ABSTRACT
BACKGROUND
Healthy individuals exhibit a significantly higher concentration of faecal bifidobacteria in comparison to celiac patients. Even though there are potential benefits in probiotic usage, they have been little explored as an adjunctive therapy in celiac disease.
OBJECTIVE
This study aimed at the comparison of faecal bifidobacteria concentration and pH among celiac patients and healthy subjects before and after the daily intake of 100 g of yogurt containing probiotic for a thirty-day period.
METHODS
Feces from 17 healthy subjects and 14 celiac patients were analyzed, in which stool culture was performed for the isolation and quantification of faecal bifidobacteria. Furthermore, Gram’s method was employed for the microscopic analysis of the colonies, while the identification of the Bifidobacterium genus was made through determination of the fructose-6-phosphate phosphoketolase enzyme. Faecal pH was measured using a calibrated pHmeter.
RESULTS
Faecal bifidobacteria concentration before probiotic consumption was significantly higher in healthy individuals (2.3x108±6.3x107 CFU/g) when compared to celiac patients (1.0x107±1.7x107 CFU/g). Faecal pH values did not show a significant difference. After the daily consumption of probiotic-containing yogurt both groups showed a significant increase in the concentration of faecal bifidobacteria, but healthy subjects presented significantly higher bifidobacteria concentrations (14.7x108±0.2x108 CFU/g) than the celiac group (0.76x108±0.1x108 CFU/g). The obtained pH values from both groups were not significantly different, being 7.28±0.518 for the celiac patients and 7.07±0.570 for healthy individuals after the probiotic intake.
CONCLUSION
The probiotic supplementation significantly increased the number of bifidobacteria in the feces of celiac patients, although it was not sufficient to reach the concentration found in healthy individuals prior to its consumption.
HEADINGS
Probiotics; Celiac disease; Bifidobacterium; Hydrogen-ion concentration; Feces, microbiology; Microbial colony count
RESUMO
CONTEXTO
Indivíduos saudáveis apresentam uma concentração de bifidobactérias fecais significativamente maior em comparação a pacientes celíacos. Apesar de haver benefícios potenciais no uso de probióticos na doença celíaca, estes têm sido pouco explorados como uma terapia adjuvante.
OBJETIVO
Este estudo objetivou a comparação do pH e concentração fecal de bifidobactérias entre pacientes celíacos e indivíduos saudáveis antes e após o consumo diário de 100 g de iogurte contendo probiótico por um período de 30 dias.
MÉTODOS
Foram analisadas fezes de 17 pessoas saudáveis e 14 pacientes celíacos, tendo sido realizada a coprocultura para o isolamento e quantificação de bifidobactérias fecais. Além disso, o método de Gram foi empregado na análise microscópica das colônias, enquanto a identificação do gênero Bifidobacterium foi feita através da determinação da enzima frutose-6-fosfato fosfocetolase. O pH fecal foi medido usando um pHmetro calibrado.
RESULTADOS
A concentração de bifidobactérias fecais antes do consumo do iogurte probiótico foi significativamente maior em indivíduos saudáveis (2.3x108±6.3x107 UFC/g) quando comparada aos celíacos (1.0x107±1.7x107 CFU/g). Por outro lado, o pH fecal de ambos os grupos não apresentou diferença significativa. Após o consumo diário de iogurte contendo probiótico, ambos os grupos tiveram um aumento significativo na concentração de bifidobactérias fecais, entretanto indivíduos saudáveis apresentaram concentrações de bifidobactérias significativamente maiores (14.7x108±0.2x108 UFC/g) do que o grupo celíaco (0.76x108±0.1x108 UFC/g). Os valores de pH obtidos de ambos os grupos não foram significativamente diferentes, sendo de 7.28±0.518 para os pacientes celíacos e de 7.07±0.570 para os indivíduos saudáveis após o consumo do probiótico.
CONCLUSÃO
A suplementação com probiótico aumentou significativamente o número de bifidobactérias nas fezes dos pacientes celíacos apesar de não ter sido suficiente para alcançar a concentração encontrada em indivíduos saudáveis antes do consumo de probióticos.
DESCRITORES
Probióticos; Doença celíaca; Bifidobacterium; Concentração de íons de Hidrogênio; Fezes, microbiologia; Contagem de colônia microbiana.
INTRODUCTION
Patients with celiac disease (CD) have an intolerance to the polipeptide fragments of gluten, mediated by T lymphocytes. Gluten is a water-insoluble substance found in wheat flour, rye, barley and oats 3030. Van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-46. . CD depends on genetic, immunological and environmental factors and it is characterized by total or partial atrophy of the intestinal villi and consequent poor absorption of nutrients 66. Di Cagno R, Rizzello CG, Gagliardi F, Ricciuti P, Ndagijimana M, Francavilla R, et al. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl Environ Microbiol. 2009;75:3963-71.,99. Ivarsson A, Hernell O, Nyström L, Persson LA. Children born in the summer have increased risk for coeliac disease. J Epidemiol Community Health. 2003;57:36-9.,2727. Silva TSG, Furlanetto TW. Diagnóstico de doença celíaca em adultos. Rev Assoc Med Bras. 2010;56:122-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302010000100027&lng=en
http://www.scielo.br/scielo.php?script=s...
