INTRODUCTION
Gluten-related diseases were recently classified into three groups according to their physiopathological mechanisms: autoimmunity (celiac disease, dermatitis herpetiformis, gluten ataxia), allergy (wheat allergy- respiratory, alimentary, contact urticary and WDEIA) and no-autoimmune no-allergic (gluten sensitivity)(3030. The First Consensus Conference on Gluten Sensitivity, London; 2011.).
Dermatitis herpetiformis (DH) is an autoimmune blistering cutaneous disease that appears as a consequence of gluten intolerance. There is evidence that DH should be considered as the specific phenotypic cutaneous expression of a gluten-sensitive enteropathy indistinguishable from celiac disease (CD)(3636. Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91.). Immunological studies demonstrated the presence of granular deposits of IgA along the dermal-epidermal junction(3232. Van der Meer JB. Granular deposits of immunoglobulins in the skin of patients with dermatitis herpetiformis. An immunofluorescent study. Br J Dermatol. 1969;81:493-503).
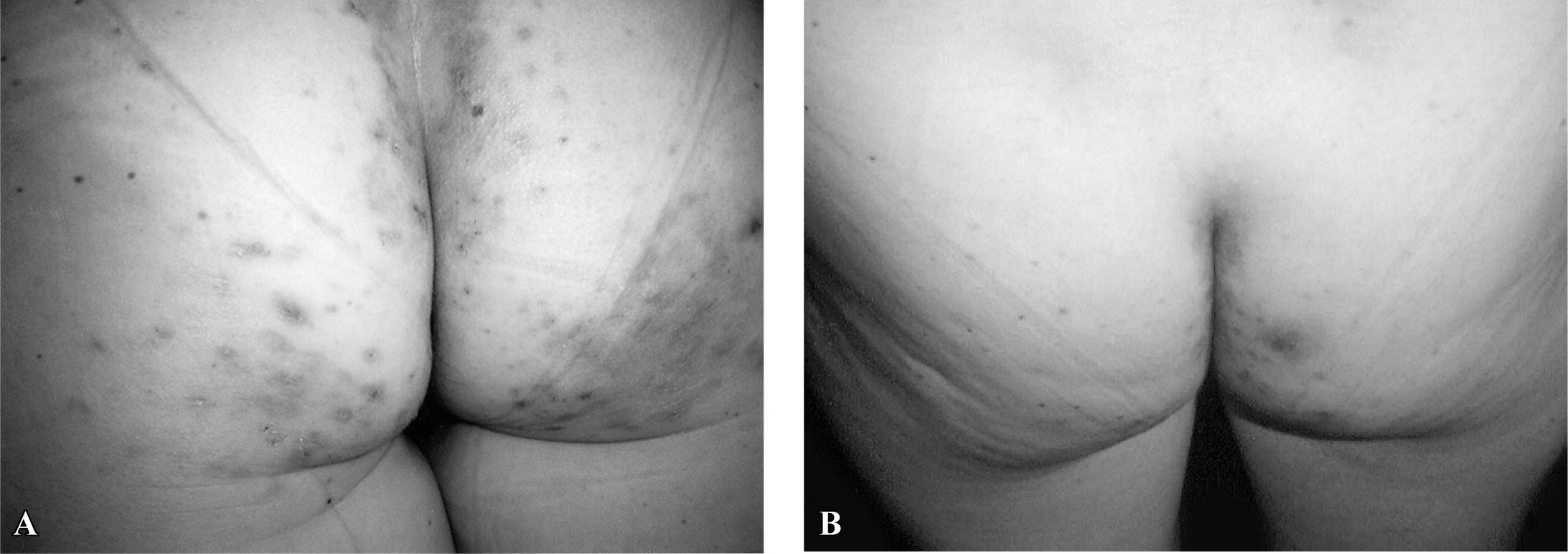
This disorder generally has a typical presentation (pruritic papulovesicular rash predominantly on extensor surfaces and on the buttocks) (Figure 1-A), but atypical presentations may occur (purpuric lesions in the palm of hands in children, and lesions in the oral and genital mucosa in adults(2323. Oxentenko AS, Murray JA. Celiac disease and dermatitis herpetiformis: the spectrum of gluten-sensitivity enteropathy. Int J Dermatol. 2003; 42:585-7.); or lesions in scalp, nuchal area, face and groin). Some celiac patients referred to changes in the skin (like thickness) related to the consumption of gluten, but without apparent lesions. It is important for the gastroenterologist to know about these facts since, in many cases, only a gluten-free diet (GFD) is not sufficient to reverse the lesions, and medication (such as dapsone) needs to be prescribed (Figure 1-B). It is crucial to diagnosis DH as early as possible because these patients are faced with reduction in the quality of life, mainly due to the need for lifelong gluten-free diet, change in eating habits and life style(1818. Kotze LMS, Dalla Vecchia LA, Nisihara NM. Alta prevalência de dermatite herpetiforme em homens com doença celíaca. GED. 2012;31(Suppl 1):28-479.).
