ABSTRACT
BACKGROUND
Helicobacter pylori (H. pylori) gastric infection is a main cause of inflammatory changes and gastric cancers.
OBJECTIVE
The aim of this study was finding the effects of curcumin on oxidative stress and histological changes in chronic gastritis associated with H. pylori.
METHODS
In a randomized clinical trial, patients were divided into two groups: a standard triple therapy group and triple therapy with curcumin group. Endoscopic and histological examinations were measured for all patients before and after 8 weeks.
RESULTS
Triple therapy with curcumin treatment group significantly decreased malondialdehyde markers, glutathione peroxides and increased total antioxidant capacity of the gastric mucosa at the end of study compared to baseline and triple regimen groups. In addition, the oxidative damage to DNA was significantly decreased in triple therapy with curcumin group at the end of study compared to baseline and compared to triple therapy (P<0.05 for both). Triple therapy group in combination with Curcumin significantly decreased all active, chronic and endoscopic inflammation scores of patients compared to the baseline and triple therapy group (P<0.05 for both). The eradication rate by triple therapy + curcumin was significantly increased compared to triple therapy alone (P<0.05).
CONCLUSION
Curcumin can be a useful supplement to improve chronic inflammation and prevention of carcinogenic changes in patients with chronic gastritis associated by H. pylori.
HEADINGS:
Gastritis; Helicobacter pylori; Curcumin; Oxidative stress; DNA damage
RESUMO
CONTEXTO
A infecção gástrica pelo Helicobacter pylori (H. pylori) é principal causa de alterações inflamatórias e de câncer gástrico.
OBJETIVO
O objetivo deste estudo foi encontrar os efeitos da cúrcuma no estresse oxidativo e as alterações histológicas na gastrite crônica associada ao H. pylori.
MÉTODOS
Em um estudo randomizado clínico experimental, pacientes foram divididos em dois grupos: um grupo de terapia tríplice padrão e outro com terapia tríplice com e cúrcuma. Exames endoscópicos e histológicos foram analisados para todos os pacientes antes e depois de 8 semanas de tratamento.
RESULTADOS
A terapia tríplice com grupo de tratamento de cúrcuma diminuiu significativamente os marcadores de malondialdeído, de peróxidos de glutationa, com aumento da capacidade antioxidante total da mucosa gástrica ao final do estudo em comparação com grupos de regime basal e tríplice. Além disso, o dano oxidativo ao DNA diminuiu significativamente em terapia tríplice com grupo de cúrcuma no final do estudo em comparação com a linha de base e comparado à terapia tríplice (P<0,05 para ambos). No grupo de terapia tríplice em combinação com cúrcuma houve diminuição significativa de todas os escores ativos de inflamação crônica e endoscópica dos pacientes em relação ao grupo de terapia de base e tríplice (P<0,05 para ambos). A taxa de erradicação por terapia tríplice + cúrcuma aumentou significativamente em relação à terapia tríplice isolada (P<0,05).
CONCLUSÃO
A cúrcuma pode ser um complemento útil para melhorar a inflamação crônica e prevenção de alterações cancerígenas em pacientes com gastrite crônica associada ao H.pylori.
DESCRITORES:
Gastrite; Helicobacter pylori; Curcumina; Estresse oxidative; Dano ao DNA
INTRODUCTION
Gastritis is a gastric mucosa inflammation that is divided into two types: acute inflammation with neutrophils infiltration and chronic inflammation with lymphocytic infiltration and plasma cells, or both1919. Pajares JM, Gisbert JP. Helicobacter pylori: its discovery and relevance for medicine. Rev Esp Enferm Dig. 2006;98:770-85.. One known cause of gastritis is infection with Helicobacter pylori (H. pylori), which is a microaerophilic spiral bacterium2828 27. Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Comparison of Helicobacter pylori infection and gastric mucosal histological features of gastric ulcer patients with chronic gastritis patients. World J Gastroenterol. 2005;11:976-81.. One of the important mechanisms of H. pylori gastritis is the production of reactive oxygen species (ROS) and lipid peroxidation products accumulation, such as malondialdehyde (MDA). These oxidative products feature genotoxic and carcinogenic properties1313. Khanzode SS, Khanzode SD, Dakhale GN. Serum and plasma concentration of oxidant and antioxidants in patients of Helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Letters. 