. Its prevalence in Brazil is shown to be 1/214 33. Cassol CA, Pellegrin CP, Wahys MLC, Pires MMS, Nassar SM. [Clinical profile of Santa Catarina members of Brazilian Celiac Association]. Arq Gastroenterol. 2007;44:257-65. .
CD diagnosis must be based on clinical, histopathological (gold standard) and serological examinations 33. Cassol CA, Pellegrin CP, Wahys MLC, Pires MMS, Nassar SM. [Clinical profile of Santa Catarina members of Brazilian Celiac Association]. Arq Gastroenterol. 2007;44:257-65.,2727. Silva TSG, Furlanetto TW. Diagnóstico de doença celíaca em adultos. Rev Assoc Med Bras. 2010;56:122-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302010000100027&lng=en
http://www.scielo.br/scielo.php?script=s...
. There are few studies on the intestinal microbiota role in CD, even though gliadin (a gluten peptide) and microorganisms similarly activate pro-inflammatory routes 1212. Medina M, Palma GD, Ribes-Koninckx C, Calabuig M, Sanz Y. Bifidobacterium strains suppress in vitro the pro-inflammatory milieu triggered by the large intestinal microbiota of coeliac patients. J inflamm. 2008;5:1-13. . The information about the intestinal microbiota of celiac patients is mainly obtained from a stool sample examination 1515. Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with celiac disease. J Med Microbiol. 2007;56:1669-74. .
Healthy subjects present a significantly higher concentration of bifidobacteria when compared to celiac patients, while faecal pH seems to remain the same in both situations 88. Golfetto L, Senna FD, Hermes J, Beserra BTS, França FS, Martinello F. Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq Gastroenterol . 2014;51:139-43. .
The only effective and possible treatment for CD is dietary, throughout the exclusion of gluten from the diet, which allows the remission of symptoms and the restoration of the regular mucosa 11. Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004; 36:492-8. . Without treatment, CD has a high morbi-mortality rate, with risks of developing complications such as anemia, infertility, osteoporosis and cancer, being the most prevalent the intestinal lymphoma 2727. Silva TSG, Furlanetto TW. Diagnóstico de doença celíaca em adultos. Rev Assoc Med Bras. 2010;56:122-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302010000100027&lng=en
http://www.scielo.br/scielo.php?script=s...
. Alternative treatments are also available and can be used simultaneously as palliatives, for instance, the use of probiotics, mainly Lactobacillus and Bifidobacterium77. Fontana L, Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr. 2013; 109 Suppl 2:S35-50.,1515. Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with celiac disease. J Med Microbiol. 2007;56:1669-74.,2424. Sanz Y. Novel perspectives in celiac disease therapy. Mini Rev Med Chem. 2009;9:359-67. .
The presence of bifidobacteria in the gastrointestinal tract seems to suffer variations throughout life and it is associated with beneficial effects to health, including the re-composition of the intestinal microbiota, the growth inhibition of pathogenic bacteria, regeneration of the epithelial barrier and anti-inflammatory effects 1111. Leahy SC, Higgins DG, Fitzgerald GF, Sinderen DV. Getting better with bifidobacteria. J Appl Microbiol. 2005;98:1303-15.,2121. Pokusaeva K, Fitzgerald GF, Sinderen DV. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6:285-306.,2525. Satokari RM, Vaughan EE, Smidt H, Saarela M, Matto J, de Vos WM. Molecular approaches for the detection and identification of bifidobacteria and lactobacilli in the human gastrointestinal tract. Syst Appl Microbiol. 2003;26:572-84.,2626. Shanahan F. Probiotics in perspective. Gastroenterology. 2010;139:1808-12.,3232. WHO. World Health Organization Food and Agriculture Organization of the United Nations: Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria. 2001. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
ftp://ftp.fao.org/docrep/fao/009/a0512e/...
. Some species have the capacity of inhibiting the increased permeability induced by gliadin, weakening its cytotoxic effect and the host autoimmune response 1010. Laparra JM, Sanz Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J Cell Biochem. 2010; 109:801-7. . Smecuol et al. showed that celiac patients on a gluten diet had experienced beneficial effects related to gastrointestinal tract symptoms (such as constipation and gastroesophageal reflux) when consuming bifidobacteria in capsules before meals 2828. Smecuol E, Hwang HJ, Sugai E, Corso L, Chernavsky AC, Bellavite FP, et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J Clin Gastroenterol . 2013;47:139-47. . Other beneficial effects of bifidobacteria consumption with a gluten diet have been described, such as the reduction of human α-defensin 5 (HD-5) and paneth cells 2020. Pinto-Sánchez MI, Smecuol EC, Temprano MP, Sugai E, González A, Moreno ML, et al. Bifidobacterium infantis NLS super strain reduces the expression of [alpha]-defensin-5, a marker of innate immunity, in the mucosa of active celiac disease patients. J Clin Gastroenterol. 2016. [Epub ahead of print]. .