Dermatitis herpetiformis. A - Pruritic papulovesicular rash on the buttocks. B - Great improvement of the lesions after 30 days on gluten-free diet and orally dapsone.
HISTORY
DH was described and named in 1884 by Dr. Louis Duhring, a dermatologist at the University of Pennsylvania(88. Duhring L. Dermatitis herpetiformis. JAMA. 1884;3:225-8.). The association of DH and enteropathy was described in 1966 by Dr. Janet Marks et al.(2222. Marks J, Shuster S, Watson AJ. Small-bowel changes in dermatitis herpetiformis. Lancet. 1966;2:1280-2.). In 1969 Van der Meer(3232. Van der Meer JB. Granular deposits of immunoglobulins in the skin of patients with dermatitis herpetiformis. An immunofluorescent study. Br J Dermatol. 1969;81:493-503) in Holland described the presence of granular immunoglobulin (Ig) A in the dermal papillary tips of skin from patients with DH, currently recognized as the hallmark of the disease. Dr. Lionel Fry et al. (England), Dr. Timo Reunala et al. (Finland), Drs. Mobacken and Kastrups et al. (Sweden), Dr. Steven Katz and Dr. Russel Hall (USA) published important papers about the affection (apud ZONE 2005)(3636. Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91.). Dr. Kumar, in 2001, at the University of Buffalo is credited with the discovery of the endomysial antibody in patients with DH(2020. Kumar V, Jarzabek-Chorzelska M, Sulej J, Rajadhyaksha M, Jablonska S. Tissue transglutaminase and endomysial antibodies-diagnostic markers of gluten sensitive enteropathy in dermatitis herpetiformis. Clin Immunol. 2001;98:378-82.). Dr. Zone has evaluated more than 800 patients since 1970(3636. Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91. 3737. Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-8.).
EPIDEMIOLOGY
The prevalence of DH has been reported to be 1.2 per 100,000 population in Great Britain (1971), 39.2 per 100,000 population in central Sweden (1984) and 11.2 per 100.000 population in Utah – USA (1992)(22. Bolontin D, Petronic-Rosic V. Dermatitis herpetiformis: part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-24.). The incidence of DH in the last report was 0.98 per 100.000 population(3636. Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91.).
DH could appear in any age with the onset of symptoms in the third or fourth decade, being an exception in children under 3 years of age. Unlike CD, DH is more frequent in males ∼1.4:1. About 5% of the patients with CD will present DH in their lifetime(1919. Kotze LMS, Nisihara RM, Kotze LR, Utiyama SRR. Celiac disease and dermatitis herpetiformis in Brazilian twins: a long-term follow-up and screening of their relatives. J Pediatr Endocrinol Metab. 2012;26:71-5. 2323. Oxentenko AS, Murray JA. Celiac disease and dermatitis herpetiformis: the spectrum of gluten-sensitivity enteropathy. Int J Dermatol. 2003; 42:585-7.). The patients may present DH before or after the diagnosis of CD(1919. Kotze LMS, Nisihara RM, Kotze LR, Utiyama SRR. Celiac disease and dermatitis herpetiformis in Brazilian twins: a long-term follow-up and screening of their relatives. J Pediatr Endocrinol Metab. 2012;26:71-5.).
In Brazil, a unique report on this subject is credited to Kotze (the author of this revision), referring to DH in 11.5% of the 157 studied patients with CD(1616. Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9.). High prevalence of DH in males was also established by the same author(1818. Kotze LMS, Dalla Vecchia LA, Nisihara NM. Alta prevalência de dermatite herpetiforme em homens com doença celíaca. GED. 2012;31(Suppl 1):28-479.).