2003;195:27-31.. Several studies have shown that the changes and damages of oxidative deoxyribonucleic acid (DNA) associated with H. pylori gastritis as well as stomach cancer are due to the imbalance between endogenous antioxidants, such as superoxide dismutase (SOD), Glutathione peroxidase (GPx), catalase (CAT), and ascorbic acid and lipid peroxidation product accumulation in the cells77. Choi MA, Kim BS, Yu R. Serum antioxidative vitamin levels and lipid peroxidation in gastric carcinoma patients. Cancer Lett. 1999;136:89-93.,2626 25. Waring AJ, Drake IM, Schorah CJ, White KLM, Axon ATR, Dixon MF. Ascorbic acid and total vitamin C concentration in plasma gastric juice and gastrointestinal mucosa: effects of gastritis and oral supplementation. Gut.1996;38:171-6.. Given the importance of oxidative stress in gastritis associated with H. pylori, one of the todays’ aspects of treatment in this context, is the use of natural antioxidants in these patients1717. Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, Andersen LP, Wadström T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine . 2008;15:391-9.,1818. Liang T, Zhang X, Xue W, Zhao S, Zhang Xand Pei J. Curcumin induced human gastric cancer BGC-823 cells apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway. Int J Mol Sci. 2014 5;15:15754-65.. Curcumin is a hydrophobic polyphenol isolated from Curcuma longa L (rhizomes of the Zingiberaceae family)55. Cai XZ, Huang WY, Qiao Y, Du SY, Chen Y, Chen D, et al. Inhibitory effects of curcumin on gastric cancer cells: a proteomic study of molecular targets. Phytomedicine. 2013;20:495-505.. It has antioxidant, anti-microbial, anti-inflammatory, and anti-carcinogenic effects22. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-18.. The antioxidant properties of curcumin are due to phenolic groups, β-diketone, and methyl that cause some free radicals’ scavenger effects11. Ak T, Gülcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27-37.. Curcumin also has a self-regulatory effect and is associated with the increased expression of nuclear factor erythroid 2 (Nrf2) and the enzymes of phase II, such as SOD, GPx, glutathione reductase (GR), glutathione S-transferases (GST), and CAT1111. García-Niño WR, Pedraza-Chaverrí J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxico. 2014;69:182-201.. Studies have been reported about curcumin inhibitory effects on the induction of apoptosis of gastric cancer cell lines55. Cai XZ, Huang WY, Qiao Y, Du SY, Chen Y, Chen D, et al. Inhibitory effects of curcumin on gastric cancer cells: a proteomic study of molecular targets. Phytomedicine. 2013;20:495-505.. It has also been shown that curcumin has beneficial effects in diabetic gastroparesis due to its antioxidant effects2727 26. Xun L, Li Z, Guo F. Curcumin improves expression of ghrelin through attenuating oxidative stress in gastric tissues of streptozotocin-induced diabetic gastroparesis rats. Eur J Pharmacol. 2013;718:219-25.. Another study showed that curcumin can cause a high rate of H. pylori eradication and gastric rearrangement effects in mice88. De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592-7.. So far, the effects of curcumin on the pathological and oxidative features of gastritis caused by H. pylori have not been determined in humans. Therefore, the objective of this study was to determine the oxidative and anti-inflammatory effects of Curcumin in patients with chronic gastritis.
METHODS
Patient’s selection
This randomized clinical trial was conducted during 2013 and 2014 on patients referred to the gastrointestinal and liver clinics in Ilam city, Iran. All clinical and demographic characteristics of participants were collected via questionnaire, and a medical history and physical examination was completed for each patient. All the goals of this study were described orally to the patients, so they were aware of the study objectives and details. Informed consent was also obtained from all patients. The Ethics Committee of Ilam University of Medical Sciences approved this study, and its Iranian clinical trial registration number was IRCT2016012826238N1. All patients with the following conditions were excluded: the recent use of antibiotics in the past 6 months, pregnancy, lactation, recent surgical procedures, alcohol use, age under 18 and over 70 years, existence of systemic diseases such as cardiovascular diseases, diabetes. All patients with gastritis, confirmed by biopsy, were selected to be a part of the sample.