Bifidobacterium and Lactobacillus are widely used in several food products, such as yogurt, milk, cheese and dietetic supplements, upon which research has been increasing. Even though there are potential benefits in their usage, probiotics have been poorly explored as an adjunctive therapy in CD 1717. OMG. Organização Mundial de Gastroenterologia. Guias Práticas da OMGE: Probióticos e Prebióticos. 2008. Available from: http://www.serdigital.com.br/gerenciador/clientes/biologicus/arquivos/25.pdf.
http://www.serdigital.com.br/gerenciador...
,3232. WHO. World Health Organization Food and Agriculture Organization of the United Nations: Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria. 2001. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
ftp://ftp.fao.org/docrep/fao/009/a0512e/...
. In this context, the hypothesis is that the intestinal microbiota of patients with controlled CD can be restored by the daily intake of probiotic-containing yogurt. The results of this study will allow the analysis of the necessity and efficacy of supplementation with probiotics to restore the intestinal microbiota equilibrium, with consequent reduction of gastrointestinal complications and infections, improving the quality of life of celiac patients.
METHODS
Study design
The Ethics Committee for Studies with Humans of the Federal University of Santa Catarina, Brazil, approved the experimental protocol for this study (number 772, 2010). The participants with CD were recruited in the local Association of Celiac People in Brazil (Associação dos Celíacos do Brasil - ACELBRA) during its monthly meetings. All celiac patients were on a controlled stage of the disease during the study, i.e ., they were on a gluten free diet, without signals and symptoms of CD. The non-celiac participants were randomly recruited from the population.
Volunteers were submitted to a clinical and sociodemographic questionnaire and the research started by collecting the first stool sample to quantify bifidobacteria and measure faecal pH. Afterwards, each volunteer consumed one unit of probiotic-containing yogurt (100g) from Piá Essence, PIÁ®, Nova Petrópolis-RS) per day, having eaten in the fasting state at morning, during one month. The yogurt delivery was made weekly. After 30 days of consumption, feces were collected again in order to quantify bifidobacteria and measure faecal pH.
Exclusion criteria
The following exclusion criteria for the participation in the study were adopted: individuals with suspicion or diagnosis of autoimmune diseases; suspicion or diagnosis of diabetes; lactose intolerance; allergy to any excipient present in the yogurt; individuals who consumed products containing prebiotics and/or probiotics three months prior to the beginning of research, and individuals who presented fever, diarrhea and/or vomit three months prior to the beginning or during study.
Determination of faecal bifidobacteria content and pH
For the isolation and quantification of bifidobacteria and measurement of faecal pH, participants collected stool samples, which were sent to the laboratory and analyzed within 8 h after collection 1313. Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 Supplementation on Body Weight, Fecal pH, Acetate, Lactate, Calprotectin, and IgA in Preterm Infants. Pediatr Res. 2008;64:418-22.,2929. Thitaram SN, Siragusa GR, Hinton AJr. Bifidobacterium-selective isolation and enumeration from chicken caeca by a modified oligosaccharide antibiotic-selective agar medium. Lett Appl Microbiol. 2005;41:355-60. .
Feces aliquot (1 g) from each volunteer was diluted in 9 mL of distilled and deionized sterile water for the measurement of faecal pH in pHmeter PHTEK®. Another feces aliquot (1 g) from each stool sample was diluted in 9 mL of phosphate buffer. The mixture was homogenized five times using the anaerobic technique. From this dilution (10-1), serial fold dilutions up to 10-7 were prepared. The stock phosphate buffer was previously prepared with 34 g of KH2PO4 in 500 mL of distilled and deionized water, having the pH adjusted to 7.2 with NaOH 1 N and the volume completed to 1 L with distilled water, being subsequently sterilized in an autoclave at 121°C during 18 minutes. For the dilution of the stool sample, the phosphate buffer was diluted a thousand times from the stock solution.
The culture media used for isolation of bifidobacteria was the RCA (Reinforced Clostridial Agar, DifcoTM BD) supplemented with antibiotics (nalidixic acid 2%, polymyxin B sulfate 0.85%, kanamycin sulfate 0.5%, iodoacetic acid 0.5%, 2,3,5-triphenyltetrazolium chloride 0.5% and amphotericin B 0.001%) 141 4.Muñoa FJ, Pares R. Selective medium for isolation and enumeration of Bifidobacterium spp. Appl Environ Microbiol . 1988;54:1715-8. .
The spread-plating of 100 µL from each dilution was prepared and the plates were incubated at 37°C for 72 hours under anaerobic conditions 141 4.Muñoa FJ, Pares R. Selective medium for isolation and enumeration of Bifidobacterium spp. Appl Environ Microbiol . 1988;54:1715-8. using a commercial anaerobic atmosphere generation system (Anaerobac from Probac®), followed by counting of bifidobacteria colonies in the plates containing between 30 and 300 colony-forming units (CFU). For the confirmation of the Bifidobacterium genus, Gram staining was made, as well as catalase proof and fructose-6-phosphate phosphoketolase (F6PPK) reaction, as stated by Orban & Patterson (2000), for all isolated colony types 1818. Orban JI, Patterson JA. Modification of the phosphoketolase assay for rapid identification of bifidobactérias. J Microbiol Methods. 2000;40:221-4. .