DIAGNOSIS
The diagnosis of DH requires a complex approach: clinical, histological and immunological, considering atypical variants of the disease frequently described in the literature(33. Bonciolini V, Bonciani D, Verdelli A, D'Errico A, Antiga E, Fabbri P, Caproni M. Newly described clinical and immunopathological feature of dermatitis herpetiformis. Clin Develop Immunol. 2012. doi: 10.1155/2012/967974.
https://doi.org/10.1155/2012/967974...
).
Digestive manifestations
Although all the patients with DH present gluten sensitivity, a great majority are asymptomatic from the digestive point of view(1313. Herrero-González JE. [Clinical guidelines for the diagnosis and treatment of dermatitis herpetiformis]. Actas Dermosifiliogr. 2010;101:820-6.). Alonso-Llamazares et al., reviewing the Mayo Clinic experience, referred to 13% of digestive complaints in 300 patients with DH (diarrhea, abdominal pain or failure to thrive in children)(11. Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. Int J Dermatol. 2007;46:910-9.). The intestinal biopsy performed in patients with DH could reveal signs of gluten sensitivity in 60% to 75%, ranging from normal - appearing epithelium to a flat mucosa (Marsh I to III)(11. Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. Int J Dermatol. 2007;46:910-9.).
Cutaneous manifestations
The most commonly involved sites in DH are those of symmetrical distribution on the extensor surfaces of the elbows (90%), knees (30%), shoulders, buttocks, sacral region, and face. The polymorphic lesions may be diffused or grouped: erythema, urticarial plaques, papules, herpetiform vesiculae and blisters (Figure 1-A). Erosions, excoriations and hyperpigmentation follow. Itching of variable intensity, scratching and burning sensation could predict the appearance of lesions(2323. Oxentenko AS, Murray JA. Celiac disease and dermatitis herpetiformis: the spectrum of gluten-sensitivity enteropathy. Int J Dermatol. 2003; 42:585-7.). Many patients referred to a burning sensation even before the onset of skin lesions(33. Bonciolini V, Bonciani D, Verdelli A, D'Errico A, Antiga E, Fabbri P, Caproni M. Newly described clinical and immunopathological feature of dermatitis herpetiformis. Clin Develop Immunol. 2012. doi: 10.1155/2012/967974.
https://doi.org/10.1155/2012/967974...
).
From the pathogenic point of view, CD4+ T cells with cytokine expression pattern belonging to the Th2 phenotype has been documented in recent DH lesions along with perilesional skin(99. Fabbri P, Calabro AS, Hashimoto T, Fasano A, Caproni M. Novel advances in dermatitis herpetiformis. Clin Develoment Immunol. 2012.).
Figure 2 shows the recommended tests confirming DH.
Tests for the diagnosis of dermatitis herpetiformis adapted from Herrero-Gonzales(1313. Herrero-González JE. [Clinical guidelines for the diagnosis and treatment of dermatitis herpetiformis]. Actas Dermosifiliogr. 2010;101:820-6.)
Histopathology
The classic features of DH under light microscopy are the subepidermal cleft with neutrophils (considered mainly responsible for the dermal-epidermal separation) and a few eosinophils at the tips of dermal papillae, often with a perivascular mixed inflammatory infiltrate. But in patients with pruritic and excoriated lesions, histological findings could not confirm DH, maybe because of an error in the site of the biopsy(3636. Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91. 3737. Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-8.).
Direct immunofluorescence of uninvolved skin collected in the perilesional site is considered the gold standard for the diagnosis of DH(44. Caproni M, Antiga E, Melani L, Fabbri P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009; 23:633-8. 55. Chang D. The need for direct immunofluorescence in the diagnosis of IgA bullous dermatosis. J Bras Patol Med Lab. 2012;48:55-7. 3434. Warren SJP, Cockerell CJ. Characterization of a subgroup of patients with dermatitis herpetiformis with non-classical histologic features. Am J Dermatopathol. 2002;24:305-8.). The choice of normal appearing perilesional skin for biopsy is fundamental as patients showed greater IgA deposition at this site than in non lesional or lesional skin, as demonstrated by Zone et al.(3737. Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-8.).
There are two different patterns: granular deposits in the dermal papillae or granular deposits along the basement membrane (a combination of both could occur). These deposits are polyclonal, mainly composed of IgA1(3333. Volta U, Molinaro N, De Franchis R, Forzenigo L, Landoni M, Fratangelo D, Bianchi FB. Correlation between IgA antiendomysial antibodies and subtotal villous atrophy in dermatitis herpetiformis. J Clin Gastroenterol. 1992;14:298-301.). Warren and Cockerell(3434. Warren SJP, Cockerell CJ. Characterization of a subgroup of patients with dermatitis herpetiformis with non-classical histologic features. Am J Dermatopathol. 2002;24:305-8.) reported the characterization of a subgroup of patients with DH with non-classical histological features.