Study design
To check the patients’ antioxidant and oxidative stress marker levels, 10 mL of blood was taken from the brachial vein. The blood samples were stored in -80°C until the markers were measured. After the patients’ examinations, a biopsy was obtained from each participant. According to the previous studies, during endoscopy biopsy samples of gastric antrum were taken for H. pylori urease culture tests. Stomach antrum and corpus biopsies were also taken for histological examination. In general, if one of the results of the three tests of H. pylori urease culture and histopathological examination was negative, the patient was considered as negative for H. pylori infection. Meanwhile, if all three of the test results were positive, the patient was considered as positive for H. pylori infection. Patients with positive H. pylori cultures were attributed into the treatment group, and patients with negative H. pylori cultures and normal gastric mucosa, after histopathological examinations, were selected for control group. Eligible patients were divided into two groups: a triple therapy group (control) and a triple therapy + curcumin group (treatment). Triple therapy was given as a one-week course of an omeprazole-based triple regimen (omeprazole/20 mg, amoxicillin/1 g, and metronidazole/800 mg, each given orally twice a day (OAM - manufactured by The Government Pharmaceutical Organization, Bangkok, Thailand). In this study, for curcumin administration, we used the Turmeric Tablet (Khaolaor Laboratory; Bangkok, Thailand) (700 mg orally three times a day) for 4 weeks88. De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592-7.. The control group received no surplus drugs, and the drug types and dosages in Curcumin group were not changed during the study. Blood samples (10 mL) were centrifuged for 10 min at 1500 g to prepare the plasma and washed three times with saline and were separated by centrifugation to prepare packed cells. Packed cell lyses were prepared with distilled water in a ratio of 1:10 to measure the markers. Successful H. pylori eradication was defined as a negative 14C-urea breath test result and a negative H. pylori stool antigen test result 4 weeks after discontinuation of therapy2222 21. Sezikli M, Cetinkaya ZA, Sezikli H, Güzelbulut F, Tiftikçi A, Ince AT, et al. Oxidative stress in Helicobacter pylori infection: does supplementation with vitamins C and E increase the eradication rate? Helicobacter. 2009;14:280-5..
Urease test
Biopsy specimens were located in CLOtests (Tri-Med Specialities, Osborne Park, Western Australia), which detect the existence of H. pylori urease. The CLO tests were read twice as 1h and 24h. According to previous studies, successful H. pylori eradication was defined as a negative 14 C-urea breath test result and a negative H. pylori stool antigen test result 4 weeks after end of treatment.
Helicobacter pylori cultivation
Samples of antrum were entered into a transport medium (Portagerm pylori, bioMérieux, Marc l’Etoile, France) for culturing and sent by courier in a cool transport container (Sarstedt, Orsay, France) to a referral laboratory. They were processed based on a previously described protocol33. Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567-98.,44. Borish ET, Cosgrove JP, Church DF, Deutsch WA, Pryor WA. Cigarette causes single-strand breaks in DNA. Biochem Biophys Res Commun. 1095;133:780-6..
Lipid peroxidation and oxidative stress measurement
Total antioxidant status (TAS) was measured via Randox Laboratories Ltd. (Cat. No. NX2332). Based on its protocol the plasma sample volume was 5 µL, in a total assay volume of 305 µL. Color production was measured at 600 nm with a read time of 5 min. Superoxide dismutase (SOD) was measured using Randox Laboratories Ltd. (Cat. No. SD125) using an appropriate whole blood SOD control (Cat. No. SD126). The reaction was measured at 500 nm, using 5 µL of sample in a total reaction volume of 230 µL. Glutathione peroxidase (GPx) was measured using its relevant kit supplied by Randox Laboratories Ltd. (Cat. No. RS505) and GPx activity was measured at 340 nm, using a sample volume of 5 µL in a total reaction volume of 285 µL. Glutathione reductase (GR) was measured via Randox Laboratories Ltd. (Cat. No. GR2368). Any decrease in absorbance was measured at 340 nm. Malondialdehyde (MDA) was assayed as a marker of lipid peroxidation using a colorimetric reaction which uses 1-methyl-2-phenylindole as chromogen.
DNA oxidative damage assessment
For measuring the amount of damages of DNA of the gastric cells: firstly, gastric tissue samples were completely homogenized with 1.5 mL of potassium phosphate buffer. The buffer contained 10 mmol of potassium phosphate and 30 mmol of potassium chloride. Oxidative damage of DNA in gastric biopsies was measured with the Kimiya Biomedical kit, which is a colorimetric method, and the ability to measure the AP from 1 to 40 was 1 × 105 BP.
Endoscopic assessment
Standard monitoring of vital signs was performed for all patients before and after eight weeks of treatment by a gastroenterologist. Macroscopic changes were observed via endoscopy according to the “modified Lanza standard” as follows: 0 = no erosion; 1 = one or two erosive lesions limited to one area, such as the antrum, corpus, or fundus; 3 = three to five lesions but in the same area; and 4 = extensive or more than 10 lesions. The first step in this system is identifying H. pylori gastritis from negative bacteria samples.
Histopathologic assessment
The specimens taken from antrum were used to study the histological effects of rebamipide on inflammation in the gastric mucosa. Hematoxylin-Eosin (H&E) staining was performed for the H. pylori diagnosis and histological findings. To confirm the presence of organism, Giemsa stain was done. Rods and curved Gram-negative organisms were seen in the mucosal surface in the pit of stomach with a ×40 zoom of a light microscope. The report of at least five bacilli per microscopic field was necessary. The infiltration of lymphocytes and plasma cells in the lamina propria was a sign of chronic gastritis, and neutrophil infiltration was a sign of active gastritis symptoms. The presence of goblet cells and the replacement of mucosal cylindrical cells with absorption cells were a sign of intestinal metaplasia. The evaluation of acute and chronic inflammation was based on the histopathologic findings as follows: 0 = normal, 1 = mild, 2 = moderate, 3 = marked, according to the updated Sydney system2020 Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-33..