The results from the bifidobacteria quantification were presented as CFU per gram of feces (CFU/g). To obtain the results, the number of CFU counted in each plate was multiplied by its respective dilution factor and corrected for the sample volume spread. They are expressed as mean ± standard deviation (n=17 for the control group and n=14 for the celiac group).
Determination of yogurt bifidobacteria content and pH
All lots of the yogurt Piá Essence donated were analyzed for the isolation and quantification of bifidobacteria and measurement of pH. One pot containing 100 g of yogurt was randomly selected from each lot and 1 g was diluted in 9 mL of distilled and deionized sterile water for measurement of pH in pHmeter PHTEK® previously calibrated.
Another yogurt aliquot (1 g) was also diluted in 9 mL of phosphate buffer. Serial dilutions were made from this solution as for the feces analysis. The spread-plating from each dilution, the counting of colonies, the confirmation of the genus and the expression of the results were made as previously described for the stool samples.
Statistical analysis
The statistical analysis was performed using the program GraphPad Prism® version 5.0 from 2007. For data distribution analysis, the D’Agostino normality test and Pearson Omnibus Normality Test were employed. Spearman’s correlation coefficient was employed to verify the correlation among the bifidobacteria concentration, faecal pH and volunteers’ ages. Wilcoxon test was used for the comparison of the results between groups. A significance level of 5% ( P <0.05) was adopted for all tests.
RESULTS
The yogurt package informs that each 100 g of yogurt contains 108 CFU of Lactobacillus acidophilus and Bifidobacterium lactis . Amongst the yogurt lots available to the volunteers, the average concentration of bifidobacteria was 6.67x108±10.3x108 CFU/g of yogurt. The average yogurt pH was 4.28±0.15 and there was a significant correlation between the bifidobacteria concentration and yogurt pH ( P =0.0121). There was growth of Gram-positive bacillus colonies in every lot of yogurt, being all catalase negative and showing F6PPK activity.
Amongst the 17 healthy control individuals, 10 were female and seven were male, aged between 18 and 58 years (average of 26 years old). This group of individuals did not have any relatives with CD.
From the group of 14 celiac patients, 10 were female and four were male, with ages ranging from 18 to 60 years, being the average being 38 years old. The prevalence of CD in their families was higher in first (father, mother and siblings) and second (grandparents, aunts, uncles and cousins) degree relatives. Two (14.4%) volunteers had first-degree celiac relatives, three (21.4%) had first and second-degree celiac relatives, one (7.1%) could not answer and eight (57.1%) did not have celiac relatives. The average age in which the diagnosis was made was 36 years old, where 100% of the patients had the small intestine biopsy done for confirmation of CD. A relation between faecal bifidobacteria concentration and age was not observed in any of the groups, either celiac or healthy subjects.
Seven (50%) of the 14 celiac patients received drug therapy after CD diagnosis, being calcium therapy the most prevalent in 57% of them, mainly related to women 30 years old or older.
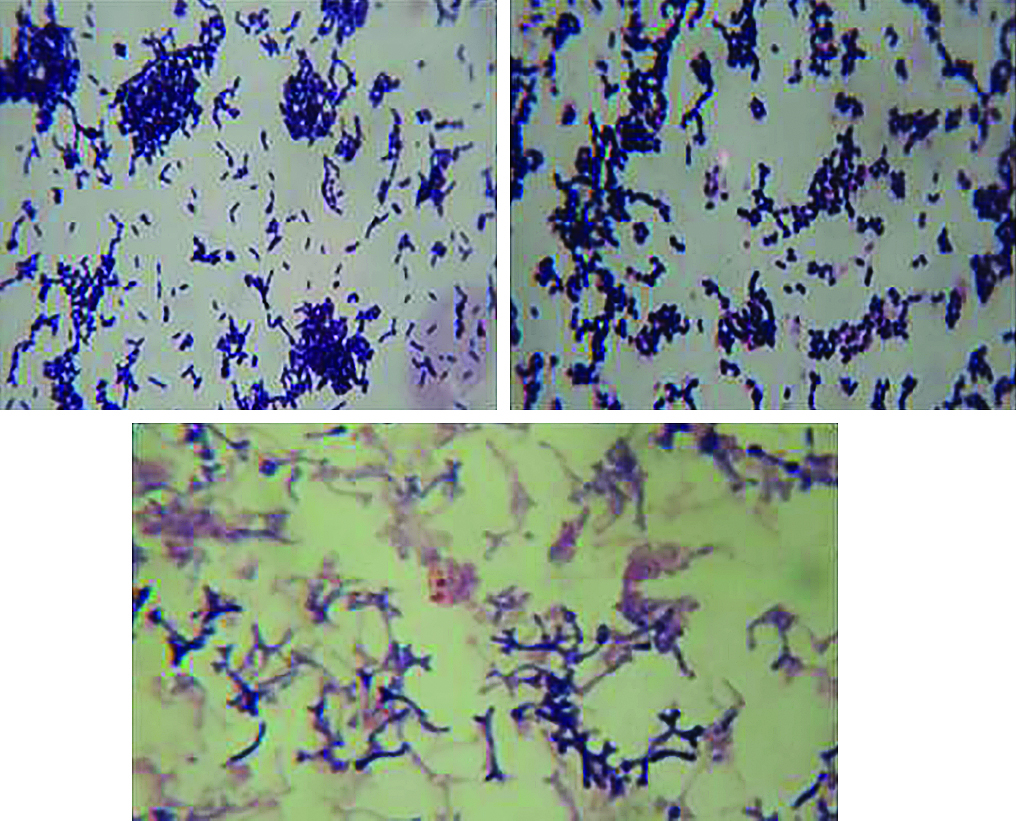
During the stool sample examination from the volunteers, bifidobacteria colonies were observed, presenting round shape, smooth surface, pink to wine color and small to medium size. The colony morphology was similar between both groups, celiac and control ( Figure 1 ). Bifidobacteria appeared in the form of short and long Gram-positive bacilli, with or without bifurcated ends V or Y-shaped, and as Gram-positive coccobacilli ( Figure 2 ).
