(13, 17). The great amount of xanthine-oxidase in the hepatic parenchyma submitted to ischemia further elucidates this phenomena(12).
Hepatic defense systems, such as antioxidant enzymes (superoxidodismutase, glutation-peroxidase, catalase, alfa-tocopherol, and coenzyme-Q10) are insufficient to sustain viability after a considerable period of severe liver ischemia(13, 20). Several attempts to reduce these mechanisms were reported in the literature(8, 10).
The anti oxidative effect of N2-mercaptopropionylglycine(N2 -MPG), a thiol compound that partially prevents the conversion of xanthine-dehydrogenase into xanthine-oxidase, was previously demonstrated by the preservation of miocutaneous graft after 12 hours of normothermic ischemia(7). The tissue protective effect of N2-MPG was also demonstrated, in the acute pancreatitis model, where low blood flow and cellular damage resembles the ischemia/reperfusion process(3).
The purpose of the present study was to analyze the effects of N2-MPG administration to animals of two different species submitted to normothermic liver ischemia/reperfusion.
MATERIAL AND METHODS
Twenty-two male Wistar rats (250 g to 350 g) and 22 mongrel dogs (12 kg to 14 kg ) were fasted 12 hours before surgery except for water.
Rats were anesthetized with 0.2 mL/100 g of ketamine hydrochloride (Park-Davis Lab, USA) by intraperitoneal route and dogs with intravenous sodium thiopental was used in a dose of 30 mg/kg.
Segmental liver ischemia was achieved in rats and dogs by appropriate clamp occlusion of both afferent and efferent blood vessels of the left segment for 25 minutes. In dogs an extensive dissection of the portal and hepatic artery left branch was needed and, after section of the hepato-phrenic ligaments the identification of the left hepatic vein permitted its appropriate clamping. A "bulldog" type clamp was used for rats and "Potts" clamps for dogs to fit the vessels size.
Animals were divided into four groups: g-I (rat control): 12 rats submitted to normothermic ischemia of the lateral segment of the left hepatic lobe 10 minutes after 0.5 mL of normal saline intravenous injection; g-II (rat experimental): 12 rats submitted to normothermic ischemia of the same liver segment 10 minutes after intravenous injection of 100 mg/kg of N2-MPG (Sigma-Chemical Company, St Louis, USA) in 0.5 mL of normal saline; g-III (dog control): 10 dogs submitted to normothermic ischemia of the lateral segment of the left hepatic lobe 10 minutes after 100 mL normal saline intravenous infusion over a 25 minute period; g-IV (dog experimental): 12 dogs submitted to normothermic ischemia of the same liver segment 10 minutes after intravenous infusion over a 25 minute period of a solution of 100 mg/kg of N2-MPG in 100 mL of normal saline.
Histological examinations
Twenty-four hours after the ischemia/reperfusion procedure the animals were re-operated and a portion of the central part of both the reperfused and normal lobes of each animal was fixed overnight in 10% formaldehyde and embedded in paraffin. Tissue slices were subjected to hematoxylin-eosin staining and to histological study by light microscopy. Slices were coded and examined in a blinded fashion by a single pathologist for grading of the histological alterations. Tissue damage was graded as follows: mild damage: low to moderate grade of necrosis, vacuolization or centrolobular lesion; severe damage: extensive to very extensive damage, with destruction of hepatocytes in extensive areas or throughout the liver.
Biochemical assay
Aspartate aminotransferase (AST) and alanine-aminotransferase (ALT) levels were determined in serum samples collected from each animal preoperatively and at 24 hours after the end of the ischemic phase.
Statistical analysis
Results expressed as means + standard error (SE) were statistically analyzed by means of Mann-Whitney test for AST and ALT and Fisher's exact test for contingents of histological data. A P value < 0.05 was considered statistically significant.
RESULTS
Observed increases of AST and ALT serum levels were significantly lower after ischemia/reperfusion in g-II when compared to g-I in rats (Table 1). Similar differences, though not as marked as in rats but still significant, were observed in dogs (g-IV vs g-III) (Table 2).