Statistical analysis
The statistical analysis was performed using SPSS software version 16 and patients’ characteristics were analyzed via tables of frequencies. Based on within (before- after) or between groups comparisons, paired or unpaired t-test was used. If the frequency numbers of certain variables were less than 5, the results were reported upon the Fisher’s exact test. For comparison of categorical variables, chi-square was used. The differences were considered as significant at P≤0.05.
RESULTS
Basic information of patients
The demographic and clinical characteristics of the studied participants are shown in Table 1. The triple therapy group (control group) included 24 (48%) male patients and 26 (52%) female patients. The triple therapy plus curcumin group (treatment group) included 28 (56%) male patients and 22 (44%) female patients. No significant difference was found in terms of gender between the two groups (P=0.076). In addition, no significant difference in the mean age was found between the control (53.65±15.65 years) and treatment groups (54.65±16.54 years, P=0.687). The mean ± standard deviation (SD), body mass index (BMI) of the control group was 24.53±3.53 kg/m2, and for the treatment group was 23.54±5.43 kg/m2 (P=0.141). We checked the patient-related risk factors for gastrointestinal diseases. The results showed that five patients in the control group (10%) and four (8%) patients in the treatment group had histories of alcohol usage (P=0.234). In addition, 13 (26%) patients in the control group and 10 (20%) patients in the treatment group had histories of NASID drug usage (P=0.365). Twelve patients (24%) in the control group and 13 (26%) patients in the treatment group reported a history of smoking, but no significant difference was found between the two groups in this regard (P=0.270).
Effect of Curcumin on MDA level
The mean ± SD of MDA levels in the two groups is shown by Figure1A. The mean ± SD of MDA levels in the triple therapy group (control) at the beginning and end of the study were 2.51±0.53 µmol/L and 2.47±0.64 µmol/L respectively, which were not significantly different (P>0.05). The mean ±SD of MDA level of gastric mucosa in patients with triple therapy plus Curcumin group (treatment) at the baseline was 2.45±0.47 μmol/L, and at the end of the study decreased to 2.31±0.33 μmol/L. The figure shows that the MDA level in the triple therapy with Curcumin group was significantly decreased at the end of the study compared to the baseline (P<0.05), and it was reduced compared to the triple therapy regimen alone.
Effect of Curcumin on GPx level
The mean ± SD of GPx in both groups is shown by Figure 1B. The mean ±SD of GPx level showed a significant increase in the triple therapy group at the end of the study (56.02±4.53 U/g HGB) compared to the baseline (38.40±6.54 U/g HGB) (P<0.05). The results showed a significant reduction in the mean ±SD of GPx + curcumin level in the triple therapy group at the end of the study (45.61±5.64 U/g HGB) compared to the baseline (52.69±4.89 U/g HGB), as well as in the mean ±SD of GPx level in the triple therapy group at the end of the study (P<0.05 for both).
Effect of Curcumin on total antioxidant capacity (TAC) level
The mean and standard deviation of the TAC level was shown by Figure 1C. As the figure shows, the mean ±SD of TAC level at the end of study was not decreased in the triple therapy group (2.22±60 µmol/L) compared to the baseline (2.33±0.53 µmol/L) (P>0.05).
Effect of Curcumin on oxidative DNA damage
The effects of curcumin and triple therapy on oxidative DNA damage was shown by Figure 1D. The results showed that the level of DNA oxidative damage was significantly increased in patients treated with triple therapy in the study compared to the baseline (P<0.05). In patients treated with triple therapy plus curcumin, the level of oxidative DNA damage was significantly decreased at the end of the study compared with the baseline (P<0.05). The findings also showed that the quantity of DNA oxidative damage in patients treated with triple therapy plus curcumin was significantly reduced at the end of the study compared to the group treated with triple therapy (P<0.05).
Effect of triple therapy or triple therapy with Curcumin treatment on gastric mucosa of malondialdehyde (MDA) (µmol/L, figure A) or Glutathione peroxidase (GPx) (U/g HGB, figure B), TCA level (U/g HGB, figure C) and DNA oxidative damege (µmol/L, figure D) in patients with chronic gastritis associated with H. pylori. (*P<0.05) level at end of study compared with at beginning of study in triple therapy group. (**P<0.05) GPx level at end of study compared with beginning of study in triple therapy with Curcumin group (***P<0.05) level at end level in triple therapy with Curcumin group compared with triple therapy group.