Bifidobacteria colonies in Reinforced Clostridial agar media supplemented with antibiotics. (A) Celiac patient plate. (B) Control subject plate.
Micromorphology of bifidobacteria colonies stained by Gram method in optical microscopy in 1000 times increase. (A) Short Gram-positive bacilli, isolated, in pairs or grouped. (B) Gram-positive cocobacilli, isolated, in pairs or grouped. (C) Long Gram-positive bacilli with bifurcated ends V or Y-shaped.
Number of colony forming units (CFU) of bifidobacteria per gram of feces from control and celiac groups, before and after probiotic intake. Results are expressed as mean ± standard deviation (control group n=17 and celiac group n=14). * P <0.05 nonparametric t test, when compared to the respective control group before probiotic intake or ** when compared to the respective control group after probiotic intake.
The results of bifidobacteria quantification in the stool samples are shown in Figure 3 . Healthy individuals presented a significantly higher concentration of bifidobacteria (2.3x108 ± 6.3x107 CFU/g) before the probiotic-containing yogurt intake when compared to the celiac group (1.0x107±1.7x107 CFU/g) ( Figure 3 ). Celiac patients presented, in average, 83% less bifidobacteria than healthy individuals. Still, celiac faecal pH (7.19±0.521) was not significantly different from the faecal pH of the control group (7.18±0.522).
After the daily intake of 100 g of probiotic-containing yogurt for 30 days, healthy individuals presented a significantly higher bifidobacteria concentration (14.7x108±0.2x108 CFU/g) than celiac patients (0.76x108±0.1x108 CFU/g) ( Figure 3 ). However, faecal pH of celiac patients (7.28±0.518) did not show significant difference from the faecal pH of healthy individuals (7.07±0.570) after the yogurt intake.
DISCUSSION
Several probiotic supplements can be found on the market; meanwhile it is still hard to find gluten free products for celiac patients. In this context, the product options for this research were limited. Amongst the companies for which support was requested, only PIÁ®, Nova Petrópolis-RS, provided the products. The average bifidobacteria concentration provided for the research participants (6.67x108±10.3x108 CFU/g of yogurt) is enough to bring benefits to their health, according to Vinderola & Reinheimer 3131. Vinderola CG, Reinheimer JA. Enumeration of Lactobacillus casei in the presence of Lactobacillus acidophilus and lactic starter in fermented dairy products. Int Dairy J. 2000;10:271-5. .
A number of factors can affect probiotic bacteria viability in yogurts. High carbohydrate concentrations added to the product before its fermentation can inhibit the bacteria, leading to long periods of fermentation and an underdevelopment of acidity 1616. Oliveira MN, Damin MR. [Effect of total solids and sucrose contents on acidity, firmness and viability of yogurt and probiotic bacteria in fermented milk]. Ciênc Tecnol Aliment. 2003;S23:172-6. Available from http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612003000400032
http://www.scielo.br/scielo.php?script=s...
. Oliveira & Damin 1616. Oliveira MN, Damin MR. [Effect of total solids and sucrose contents on acidity, firmness and viability of yogurt and probiotic bacteria in fermented milk]. Ciênc Tecnol Aliment. 2003;S23:172-6. Available from http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612003000400032
http://www.scielo.br/scielo.php?script=s...
found that the number of probiotic bacteria remained stable for at least seven days of storage. However, in this study volunteers consumed the probiotics up to their expiration date, which simulates the acquisition of products commercialized for the general population. A yogurt of a lot provided for the volunteers was randomly tested six days after its expiration date, in which a bifidobacteria concentration of 1.74x106 CFU/g of yogurt was found. Coupled with the likely concentration of Lactobacillus , this would still be a probiotic food and bring benefits to people’s health 1919. Pérez N, Iannicelli JC, Girard-Bosch C, González S, Varea A, Disalvo L. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio-economic status. Eur J Nutr. 2010;49:173-9. , including celiac patients.