N2-MPG pre-treatment reduced the severity of histological liver damage induced by the ischemia/reperfusion process in both rat and dog experimental groups. Low grade histological lesions (mild damage) were predominant in g-II when compared to g-I in rats and g-IV whereas extensive and very extensive necrosis (severe damage) predominated in g-I and g-III (Tables 3, 4).
DISCUSSION
Oxygen free radicals are usually produced by the aerobic metabolism of living cells. An overproduction of these radicals has been reported in ischemic/reperfused organs, leading to a high degree of tissue damage(6, 12, 21, 22). Prevention of these deleterious effects, by the administration of antioxidative substances has been attempted in organ transplantation and surgical emergencies(17, 23).
After a significant period of ischemia, blood recirculation produces large amounts of super oxide residues derived from the abnormal activity of the newly formed xanthine-oxidase and responsible for tissue lesion(6).
The experimental model of this study was chosen to investigate the possible different behavior between different animal species submitted to organ ischemia/reperfusion and ant oxidative drug therapy. Partial liver ischemia was used to avoid visceral damage determined by total portal vein obstruction.
The lower increase of AST and ALT levels observed in animals of g-II and g-IV when compared to their respective controls, g-I and g-III, suggests a possible protective effect of the N2-MPG administration in the organ ischemia/reperfusion condition. The increase of AST and ALT observed in control groups can be explained by the cytolysis, probably secondary to the lipid per-oxidation caused by the oxygen free radical formed during the reperfusion phase.
It might be speculated that the smaller but still significant differences observed in AST and ALT serum levels (Table 2) between g-III and g-IV (dog groups) and g-II and g-I (rat groups) may be due to the greater resistance of large animals to transient hepatic ischemia when compared to small animals.
The histological results also support the theory of an inhibitory activity of N2-MPG in the formation of xanthine-oxidase and the hydroxyl radical responsible for tissue destruction(2, 11).
Beneficial effects of N2-MPG have been extensively reported in several organs such as heart(14, 16, 26), pancreas(1) and lung(4). The demonstration, in the present study, that the antioxidant agent N2-MPG attenuates the process of liver cell damage, strongly suggests the participation of oxygen free radical as a factor of experimental hepatocelular ischemic aggression
CONCLUSIONS
The findings of the present study, that demonstrate an inhibitory effect of N2-Mercaptopropyonilglycine upon cytolysis and hepatic necrosis in different animal species submitted to liver ischemia/reperfusion, may represent an important accomplishment for further understanding the metabolic aspects of organ preservation, justifying further studies of this promising chemical group.
Recebido em 22/10/2002.
Aprovado em 27/1/2003.
- 1. Abdo EE, Sampietri SN, Coelho AM, Molan N, Kubrusly M. N2-Mercaptopropionylglycine(N2-MPG) in experimental acute pancreatitis. Rev Hosp Clin Fac Med S Paulo 1998;53:169-73.
- 2. Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 1988;45:670-3.
- 3. Blankensteijn JD, Terpstra OT. Liver preservation: the past and the future. Hepatology 1991;13:1235-50.
- 4. Cai M, Ogawa R. Effect of free radical scavengers, methylprednisolone and ulinastin on acute xanthine and xanthine-oxidase induced lung injury in rats. Circ Shock 1994;43:71-8.
- 5. Del Maestro RF. An approach to free radicals in medicine and biology. Acta Physiol Scand 1980;Suppl 492:153-68.
- 6. Epstein FH. Oxygen-derived free radicals in post ischemic tissue injury. N Engl J Med 1985,312:159-63.
- 7. Fontana C. Efeito do N2-Mercaptopropionylglyccine na sobrevivência de retalhos cutâneos submetidos à oclusão temporária do pedículo vascular. Estudo experimental em ratos [tese]. São Paulo: Faculdade de Medicina da Universidade de São Paulo; 1992.