Immunological determinations in the sera
DH patients have no circulating autoantibodies binding to the cutaneous basement membrane components or to other adherent structures of the skin, even considering that epidermal transglutaminase (TGase 3) is the autoantigen of DH(1515. Kàrpàti S. Dermatitis herpetiformis. Clin Dermatol. 2012;30:56-9. 2626. Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal thansglutaminase(TGase 3) is the autoantigen of dermatitis herpetiformis. J Experim Med. 2002;195:747-57.).
As DH presents variable degrees of enteropathy, the correlation with serological tests is difficult(3333. Volta U, Molinaro N, De Franchis R, Forzenigo L, Landoni M, Fratangelo D, Bianchi FB. Correlation between IgA antiendomysial antibodies and subtotal villous atrophy in dermatitis herpetiformis. J Clin Gastroenterol. 1992;14:298-301.). The tests indicated are IgA antiendomysial (detected by indirect immunofluorescence)(3333. Volta U, Molinaro N, De Franchis R, Forzenigo L, Landoni M, Fratangelo D, Bianchi FB. Correlation between IgA antiendomysial antibodies and subtotal villous atrophy in dermatitis herpetiformis. J Clin Gastroenterol. 1992;14:298-301.) and/or anti-tissue transglutaminase (detected by ELISA)(77. Dieterich W, Laag E, Bruckner-Tuderman L, Reunala T, Karpati S, Zagoni T, Riecken EO, Schuppan D. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Invest Dermatol. 1999;113:133-36.). Both correlate with small bowel damage and adherence to gluten-free diet. Sugai et al. suggested that deamidated synthetic gliadin-derived peptide (a-GDP) was more sensitive(2929. Sugai E, Smecuol E, Niveloni S, Vazquez H, Label M, Mazure R, Czech A, Kogan Z, Mauriño E, Bai JC. Celiac disease serology in dermatitis herpetiformis. Which is the best option for detecting gluten sensitivity? Acta Gastroenterol Latinoam. 2006;36:197-201.). Volta et al. reported the correlation between IgA antiendomysial antibodies and subtotal villous atrophy in DH(3333. Volta U, Molinaro N, De Franchis R, Forzenigo L, Landoni M, Fratangelo D, Bianchi FB. Correlation between IgA antiendomysial antibodies and subtotal villous atrophy in dermatitis herpetiformis. J Clin Gastroenterol. 1992;14:298-301.).
HLA typing
The prevalence of HLA DQ2 and DQ8 is the same as in CD, supporting the concept that DH is a skin manifestation of CD. About 90% of the patients with DH express HLA DQ2 (∼20% of controls); the others, DQ8. Patients without the two predisposing HLA types are extremely rare(66. Collin P, Reunala T. Recognition and manegment of cutaneous manifestations of celiac disese: a guide for dermatologists. Am J Clin Dermatol. 2003; 4:13-20.).
DH, like CD, is familial, since 10%-15% of the first-degree relatives of patients with DH present CD or DH. In the experience of Kotze et al., among fourteen cases of DH, one male patient referred to a sister with DH (7.15%)(1818. Kotze LMS, Dalla Vecchia LA, Nisihara NM. Alta prevalência de dermatite herpetiforme em homens com doença celíaca. GED. 2012;31(Suppl 1):28-479.).
Digestive approaches
Tests studying intestinal malabsorption are recommended in some patients for detection of deficiencies (iron, vitamin B12, folic acid, calcium etc)(1111. Fry L, Keir P, McMinn RM, Cowan JD, Hoffbrand AV. Small intestinal structure and function and haematological changes in dermatitis herpetiformis. Lancet. 1967;2:29-33.).
Small bowel biopsy
Small bowel biopsy is considered unnecessary for DH patients by some authors(44. Caproni M, Antiga E, Melani L, Fabbri P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009; 23:633-8.). It is not the opinion of the author of this study as DH can present lymphoma with time and it is important to compare the findings in intestinal biopsies at the diagnosis stage and when a neoplasia is suspected(1616. Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9.). The adherence to a GFD could be monitored by serological tests and skin lesions observations, if the skin lesions recur within few days of gluten ingestion(44. Caproni M, Antiga E, Melani L, Fabbri P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009; 23:633-8.).
