ABSTRACT
BACKGROUND:
There is increasing evidence to show that TNF-α -308G>A polymorphism may be a risk factor for celiac disease, but the results are inconsistent.
OBJECTIVE:
Thus, we aimed to perform a meta-analysis involving published studies up to January 2019 to elucidate the association.
METHODS:
To assess the effect of TNF-α -308G>A polymorphism on celiac disease susceptibility, we searched PubMed, ISI Web of Knowledge, Chinese National Knowledge Infrastructure (CNKI) databases to identify eligible studies, without restriction. Summary odds ratios (ORs) and 95% confidence intervals (CIs) were used to evaluate the susceptibility to celiac disease.
RESULTS:
A total of 11 studies with 1147 cases and 1774 controls were selected for this meta-analysis. The pooled results indicated that TNF-α -308G>A polymorphism was associated with increased risk of celiac disease (A vs G: OR=2.077, 95% CI=1.468-2.939, P=≤0.001; AA vs GG: OR=8.512, 95% CI=3.740-19.373, P=≤0.001; AA+AG vs GG: OR=1.869, 95% CI=1.161-3.008, P=0.010; and AA+AG vs GG: OR=4.773, 95% CI=3.181-7.162, P≤0.001). Subgroup analysis by ethnicity also revealed significant association in Caucasians. In addition, there was a significant association between TNF-α -308G>A polymorphism and celiac disease risk in Italy, Spain and PCR-FRLP group studies.
CONCLUSION:
Our meta-analysis suggests that the TNF-α -308G>A polymorphism plays an important role in celiac disease susceptibility. However, our results are still needed to strengthen by further studies in different ethnicities and larger sample sizes.
HEADINGS:
Celiac disease; Genetic polymorphism; European Continental ancestry group; Meta-analysis
RESUMO
CONTEXTO:
Há evidências crescentes para mostrar que o TNF-α-308G>A polimorfismo pode ser um fator de risco para a doença celíaca, mas os resultados são inconsistentes.
OBJETIVO:
Por isto objetivou-se realizar uma meta-análise envolvendo estudos publicados até janeiro de 2019 para elucidar esta associação.
MÉTODOS:
Para avaliar o efeito do TNF-α-308G>A polimorfismo na suscetibilidade da doença celíaca, pesquisou-se os bancos de dados do PubMed, ISI Web of Knowledge e Chinese National Knowledge Infrastructure (CNKI) para identificar estudos elegíveis, sem restrições. Para avaliar a suscetibilidade à doença celíaca, foram utilizadas os odds ratio sumários (ORs) e os intervalos de confiança de 95% (ICs).
RESULTADOS:
Um total de 11 estudos com 1147 casos e 1774 controles foram selecionados para esta meta-análise. Os resultados agrupados indicaram que o TNF-α-308G>A polimorfismo associou-se ao aumento do risco de doença celíaca (A vs G: OR=2,077; 95% IC=1,468-2,939; P=≤0,001; AA vs GG: OR=8,512; 95% IC=3,740-19,373; P=≤0,001; AA+AG vs GG: OR=1,869; 95% IC=1,161-3,008; P=0,010; e AA+AG vs GG: OR=4,773; 95% IC=3,181-7,162; P≤0,001). A análise de subgrupos por etnia também revelou associação significativa em caucasianos. Além, havia uma associação significativa entre o TNF-α-308G>A um polimorfismo e o risco do doença celíaca na Italia, na Espanha e em estudos do grupo do PCR-FRLP.
CONCLUSÃO:
Nossa meta-análise sugere que o TNF-α-308G>A polimorfismo desempenha um papel importante na suscetibilidade da doença celíaca. No entanto, nossos resultados necessitam de mais dados e de serem fortalecidos por outros estudos em diferentes etnias e tamanhos amostrais maiores.
DESCRITORES:
Doença celíaca; Polimorfismo genético; Grupo com ancestrais do continente europeu; Metanálise
INTRODUCTION
Coeliac disease (CD), also known as celiac sprue, is an autoimmune and chronic small intestinal disorder which causing malnutrition and severe complications in adults and children11. Zupin L, Polesello V, Catamo E, Crovella S, Segat L. Interleukin-10 gene promoter polymorphisms in celiac patients from north-eastern Italy. Hum Immunol. 2014;75:656-61.,22. Hermann C, Krikovszky D, Vásárhelyi B, Dezsofi A, Madácsy L. Polymorphisms of the TNF-alpha gene and risk of celiac disease in T1DM children. Pediatr Diabetes. 2007;8:138-41.. CD is induced by dietary protein gluten in genetically predisposed individuals. It is considered a public health problem affecting about 1% of general population in Europe with some regional variations and a higher rate in female gender33. Dieli-Crimi R, Cénit MC, Núñez C. The genetics of celiac disease: A comprehensive review of clinical implications. J Autoimmun. 2015;64:26-41.. Lifelong gluten-free diet is the only known effective treatment of CD, which leads to recovery of the intestinal mucosa, improves symptoms and reduces risk of developing complications in most individuals44. Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412-20..