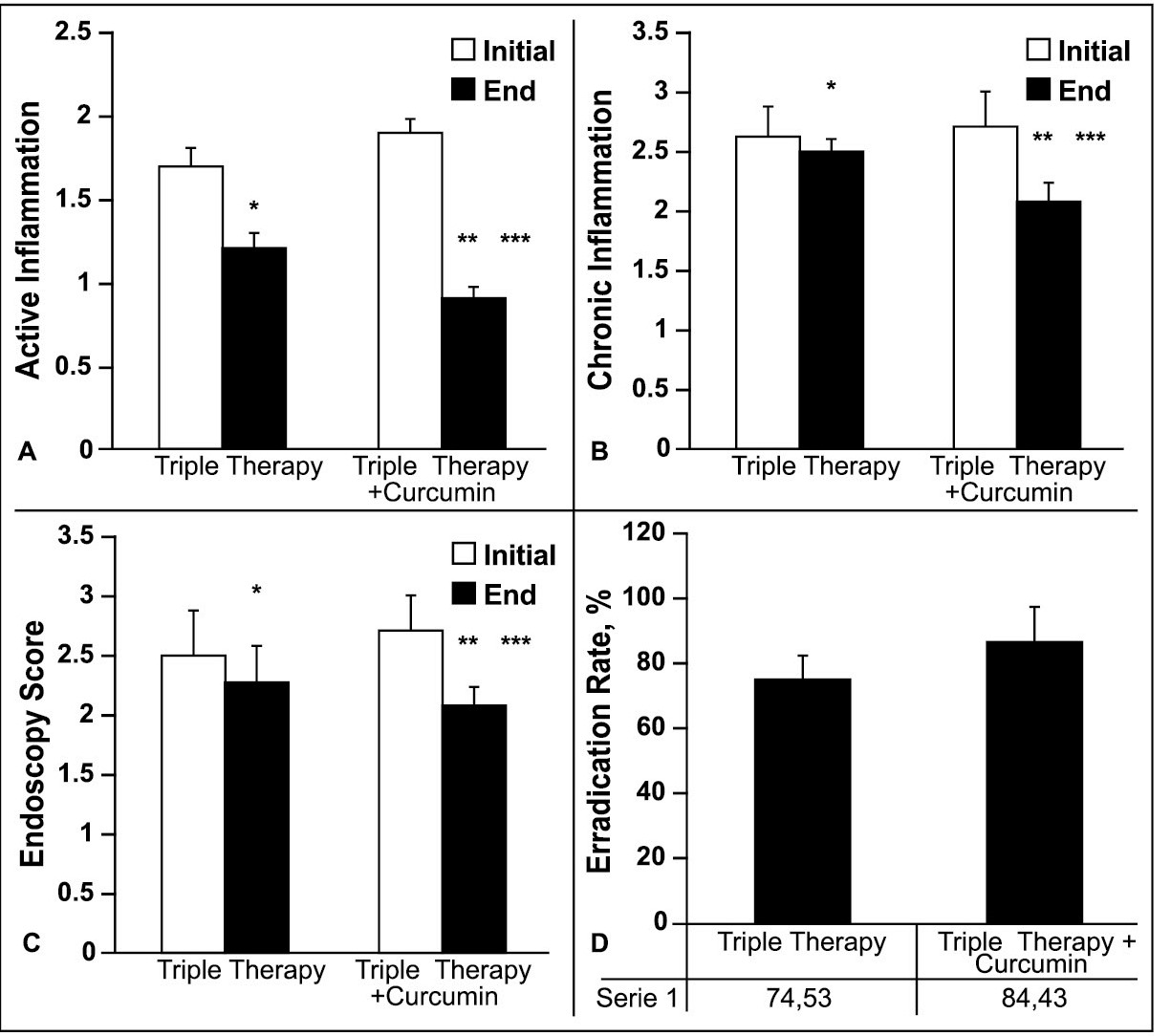
Effect of Curcumin on gastric mucosal according to endoscopic and histological assessment
The effects of triple therapy + curcumin on active inflammation of gastric mucosa based on the histological assessment was shown by Figure 2A and the study showed that combination therapy of triple therapy + curcumin decreased the active inflammation of gastric mucosa compared to triple therapy regimen significantly (P<0.05). The mean inflammation activity of gastric mucosa, at the end of study compared to baseline figure, was also decreased in triple therapy + curcumin group significantly (P<0.05). A similar finding was revealed for the chronic inflammation of gastric mucosa index.