The largest number of female celiac patients in this study is consistent with literature, which shows a higher prevalence of CD in women 33. Cassol CA, Pellegrin CP, Wahys MLC, Pires MMS, Nassar SM. [Clinical profile of Santa Catarina members of Brazilian Celiac Association]. Arq Gastroenterol. 2007;44:257-65. . About 30% of celiac patients evaluated in this study have a relative with CD, which is similar to a study made with patients from Association of Celiac People in Brazil, section from Santa Catarina, (ACELBRA-SC) in 2004, revealing that 27% of associates had relatives with CD 33. Cassol CA, Pellegrin CP, Wahys MLC, Pires MMS, Nassar SM. [Clinical profile of Santa Catarina members of Brazilian Celiac Association]. Arq Gastroenterol. 2007;44:257-65. . This data reinforces the idea that genetic determinants of CD are associated with environmental factors 1515. Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with celiac disease. J Med Microbiol. 2007;56:1669-74. . It is important to note that 100% of the celiac patients who participated in the research had the intestinal biopsy done for their diagnosis, which is recommended by the literature 3030. Van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-46. .
The poor intestinal absorption of most nutrients resulting from the inflammatory response on CD can explain why most celiac patients reported having osteoporosis and osteopenia 33. Cassol CA, Pellegrin CP, Wahys MLC, Pires MMS, Nassar SM. [Clinical profile of Santa Catarina members of Brazilian Celiac Association]. Arq Gastroenterol. 2007;44:257-65.,3030. Van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-46. . It also explains why most participants of this research have replenished calcium and vitamin D after CD diagnosis. The supplementation with probiotic-containing yogurt could bring not only the benefits from the probiotics for celiac patients but also a greater amount of calcium absorbed from their diet.
The mechanisms of action of probiotics have not been completely elucidated, even though many have been suggested and possibly operate individually or associated 3232. WHO. World Health Organization Food and Agriculture Organization of the United Nations: Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria. 2001. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
ftp://ftp.fao.org/docrep/fao/009/a0512e/...
. There is evidence that probiotics have antimicrobial action, compete for limited nutritional resources from the intestinal microbiota, block adhesion of pathogens in the intestinal mucosa and have antitoxin effects of pathogens 2222. Saad SMI. Probióticos e prebióticos: o estado da arte. Rev Bras Cienc Saude. 2006;42:1-16. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-93322006000100002&lng=en
http://www.scielo.br/scielo.php?script=s...
. Bifidobacteria can also benefit people’s health by lowering intestinal pH through the production of short chain fatty acids (acetate and lactate), thus inhibiting pathogenic bacteria growth. This is a digestive system self-mechanism for population control and selectivity of bacterial colonization 1313. Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 Supplementation on Body Weight, Fecal pH, Acetate, Lactate, Calprotectin, and IgA in Preterm Infants. Pediatr Res. 2008;64:418-22. . Indeed, a significant correlation between faecal pH and bifidobacteria concentration was not seen in this study.
Macro and micromorphology of bifidobacteria colonies found in the stool samples were similar in both groups and were as described in the literature. However, the results show a significant lower quantity of bifidobacteria CFU per gram of feces of celiac patients than in the control group. Some studies show that allergic children and patients with atopic diseases are frequently colonized by a reduced number of bifidobacteria when compared to healthy children, showing a close relationship between bifidobacteria concentration and host immune disorders 55. Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008; 8:232. .
Nadal et al. 1515. Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with celiac disease. J Med Microbiol. 2007;56:1669-74. reported an imbalance in the intestinal biota of celiac children, especially the reduction of faecal Bifidobacterium spp. concentration. Similarly, Collado et al. 44. Collado MC, Calabuig M, Sanz Y. Differences between the fecal microbiota of coeliac infants and healthy controls. Curr Issues Intest Microbiol. 2007;8:9-14. have reported that celiac children with active or inactive disease had inferior bifidobacteria counting than control groups for both analyzed samples, either feces or intestinal biopsy specimens. Therefore, this imbalance seems to be independent on the activity of the disease. This explains the lower bifidobacteria concentration found in feces of adult celiac patients in this study, all in a controlled phase of CD.
The results found in this study for bifidobacteria concentration without probiotic consumption show a significantly higher bifidobacteria count in healthy subjects when compared to celiac patients, which is consistent with literature 88. Golfetto L, Senna FD, Hermes J, Beserra BTS, França FS, Martinello F. Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq Gastroenterol . 2014;51:139-43. . Even after probiotic consumption, the faecal bifidobacteria count in celiac patients from this study has not reached the counting in healthy individuals without probiotic consumption ( Figure 3 ).
The values of faecal pH for both groups before probiotic intake had no significant difference, having them remained very similar even after probiotic intake. These results suggest that the higher faecal bifidobacteria concentration after probiotic consumption did not increase intestinal fermentation, which would lower the pH and ease bifidobacteria growth 1313. Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 Supplementation on Body Weight, Fecal pH, Acetate, Lactate, Calprotectin, and IgA in Preterm Infants. Pediatr Res. 2008;64:418-22. . However, it is worth noting that the pH from the control group was slightly more acidic than the pH from the celiac patients. The increase in bifidobacteria count favors the lowering of faecal pH due to the fermentation done by these bacteria 1313. Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 Supplementation on Body Weight, Fecal pH, Acetate, Lactate, Calprotectin, and IgA in Preterm Infants. Pediatr Res. 2008;64:418-22. . The results of pH values from both groups, celiac and control, suggest that the smaller amount of bifidobacteria in the intestine of celiac patients is probably not related to faecal pH, but to the pathogenesis of CD. Thus, the relationship between bifidobacteria counting and CD has yet to be elucidated.