Associated autoimmune diseases
DH, like CD, can be associated with other autoimmune diseases (Hashimoto thyroiditis, insulin-dependent diabetes mellitus, pernicious anemia, nephropathies, liver diseases, multiple sclerosis, Sjogren's syndrome, lupus erythematous, rheumatoid arthritis, vitiligo, psoriasis) or with Down syndrome). It is important to screen for these disorders mainly if there is a complaint or familial report(1616. Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9. 1818. Kotze LMS, Dalla Vecchia LA, Nisihara NM. Alta prevalência de dermatite herpetiforme em homens com doença celíaca. GED. 2012;31(Suppl 1):28-479.).
Complications
Like in CD, patients with DH are at risk of contracting lymphoma (10.3), 5.4 in men. Hervonen et al. reported 1.0% among 1104 cases in Finland(1414. Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Brit J Dermatol. 2005;152:82-6.). Although the lymphoma most associated with CD is EATL, in DH, diffused large B-cell lymphoma is observed(1616. Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9.). This disorder can occur both inside and outside the gastrointestinal tract as a nodal or extranodal disease.
Whether a GFD protects against the development of lymphoma in CD is controversial. In DH patients, the adherence to a GFD could be not strict and most of them needed dapsone for longer periods. These facts could increase the risk of neoplasias. The occurrence of lymphoma in the first-degree relatives did not seem to increase when compared with that of the general population(1414. Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Brit J Dermatol. 2005;152:82-6.).
Differential diagnosis
The differential diagnosis is concerned with scabies, atopic dermatitis, contact eczema, impetigo and other autoimmune bullous disorders like lineal IgA dermatosis and penfigo. The histopathological findings are fundamental as are the direct immunofluorescence findings(55. Chang D. The need for direct immunofluorescence in the diagnosis of IgA bullous dermatosis. J Bras Patol Med Lab. 2012;48:55-7.).
Treatment
The treatment of DH involves lifelong GFD for all the patients (gluten from wheat, barley, rye, and oats because of contamination)(44. Caproni M, Antiga E, Melani L, Fabbri P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009; 23:633-8.). The treatment is efficient for the disappearance of cutaneous and digestive manifestations, resulting in general well-being, reduction in or elimination of medication, resolution of enteropathy and the correlated malabsorption of essential nutrients and prevention of lymphomas(1414. Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Brit J Dermatol. 2005;152:82-6.). More studies are required to determine whether a long-term GFD will decrease the concurrent autoimmune conditions in patients with DH(1616. Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9.).
GFD alleviates digestive symptoms much more rapidly than the rash: it takes an average of 2 years of GFD for complete elimination of cutaneous lesions, which invariably recurs within 12 weeks after the reintroduction of gluten(1212. Handler MZ, Chacon AH, Shiman MI, Schachner LA. Application of dapsone 5% gel in a patient with dermatitis herpetiformis. J Dermatol. 2012;31:132-3.). IgA antibodies may disappear from the dermal-epidermal junction after many years of a strict GFD, although on reintroduction of gluten, IgA deposits reappear in the skin and are also present when the rash recurs(3131. Turchin I, Barankin B. Dermatitis herpetiformis and gluten-free diet. Dermatol Online J. 2005;11:6.). But only 10%-20% of the patients develop immunological tolerance and are capable of having a normal diet after years of GFD.
Despite the benefits of a GFD, it is not easy for the DH patients to maintain strict adherence to it: it is time-consuming and socially restricting. Consultation with a dietician and participation in support groups are strongly recommended(1616. Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9. 2525. Rottmann LH. Details of the gluten-free diet for the patients with dermatitis herpetiformis. Clin Dermatol. 1991;9:404-14.).
Although GFD is considered the only effective treatment for individuals with DH and CD, a better understanding of the complexity of the genetic/environmental interactions responsible for both diseases opened up possibilities to a novel therapy(1010. Fasano A. Novel therapeutic/integrative approaches for celiac disease and dermatitis herpetiformis. Clin Develop Immunol. 2012. doi: 10.1155/2012/959061
https://doi.org/10.1155/2012/959061...
).