The mean ±SD of chronic inflammation score according to endoscopic findings was shown by Figure 2B. The inflammation score for patients treated with triple therapy was reduced at the end of the study (2.3±0.30) compared to the baseline (2.5±0.44), but this reduction was not significant (P>0.05). For the combination treatment of triple therapy plus Curcumin, the mean ±SD of chronic inflammation score was significantly decreased at the end of the study (2.1±0.27) compared to the baseline (2.7±0.22) (P<0.05). The results of the study showed that the chronic inflammation score was significantly reduced in patients treated by the combination of triple therapy plus Curcumin compared to the triple therapy alone (P<0.05). Based on the findings of endoscopic evaluation of gastric mucosa, the triple therapy regimen could decrease the mean ±SD of gastric endoscopic marker, at the end of study (2.3±0.3) compared to the start of study (2.5±0.4) but insignificantly (P>0.05). While, the triple therapy + curcumin regimen could decrease either the gastric endoscopic marker at the end of study (2.1±0.27) compared to the start of study (2.7±0.22) significantly (P<0.05) or compared to the triple therapy regimen alone (P<0.05).
The eradication rate of H. pylori infection at the end of study was compared between treatment and control groups by Figure 2D. As the figure shows, the eradication rate by the triple therapy + curcumin regimen (86.4%) was significantly higher than the triple therapy regimen alone (74.5%) (P<0.05).
Effect of triple therapy or triple therapy + Curcumin treatment on activity inflammation (figure A) or chronic Inflammation score (figure B) of gastric mucosal, endoscopy score (figure C) and eradication rate (figure D) of H. pylori infection in patients with chronic gastritis associated with H. pylori. *P<0.05 in end of study compared with initial of study in triple therapy group. **P<0.05 end level compared with initial of study in triple therapy + Curcumin group. ***P<0.05 end of study in triple therapy + Curcumin group compared with end of study in triple therapy group.
DISCUSSION
The aim of this study was to investigate the effects of curcumin on oxidative stress marker levels and gastritis histopathologic features in patients with chronic gastric associated with H. pylori infection44. Borish ET, Cosgrove JP, Church DF, Deutsch WA, Pryor WA. Cigarette causes single-strand breaks in DNA. Biochem Biophys Res Commun. 1095;133:780-6.,1616. Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Letters. 2008;269;199-225.,2424 23. Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis at the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591-8.. Antioxidants can decrease the risk of H. pylori infections and gastric cancer by inhibiting oxidative stress and DNA damage33. Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567-98.. The results showed that curcumin can increase TAC and reduce GPx levels2727 26. Xun L, Li Z, Guo F. Curcumin improves expression of ghrelin through attenuating oxidative stress in gastric tissues of streptozotocin-induced diabetic gastroparesis rats. Eur J Pharmacol. 2013;718:219-25.. Curcumin also reduced the level of MDA associated with lipid peroxidation markers. Curcumin antioxidant effect has not been investigated in chronic gastritis associated with H. pylori in humans heretofore, and this study is the first report in this regard. However, the study of Lu Xu and colleagues showed that curcumin adminstration in an in vitro diabetic gastroparesis rat model could improve oxidative stress, improve glutation resuscitation (GSH), and reduce the levels of MDA2727 26. Xun L, Li Z, Guo F. Curcumin improves expression of ghrelin through attenuating oxidative stress in gastric tissues of streptozotocin-induced diabetic gastroparesis rats. Eur J Pharmacol. 2013;718:219-25.. We studied the effects of curcumin on the gastric histological and endoscpic characteristics of chronic gastritis patients. The results showed that curcumin application, after two months, could significantly improve the patients’ histological characteristics by decreasing the active and chronic inflammation properties compared with the control group. No study has been done on the effects of curcumin on the histologic characteristics of chronic gastritis associated with H. pylori. However, Di Mario and colleagues demonstrated that curcumin can improve dyspepsia symptoms and also can reduce the serologic markers associated with the inflammation of gastritis33. Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567-98.. Santos and colleagues also studied chronic gastritis caused by H. pylori in rats. They showed that curcumin can improve chronic inflammation caused by H. pylori via the inhibition of the gene expression of inflammatory markers1010. Everett SM, Drake IM, White KL, Mapstone NP, Chalmers DM, Schorah CJ, Axon AT. Antioxidant vitamin supplements do not reduce reactive oxygen species activity in Helicobacter pylori gastritis in the short term. Br J Nutr. 2002;87:3-11.. We have also shown that curcumin can improve the oxidative DNA damage of the gastric mucosa. These findings constitute the first report of the beneficial effects of curcumin on the gastric mucosa in patients with chronic gastritis. Previous studies have shown that in H. pylori gastritis patients, accumulations of ROS and MDA can be increased in DNA oxidative damage and this change makes DNA content prone to mutations and carcinogenesis44. Borish ET, Cosgrove JP, Church DF, Deutsch WA, Pryor WA. Cigarette causes single-strand breaks in DNA. Biochem Biophys Res Commun. 