The maintenance of pH values before and after probiotic ingestion may be related to time or quantity/concentration of the daily-consumed probiotic, being suggested that probiotic effects are dose-dependent 1919. Pérez N, Iannicelli JC, Girard-Bosch C, González S, Varea A, Disalvo L. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio-economic status. Eur J Nutr. 2010;49:173-9. . However, the recommended dose by the literature was consumed in this study, which is between 106 e 1011 CFU/day, depending on the desired effect 2222. Saad SMI. Probióticos e prebióticos: o estado da arte. Rev Bras Cienc Saude. 2006;42:1-16. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-93322006000100002&lng=en
http://www.scielo.br/scielo.php?script=s...
.
In order to have the metabolism and intestinal content reflected in feces, variables must be taken into account, including intestinal motility, total fiber ingestion, intestinal secretion, and duration of dietetic intervention. Because of that, faecal pH may not exactly reflect colon pH. In fact, Bouhnik et. al. 22. Bouhnik Y, Raskine L, Simoneau G, Paineau D, Bornet F. The capacity of shortchain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutr J. 2006;5:8. have not considered the faecal pH as a good indicator of intestinal acidification, since it has not changed after ingestion of nondigestible carbohydrates by 200 healthy volunteers, despite the increase in the number of faecal bifidobacteria.
Although the healthy intestinal microbiota remains to be defined, there are many diseases related to its imbalance. In most cases, there is no information yet if microbiota imbalance has a triggering role or if it is a disease consequence. Anyway, both relationships lead to the hypothesis that an intervention to restore the microbiota to the healthiest state could mitigate the disease. The consumption of properly selected probiotics could be used with such role 2323. Sanders ME. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol . 2011;45:S115-9. .
There is indication, amongst research to elucidate activity of bifidobacteria, that intestinal microbiota change can influence the typical inflammatory reactions in CD in a specie-specific way 44. Collado MC, Calabuig M, Sanz Y. Differences between the fecal microbiota of coeliac infants and healthy controls. Curr Issues Intest Microbiol. 2007;8:9-14. . Therefore, it is thought that bifidobacteria has a great therapeutic potential, and manipulation of intestinal biota, as with probiotic supplementation, might improve quality of life of celiac patients.
However, it should be noted that the inclusion of a small number of participants, the evaluation of pH and bifidobacteria contents during a short period of time and the availability of molecular methods, more accurate to evaluate the intestinal microbiota, may be considered limitations of the present study. Therefore, we suggest that additional studies should be performed in order to evaluate all the aspects regarding intestinal microbiota and probiotic supplementation in CD.
It is still not clear why celiac patients who are in a controlled phase of the disease - i.e., on a gluten free diet, with restored intestinal villi and with no symptoms -, present less bifidobacteria.
CONCLUSION
The results obtained in the present study allow the conclusion that there is a lower bifidobacteria count in the intestinal microbiota of celiac patients, even when they are on a gluten free diet and consuming probiotic-containing food, when compared to the control group. This disturbance is independent on the faecal pH.
Supplementation with probiotics increased the number of faecal bifidobacteria, which reflects its intestinal concentration. Further research must be performed in order to evaluate the equilibrium of other bacteria (for instance, the pathogenics); to verify how long bifidobacteria count remains elevated after probiotic consumption; to correlate small intestine biopsy results with bifidobacteria concentration, since celiacs were on a gluten free diet; and evaluate if the microbiota imbalance was due to gluten contamination in food.
In summary, this information will help develop specific dietetic recommendations to celiac patients based on their microbiota composition.
REFERENCES
-
1Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004; 36:492-8.
-
2Bouhnik Y, Raskine L, Simoneau G, Paineau D, Bornet F. The capacity of shortchain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutr J. 2006;5:8.
-
3Cassol CA, Pellegrin CP, Wahys MLC, Pires MMS, Nassar SM. [Clinical profile of Santa Catarina members of Brazilian Celiac Association]. Arq Gastroenterol. 2007;44:257-65.
-
4Collado MC, Calabuig M, Sanz Y. Differences between the fecal microbiota of coeliac infants and healthy controls. Curr Issues Intest Microbiol. 2007;8:9-14.
-
5Collado MC, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalances in faecal and duodenal Bifidobacterium species composition in active and non-active coeliac disease. BMC Microbiol. 2008; 8:232.
-
6Di Cagno R, Rizzello CG, Gagliardi F, Ricciuti P, Ndagijimana M, Francavilla R, et al. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl Environ Microbiol. 2009;75:3963-71.
-
7Fontana L, Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gil A. Sources, isolation, characterisation and evaluation of probiotics. Br J Nutr. 2013; 109 Suppl 2:S35-50.
-
8Golfetto L, Senna FD, Hermes J, Beserra BTS, França FS, Martinello F. Lower bifidobacteria counts in adult patients with celiac disease on a gluten-free diet. Arq Gastroenterol . 2014;51:139-43.