Dapsone
Dapsone (diaminodiphenylsulfone) is a valid therapeutic option for DH during the initial 1 or 2 years until GFD is effective(1212. Handler MZ, Chacon AH, Shiman MI, Schachner LA. Application of dapsone 5% gel in a patient with dermatitis herpetiformis. J Dermatol. 2012;31:132-3.). The dosages of 1 mg/kg/day can control itching and blister development (Figure 1-B). It is prudent to start the treatment with low dosage like 50 mg per day, increasing it to 200 mg according to the necessity and tolerance of the patient. Before starting the use of this drug, determination of the levels of glucose-6-phosphate-desydrogenase and renal and hepatic profile are indicated(1313. Herrero-González JE. [Clinical guidelines for the diagnosis and treatment of dermatitis herpetiformis]. Actas Dermosifiliogr. 2010;101:820-6.). During treatment, regular controls include clinical evaluation and laboratory tests according to the response to treatment(2525. Rottmann LH. Details of the gluten-free diet for the patients with dermatitis herpetiformis. Clin Dermatol. 1991;9:404-14.). The contraindications of the use of dapsone are allergy to sulfonamides, paraminobenzoic acid, acute porfiria, anemia or severe cardiopulmonary disorder and deficit of glucose-6-fosta-todeshidrogenase. Dapsone is not indicated in pregnancy or lactation. It presents interactions with probenecid and trimetoprim. The toxicity depends on the dosage for hemolisis and metahemoglobinemia(2828. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe dapsone hypersentivity syndrome. J Invest Allerg Clin Immunol. 2006;16:268-70.).
Because of severe adverse effects, patients need to be monitored specially for renal and liver functions. The adverse reactions to dapsone are classified as toxic (metahemoglobinemia, hemolytic anemia) or idiosyncratic (dapsone hypersensitivity syndrome: general malaise, exanthematous eruption, photosensitivity, neurological effects, nephropathy, hypothyroidism, gastrointestinal effects). In general, 5% of the patients develop problems 2-6 weeks after the beginning of treatment(2828. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe dapsone hypersentivity syndrome. J Invest Allerg Clin Immunol. 2006;16:268-70.).
Dapsone suppresses inflammation in the skin, but has no influence on intestinal abnormality. So, many patients with DH choose to take dapsone chronically and not restrict gluten intake, despite knowing that gluten is the causative agent of their eruption(2828. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe dapsone hypersentivity syndrome. J Invest Allerg Clin Immunol. 2006;16:268-70.).
Dapsone is clinically useful in diseases containing neutrophilic infiltrates, inhibiting neutrophil myeloperoxidase, decreasing the damage from the neutrophil respiratory burst pathway mediated by this enzyme. The anti-inflammatory properties of topical dapsone benefit patients with acne and could also hinder the immunologic cascade and accompanying inflammatory process that occurs in DH. So, Handler et al. using dapsone 5% gel, reported an adjuvant effect in the treatment of a teenage patient with low doses of oral dapsone. As facial disease may be refractory to oral dapsone therapy, it is a new option for treatment(1212. Handler MZ, Chacon AH, Shiman MI, Schachner LA. Application of dapsone 5% gel in a patient with dermatitis herpetiformis. J Dermatol. 2012;31:132-3.).
The oral association of vitamin E (800 U/day) or cimetidine (1.2 to 1.6 g/day) could be recommended to minimize the risk of hemolytic anemia and metahemoglobinemia(2424. Rhodes LE, Tingle MD, Park BK, Chu P, Verbov JL, Friedmann PS. Cimetidine improves the therapeutic/toxic ratio of dapsone in patients on chronic dapsone therapy. Br J Dermatol.1995;132:237-62.). Taking the dosage divided into two occasions could reduce the blood concentration and the toxicity of the medication.
Sulfasalazine and sulphamethoxypyridazine
These drugs could be an effective alternative for the treatment of DH when the use of dapsone fails or presents side effects. The suggested dosages are 1-2 g/day for sulfasalazine and 0.25-1.5 g/day for sulphamethoxypyridazine. As these mentioned medications also induce adverse effects, controls are necessary monthly or 6 monthly. Enteric-coated forms of the drugs could be prescribed(3535. Willsteed E, Lee M, Wong LC, Cooper A. Sulfasalazine and dermatitis herpetiformis. Australas J Dermatol. 2005;46:101-3.).