1095;133:780-6.. This study showed that curcumin could significantly reduce the MDA content and the amount of DNA oxidative damage of the gastric mucosa. As it was mentioned, antioxidant substances by reducing the cellular and histological oxidative stress can inhibit the changes of cellular DNA content and by inhibition of oxidative changes of DNA can prevent the cellular and molecular carcinogenic reactions33. Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567-98.. Therefore, based on findings of the current study can assume that curcumin has a beneficial antioxidant effect on gastric mucosa. In addition, curcumin can have anti-carcinogenic effects through the inhibition of the growth factor and the down regulation of the nuclear factor (NF-ƙB)99. Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, Maino M, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12:238-43.,2121 20. Santos AM, Lopes T, Oleastro M, Gato IV, Floch P, Benejat L, Chaves P, et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients. 2015;7:306-20.. So far, several studies have been done on the protective effects of gastric mucosa1515. Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol. 2010;10:815-8.. Although most of these studies were conducted as in vitro studies, clinical trial studies were not completely reviewed. Many studies investigated the anti-inflammatory and antioxidant effects of curcumin in a variety of conditions1818. Liang T, Zhang X, Xue W, Zhao S, Zhang Xand Pei J. Curcumin induced human gastric cancer BGC-823 cells apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway. Int J Mol Sci. 2014 5;15:15754-65.. For example, it was reported that curcumin may be useful for treating gastric injuries by gastric peroxide inhibition, ROS, leukocyte infiltration inhibition, and reduction of the cell adhesion molecule level (ICAM-1) and tumor necrosis factor alpha (TNF-a)2323 22. Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N. Curcumin Attenuates Gastric Cancer Induced by N -Methyl- N -Nitrosourea and Saturated Sodium Chloride in Rats. J Biomed Biotechnol. 2012;91:53-80.. Another study about NSAID-induced gastric damage showed that curcumin can increase catalase and superoxide as well as nitric oxide levels in the gastric mucosa66. Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397-408.,2525 24. Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World J. Gastroenterology. 2012;18:1479-84.. The beneficial effects of curcumin on gastritis can be created by the inhibition of gastric acid production due to inhibition of histamine receptors1414. Kim DC, Kim SH, Choi BH, Baek NI, Kim D, Kim MJ, et al. Curcuma longa Extract Protects against Gastric Ulcers by Blocking H2 Histamine Receptors. Biol. Pharm. Bull. 2005;28:2220-4.. Another strength point of this study was comparison of the H. pylori eradication rates between triple therapy regimen plus curcumin and triple therapy regimen. The results showed that the eradication rate of H. pylori was significantly higher by the regimen compromised curcumin and this property can help to consider an anti-carcinogenic property for curcumin, so that previous studies have shown the H. pylori infection as a potential factor associated with precancerous or cancerous lesions of gastric mucosa1212. Jung DH, Kim JH, Lee YC, Lee SK, Shin SK, Park JC, et al. Helicobacter pylori Eradication Reduces the Metachronous Recurrence of Gastric Neoplasms by Attenuating the Precancerous Process. J Gastric Cancer. 2015;15:246-55.. It is the first time that a higher eradication rate and antibacterial effects of curcumin for H. pylori infection was reported by this study via a clinical trial study assessment. As mentioned earlier, most studies on the effects of curcumin on gastritis were in vitro and there is a lack of comprehensive studies on the inhibitory effects of curcumin on chronic inflammation in human via endoscopy and histology investigations. In this study, curcumin was administered for 2 months with a standard triple drug regimen, and the macroscopic and microscopic histological characters of gastric mucosa damage were evaluated. DNA oxidative damage was also examined. The methods and results of this study showed that curcumin plus standard triple therapy can be a perfect complement for patients with chronic gastritis caused by H. pylori. In this study we did not evaluate the genes expression associated with gastric cancer and the anti-carcinogenic properties of curcumin in a human study. Also, we did not consider the effect of curcumin on the levels of other markers related to gastric injury, such as the vein endothelial growth factor (VEGF) or matrix metalloproteinase (MMP). It is hoped that these issues will be considered in future studies. As a conclusion, this study via a clinical trial method, revealed that curcumin has an antioxidant effect on oxidative stress markers, an antibacterial effect on H. pylori infection and a higher eradication rate for this infection, an anti-carcinogenic effect via inhibition of oxidative damage of cellular DNA and finally an improving effect on gastric mucosal damages. Overall, in this study, and according to previous reports on the protective effects of curcumin on the stomach and on human models, it can be concluded that curcumin, as a completely natural substance, can be a suitable and safe treatment for patients. Clinical application can be considered in the future after the completion of clinical trials.
ACKNOWLEDGEMENTS
The School of Medicine, Ilam University of Medical Science, supported this study.
REFRENCES
-
1Ak T, Gülcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27-37.