- 8. Galley HF, Richardson N, Howdle PD, Walter BE, Webster NR. Total antioxidant capacity and lipid peroxidation in liver transplantation. Clin Sci 1995;89:329-32.
- 9. Gao W, Takey Y, Marzi I, Lindert KA, Caldwell-Kenkel JL, Currin RT, Tanaka Y, Lemasters JJ, Thurman RG. Carolina rinse solution - a new strategy to increase survival time after orthotopic liver transplantation in the rat. Transplantation 1991;52:417-24.
- 10. Goode HF, Webster NR, Howdle PD, Leek PJ, Sadek AS, Walker BE. Reperfusion injury, antioxidants and hemodynamics during orthotopic liver transplantation. Hepatology 1994;19:354-9.
- 11. Goodrich EO Jr, Welch HF, Nelson JA, Beecher TS, Welch CS. Homotransplantation of the canine liver. Surgery 1956;39:244-51.
- 12. Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol 1989;257:G683-8.
- 13. Halliwell B. Oxygen radicals and metal ions: potential antioxidant intervention strategies. Ann Intern Med 1987;107:525-45.
- 14. Horwitz LD, Fenessey PV, Shikes RH, Kong Y. Marked reduction in myocardial infarct size due to prolonged infusion of an antioxidant during reperfusion. Circulation 1994;89:1792-801.
- 15. Ichicawa R. Effects of thiopronin on senile cataracts. Ophtalmologica 1980;180:293-8.
- 16. Ihnken K, Morita K, Buckberg GD, Sherman MP, Young HH. Studies of hypoxemic/reoxigenation injury without aortic clamping. Counteraction of oxidant damage by exogenous antioxidants: N2-mercaptopropionylglycine and catalase. J Thorac Cardiovasc Surg 1995;110:1212-20.
- 17. Kobelt F, Schreck U, Henrich HA. Involvement of liver in the decompensation of hemorrhagic shock. Shock 1994;2:281-8.
- 18. Lefer AM, Lefer DJ. Pharmacology of the endothelium ischemia-reperfusion and circulatory shock. Ann Rev Pharmacol Toxicol 1993;33:71-90.
- 19. McCord JM, Fridovich I. The reduction of cytochrome by milk xanthine-oxidase. J Biol Chem 1968;243:5753-60.
- 20. McCord JM. Oxygen-derived free radicals in post ischemic tissue injury. N Engl J Med 1985;312:159-63.
- 21. Mito M, Tamaki A, Kon T, Ohira S. Experimental studies on differential hypothermia of the liver. J Surg Res 1965;5:207.
- 22. Morimoto T, Kusumoto K, Issrlhard W. Impairment of grafts by short-term warm ischemia in rat liver transplantation. Transplantation 1991;52:424-31.
- 23. Vento AE, Rämö OJ, Nemlander AT, Ahotupa M, Nissinen E, Holopainen A, Mattila SP. Nitecapone inhibits myeloperoxidase in vitro and enhances functional performance after 8 h of ischemia in experimental heart transplantation. Res Exp Med (Berl)1999;198:299-306.
- 24. Souza AP, Coelho ARB, Camara-Neto RD, Ferraz EM, Rodrigues JV, Lima-Filho JFC, Telles AMS. Complete acute occlusion of the portal vein in dogs. A study based on hemodynamics and hematologic data aimed at its application in liver transplantation. ABCD Arq Bras Cir Dig 1991;6:15-9.
- 25. Starzl TE, Iwatsuki S, Vanthiel DL. Liver and pancreas transplantation. Transpl Proc 1983;15:2582-5.
- 26. Widman SC, Liang CS, Schenk EA, Hood WB Jr. Contraction band necrosis: its modification by the free radical scavenger N2-MPG. J Cardiovasc Pharmacol 1994;24:694-701.
Publication Dates
-
Publication in this collection
11 Mar 2004 -
Date of issue
Sept 2003
History
-
Accepted
27 Jan 2003 -
Received
22 Oct 2002