Other drugs
Topic corticosteroids (clobetasol propionate) could be of relative utility, but systemic glucocorticosteroids are not indicated to treat DH. Although with not high efficacy, third-generation antihistamines with specific activity on eosinophilic granulocytes may be used to control the pruritus and itching(44. Caproni M, Antiga E, Melani L, Fabbri P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009; 23:633-8.). Immunosuppressants such as azathioprine, mycophenolate mofetil or methotrexate could be indicated in cases of non-response to a GFD plus dapsone. The author has a patient who used azathioprine for 2 years and then could be free of lesions only with a GFD (personal communication). Rituximab could be another possibility of treatment(2727. Schmidt E. [Optimizing therapy in patients with severe autoimmune blistering skin diseases]. Hautarzt. 2009;60:633-40.). But DH, as CD, can appear in patients using infliximab for ankylosing spondylitis or inflammatory bowel disease, probably because TNF inhibitors could alter immunity and this would promote an inflammatory autoimmune response in the skin of predisposed individuals(2121. Marakli SS, Uzun S, Ozbek S, Tuncer I. Dermatitis herpetiformis in a patient receiving infliximab for ankylosing spondylitis. Eur J Dermatol. 2008;18:88-9.).
Follow-up
Patients with DH should be evaluated at regular intervals (6 months after diagnosis and then yearly) by a physician and a dietician. The purpose includes compliance with GFD, reinforcing the importance of the diet, and to detect early signs of malabsorption and/or associated diseases. Monitoring the diet with serological tets (IgA EmA or IgA anti-tTG) is sensitive for major but not for minor transient dietary indiscretions(1717. Kotze LMS, Brambila-Rodrigues AP, Kotze LR, Nisihara RM. A Brazilian experience of the self transglutaminase-based test for celiac disease finding and diet monitoring. World J Gastroenterol. 2009;15:4423-8.).
CONCLUSIONS
Physicians treating patients with CD should be alert for skins manifestations of gluten sensitivity, not always characteristic of DH. On the other hand, dermatologists may be aware of digestive implications of DH per se and the systemic actions of dapsone, including liver function alterations. For men with DH, periodic control is fundamental as lymphoma needs to be diagnosed as soon as possible to give opportunity of treatment.
REFERENCES
-
1Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: review of the Mayo Clinic experience. Int J Dermatol. 2007;46:910-9.
-
2Bolontin D, Petronic-Rosic V. Dermatitis herpetiformis: part I. Epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-24.
-
3Bonciolini V, Bonciani D, Verdelli A, D'Errico A, Antiga E, Fabbri P, Caproni M. Newly described clinical and immunopathological feature of dermatitis herpetiformis. Clin Develop Immunol. 2012. doi: 10.1155/2012/967974.
» https://doi.org/10.1155/2012/967974 -
4Caproni M, Antiga E, Melani L, Fabbri P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009; 23:633-8.
-
5Chang D. The need for direct immunofluorescence in the diagnosis of IgA bullous dermatosis. J Bras Patol Med Lab. 2012;48:55-7.
-
6Collin P, Reunala T. Recognition and manegment of cutaneous manifestations of celiac disese: a guide for dermatologists. Am J Clin Dermatol. 2003; 4:13-20.
-
7Dieterich W, Laag E, Bruckner-Tuderman L, Reunala T, Karpati S, Zagoni T, Riecken EO, Schuppan D. Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Invest Dermatol. 1999;113:133-36.
-
8Duhring L. Dermatitis herpetiformis. JAMA. 1884;3:225-8.
-
9Fabbri P, Calabro AS, Hashimoto T, Fasano A, Caproni M. Novel advances in dermatitis herpetiformis. Clin Develoment Immunol. 2012.
-
10Fasano A. Novel therapeutic/integrative approaches for celiac disease and dermatitis herpetiformis. Clin Develop Immunol. 2012. doi: 10.1155/2012/959061
» https://doi.org/10.1155/2012/959061 -
11Fry L, Keir P, McMinn RM, Cowan JD, Hoffbrand AV. Small intestinal structure and function and haematological changes in dermatitis herpetiformis. Lancet. 1967;2:29-33.
-
12Handler MZ, Chacon AH, Shiman MI, Schachner LA. Application of dapsone 5% gel in a patient with dermatitis herpetiformis. J Dermatol. 2012;31:132-3.
-
13Herrero-González JE. [Clinical guidelines for the diagnosis and treatment of dermatitis herpetiformis]. Actas Dermosifiliogr. 2010;101:820-6.