-
2Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807-18.
-
3Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567-98.
-
4Borish ET, Cosgrove JP, Church DF, Deutsch WA, Pryor WA. Cigarette causes single-strand breaks in DNA. Biochem Biophys Res Commun. 1095;133:780-6.
-
5Cai XZ, Huang WY, Qiao Y, Du SY, Chen Y, Chen D, et al. Inhibitory effects of curcumin on gastric cancer cells: a proteomic study of molecular targets. Phytomedicine. 2013;20:495-505.
-
6Chattopadhyay I, Bandyopadhyay U, Biswas K, Maity P, Banerjee RK. Indomethacin inactivates gastric peroxidase to induce reactive-oxygen-mediated gastric mucosal injury and curcumin protects it by preventing peroxidase inactivation and scavenging reactive oxygen. Free Radic Biol Med. 2006;40:1397-408.
-
7Choi MA, Kim BS, Yu R. Serum antioxidative vitamin levels and lipid peroxidation in gastric carcinoma patients. Cancer Lett. 1999;136:89-93.
-
8De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB, Mukhopadhyay AK. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother. 2009;53:1592-7.
-
9Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, Maino M, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter. 2007;12:238-43.
-
10Everett SM, Drake IM, White KL, Mapstone NP, Chalmers DM, Schorah CJ, Axon AT. Antioxidant vitamin supplements do not reduce reactive oxygen species activity in Helicobacter pylori gastritis in the short term. Br J Nutr. 2002;87:3-11.
-
11García-Niño WR, Pedraza-Chaverrí J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem Toxico. 2014;69:182-201.
-
12Jung DH, Kim JH, Lee YC, Lee SK, Shin SK, Park JC, et al. Helicobacter pylori Eradication Reduces the Metachronous Recurrence of Gastric Neoplasms by Attenuating the Precancerous Process. J Gastric Cancer. 2015;15:246-55.
-
13Khanzode SS, Khanzode SD, Dakhale GN. Serum and plasma concentration of oxidant and antioxidants in patients of Helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Letters. 2003;195:27-31.
-
14Kim DC, Kim SH, Choi BH, Baek NI, Kim D, Kim MJ, et al. Curcuma longa Extract Protects against Gastric Ulcers by Blocking H2 Histamine Receptors. Biol. Pharm. Bull. 2005;28:2220-4.
-
15Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol. 2010;10:815-8.
-
16Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Letters. 2008;269;199-225.
-
17Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, Andersen LP, Wadström T. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine . 2008;15:391-9.
-
18Liang T, Zhang X, Xue W, Zhao S, Zhang Xand Pei J. Curcumin induced human gastric cancer BGC-823 cells apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway. Int J Mol Sci. 2014 5;15:15754-65.
-
19Pajares JM, Gisbert JP. Helicobacter pylori: its discovery and relevance for medicine. Rev Esp Enferm Dig. 2006;98:770-85.
-
20Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-33.
-
2120. Santos AM, Lopes T, Oleastro M, Gato IV, Floch P, Benejat L, Chaves P, et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients. 2015;7:306-20.
-
2221. Sezikli M, Cetinkaya ZA, Sezikli H, Güzelbulut F, Tiftikçi A, Ince AT, et al. Oxidative stress in Helicobacter pylori infection: does supplementation with vitamins C and E increase the eradication rate? Helicobacter. 2009;14:280-5.
-
2322. Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N. Curcumin Attenuates Gastric Cancer Induced by N -Methyl- N -Nitrosourea and Saturated Sodium Chloride in Rats. J Biomed Biotechnol. 2012;91:53-80.
-
2423. Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis at the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591-8.
-
2524. Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World J. Gastroenterology. 2012;18:1479-84.
-
2625. Waring AJ, Drake IM, Schorah CJ, White KLM, Axon ATR, Dixon MF. Ascorbic acid and total vitamin C concentration in plasma gastric juice and gastrointestinal mucosa: effects of gastritis and oral supplementation. Gut.1996;38:171-6.
-
2726. Xun L, Li Z, Guo F. Curcumin improves expression of ghrelin through attenuating oxidative stress in gastric tissues of streptozotocin-induced diabetic gastroparesis rats. Eur J Pharmacol. 2013;718:219-25.
-
2827. Zhang C, Yamada N, Wu YL, Wen M, Matsuhisa T, Matsukura N. Comparison of Helicobacter pylori infection and gastric mucosal histological features of gastric ulcer patients with chronic gastritis patients. World J Gastroenterol. 2005;11:976-81.
-
Disclosure of funding: no funding received
Publication Dates
-
Publication in this collection
08 May 2017 -
Date of issue
July-Sept 2017
History
-
Received
01 Aug 2016 -
Accepted
15 Feb 2017