-
9Ivarsson A, Hernell O, Nyström L, Persson LA. Children born in the summer have increased risk for coeliac disease. J Epidemiol Community Health. 2003;57:36-9.
-
10Laparra JM, Sanz Y. Bifidobacteria inhibit the inflammatory response induced by gliadins in intestinal epithelial cells via modifications of toxic peptide generation during digestion. J Cell Biochem. 2010; 109:801-7.
-
11Leahy SC, Higgins DG, Fitzgerald GF, Sinderen DV. Getting better with bifidobacteria. J Appl Microbiol. 2005;98:1303-15.
-
12Medina M, Palma GD, Ribes-Koninckx C, Calabuig M, Sanz Y. Bifidobacterium strains suppress in vitro the pro-inflammatory milieu triggered by the large intestinal microbiota of coeliac patients. J inflamm. 2008;5:1-13.
-
13Mohan R, Koebnick C, Schildt J, Mueller M, Radke M, Blaut M. Effects of Bifidobacterium lactis Bb12 Supplementation on Body Weight, Fecal pH, Acetate, Lactate, Calprotectin, and IgA in Preterm Infants. Pediatr Res. 2008;64:418-22.
-
14.Muñoa FJ, Pares R. Selective medium for isolation and enumeration of Bifidobacterium spp. Appl Environ Microbiol . 1988;54:1715-8.
-
15Nadal I, Donant E, Ribes-Koninckx C, Calabuig M, Sanz Y. Imbalance in the composition of the duodenal microbiota of children with celiac disease. J Med Microbiol. 2007;56:1669-74.
-
16Oliveira MN, Damin MR. [Effect of total solids and sucrose contents on acidity, firmness and viability of yogurt and probiotic bacteria in fermented milk]. Ciênc Tecnol Aliment. 2003;S23:172-6. Available from http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612003000400032
» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0101-20612003000400032 -
17OMG. Organização Mundial de Gastroenterologia. Guias Práticas da OMGE: Probióticos e Prebióticos. 2008. Available from: http://www.serdigital.com.br/gerenciador/clientes/biologicus/arquivos/25.pdf.
» http://www.serdigital.com.br/gerenciador/clientes/biologicus/arquivos/25.pdf. -
18Orban JI, Patterson JA. Modification of the phosphoketolase assay for rapid identification of bifidobactérias. J Microbiol Methods. 2000;40:221-4.
-
19Pérez N, Iannicelli JC, Girard-Bosch C, González S, Varea A, Disalvo L. Effect of probiotic supplementation on immunoglobulins, isoagglutinins and antibody response in children of low socio-economic status. Eur J Nutr. 2010;49:173-9.
-
20Pinto-Sánchez MI, Smecuol EC, Temprano MP, Sugai E, González A, Moreno ML, et al. Bifidobacterium infantis NLS super strain reduces the expression of [alpha]-defensin-5, a marker of innate immunity, in the mucosa of active celiac disease patients. J Clin Gastroenterol. 2016. [Epub ahead of print].
-
21Pokusaeva K, Fitzgerald GF, Sinderen DV. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011;6:285-306.
-
22Saad SMI. Probióticos e prebióticos: o estado da arte. Rev Bras Cienc Saude. 2006;42:1-16. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-93322006000100002&lng=en
» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-93322006000100002&lng=en -
23Sanders ME. Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol . 2011;45:S115-9.
-
24Sanz Y. Novel perspectives in celiac disease therapy. Mini Rev Med Chem. 2009;9:359-67.
-
25Satokari RM, Vaughan EE, Smidt H, Saarela M, Matto J, de Vos WM. Molecular approaches for the detection and identification of bifidobacteria and lactobacilli in the human gastrointestinal tract. Syst Appl Microbiol. 2003;26:572-84.
-
26Shanahan F. Probiotics in perspective. Gastroenterology. 2010;139:1808-12.
-
27Silva TSG, Furlanetto TW. Diagnóstico de doença celíaca em adultos. Rev Assoc Med Bras. 2010;56:122-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302010000100027&lng=en
» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302010000100027&lng=en -
28Smecuol E, Hwang HJ, Sugai E, Corso L, Chernavsky AC, Bellavite FP, et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J Clin Gastroenterol . 2013;47:139-47.
-
29Thitaram SN, Siragusa GR, Hinton AJr. Bifidobacterium-selective isolation and enumeration from chicken caeca by a modified oligosaccharide antibiotic-selective agar medium. Lett Appl Microbiol. 2005;41:355-60.
-
30Van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-46.
-
31Vinderola CG, Reinheimer JA. Enumeration of Lactobacillus casei in the presence of Lactobacillus acidophilus and lactic starter in fermented dairy products. Int Dairy J. 2000;10:271-5.
-
32WHO. World Health Organization Food and Agriculture Organization of the United Nations: Joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk and live lactic acid bacteria. 2001. Available from: ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
» ftp://ftp.fao.org/docrep/fao/009/a0512e/a0512e00.pdf.
-
Disclosure of funding: no funding received
Publication Dates
-
Publication in this collection
23 Feb 2017 -
Date of issue
Apr-Jun 2017
History
-
Received
07 Sept 2016 -
Accepted
19 Jan 2017