-
14Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Brit J Dermatol. 2005;152:82-6.
-
15Kàrpàti S. Dermatitis herpetiformis. Clin Dermatol. 2012;30:56-9.
-
16Kotze LMS. Celiac disease in Brazilian patients: associations, complications and causes of death. Forty-years of clinical experience. Arq Gastroenterol. 2009;46:261-9.
-
17Kotze LMS, Brambila-Rodrigues AP, Kotze LR, Nisihara RM. A Brazilian experience of the self transglutaminase-based test for celiac disease finding and diet monitoring. World J Gastroenterol. 2009;15:4423-8.
-
18Kotze LMS, Dalla Vecchia LA, Nisihara NM. Alta prevalência de dermatite herpetiforme em homens com doença celíaca. GED. 2012;31(Suppl 1):28-479.
-
19Kotze LMS, Nisihara RM, Kotze LR, Utiyama SRR. Celiac disease and dermatitis herpetiformis in Brazilian twins: a long-term follow-up and screening of their relatives. J Pediatr Endocrinol Metab. 2012;26:71-5.
-
20Kumar V, Jarzabek-Chorzelska M, Sulej J, Rajadhyaksha M, Jablonska S. Tissue transglutaminase and endomysial antibodies-diagnostic markers of gluten sensitive enteropathy in dermatitis herpetiformis. Clin Immunol. 2001;98:378-82.
-
21Marakli SS, Uzun S, Ozbek S, Tuncer I. Dermatitis herpetiformis in a patient receiving infliximab for ankylosing spondylitis. Eur J Dermatol. 2008;18:88-9.
-
22Marks J, Shuster S, Watson AJ. Small-bowel changes in dermatitis herpetiformis. Lancet. 1966;2:1280-2.
-
23Oxentenko AS, Murray JA. Celiac disease and dermatitis herpetiformis: the spectrum of gluten-sensitivity enteropathy. Int J Dermatol. 2003; 42:585-7.
-
24Rhodes LE, Tingle MD, Park BK, Chu P, Verbov JL, Friedmann PS. Cimetidine improves the therapeutic/toxic ratio of dapsone in patients on chronic dapsone therapy. Br J Dermatol.1995;132:237-62.
-
25Rottmann LH. Details of the gluten-free diet for the patients with dermatitis herpetiformis. Clin Dermatol. 1991;9:404-14.
-
26Sárdy M, Kárpáti S, Merkl B, Paulsson M, Smyth N. Epidermal thansglutaminase(TGase 3) is the autoantigen of dermatitis herpetiformis. J Experim Med. 2002;195:747-57.
-
27Schmidt E. [Optimizing therapy in patients with severe autoimmune blistering skin diseases]. Hautarzt. 2009;60:633-40.
-
28Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe dapsone hypersentivity syndrome. J Invest Allerg Clin Immunol. 2006;16:268-70.
-
29Sugai E, Smecuol E, Niveloni S, Vazquez H, Label M, Mazure R, Czech A, Kogan Z, Mauriño E, Bai JC. Celiac disease serology in dermatitis herpetiformis. Which is the best option for detecting gluten sensitivity? Acta Gastroenterol Latinoam. 2006;36:197-201.
-
30The First Consensus Conference on Gluten Sensitivity, London; 2011.
-
31Turchin I, Barankin B. Dermatitis herpetiformis and gluten-free diet. Dermatol Online J. 2005;11:6.
-
32Van der Meer JB. Granular deposits of immunoglobulins in the skin of patients with dermatitis herpetiformis. An immunofluorescent study. Br J Dermatol. 1969;81:493-503
-
33Volta U, Molinaro N, De Franchis R, Forzenigo L, Landoni M, Fratangelo D, Bianchi FB. Correlation between IgA antiendomysial antibodies and subtotal villous atrophy in dermatitis herpetiformis. J Clin Gastroenterol. 1992;14:298-301.
-
34Warren SJP, Cockerell CJ. Characterization of a subgroup of patients with dermatitis herpetiformis with non-classical histologic features. Am J Dermatopathol. 2002;24:305-8.
-
35Willsteed E, Lee M, Wong LC, Cooper A. Sulfasalazine and dermatitis herpetiformis. Australas J Dermatol. 2005;46:101-3.
-
36Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87-S91.
-
37Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-8.
Publication Dates
-
Publication in this collection
July-Sept 2013
History
-
Received
24 May 2013 -
Accepted
12 July 2013