The etiology of CD is still not completely understood55. Khan S, Mandal RK, Jawed A, Dar SA, Wahid M, Panda AK, et al. TNF-α -308 G > A (rs1800629) Polymorphism is Associated with Celiac Disease: A Meta-analysis of 11 Case-Control Studies. Sci Rep. 2016;6:32677.. CD has a strong heritable component and the causative genetic component being polygenic and interacting with environmental factors22. Hermann C, Krikovszky D, Vásárhelyi B, Dezsofi A, Madácsy L. Polymorphisms of the TNF-alpha gene and risk of celiac disease in T1DM children. Pediatr Diabetes. 2007;8:138-41.,66. Capilla A, Donat E, Planelles D, Espinós C, Ribes-Koninckx C, Palau F. Genetic analyses of celiac disease in a Spanish population confirm association with CELIAC3 but not with CELIAC4. Tissue Antigens. 2007;70:324-9.. Recent advances in genomic research and high-throughput genome-wide technologies have increased our understanding of potential genetic risk factors in CD. The HLA-DQ2 and HLA-DQ8 gene polymorphisms are among the most polymorphic loci currently described in pathogenesis of CD (40%)77. Huang S-Q, Zhang N, Zhou Z-X, Huang CC, Zeng CL, Xiao D, et al. Association of LPP and TAGAP Polymorphisms with Celiac Disease Risk: A Meta-Analysis. Int J Environ Res Public Health. 2017;14(2).. This suggests that several susceptibility loci to CD are outside or close the HLA region, which should be evaluated as a disease marker. Furthermore, TNF-α is also thought to play an important role in the mechanisms of CD development88. de la Concha EG, Fernández-Arquero M, Vigil P, Rubio A, Maluenda C, Polanco I, et al. Celiac disease and TNF promoter polymorphisms. Hum Immunol. 2000;61:513-7.,99. Rossi E, Basso D, Zambon CF, Navaglia F, Greco E, Pelloso M, et al. TNFA Haplotype Genetic Testing Improves HLA in Estimating the Risk of Celiac Disease in Children. PLoS One. 2015;10:e0123244..
TNF-α is a potential pro-inflammatory cytokine, which plays a critical role in a wide range of inflammatory, malignancies, and autoimmune diseases1010. Lis K, Kuzawińska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors - state of knowledge. Arch Med Sci. 2014;10:1175-85.,1111. Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H. A survey on the role of interleukin-10 in breast cancer: A narrative. Reports Biochem Mol Biol. 2017;7(1).. The TNF-α gene is located on chromosome 6p21.3, contains 8 exons, and encodes 382 amino acids1212. Azarpira MR, Ghilian MM, Sobhan MR, Mehdinezhad-Yazdi M, Aghili K, Miresmaeili SM4, Neamatzadeh H. Association of MTHFR and TNF-α genes polymorphisms with susceptibility to Legg-Calve-Perthes disease in Iranian children: a case-control study. J Orthop. 2018;15:984-7.,1313. Aslebahar F, Neamatzadeh H, Meibodi B, Karimi-Zarchi M, Tabatabaei RS, Noori-Shadkam M, et al. Association of Tumor Necrosis Factor-α (TNF-α) -308G>A and -238G>A Polymorphisms with Recurrent Pregnancy Loss Risk: A Meta-Analysis. Int J Fertil Steril. 2019;12:284-92.. The human TNF-α gene has close linkage with HLA class I and class II88. de la Concha EG, Fernández-Arquero M, Vigil P, Rubio A, Maluenda C, Polanco I, et al. Celiac disease and TNF promoter polymorphisms. Hum Immunol. 2000;61:513-7.. There are several polymorphisms in the upstream proximal promoter of TNF-α that may have an effect on its production and bioactivity1313. Aslebahar F, Neamatzadeh H, Meibodi B, Karimi-Zarchi M, Tabatabaei RS, Noori-Shadkam M, et al. Association of Tumor Necrosis Factor-α (TNF-α) -308G>A and -238G>A Polymorphisms with Recurrent Pregnancy Loss Risk: A Meta-Analysis. Int J Fertil Steril. 2019;12:284-92.. TNF-α genetic variation has been studied in the context of CD. Nevertheless, their results were inconsistent and conflicting. Therefore, we performed a systematic review and meta-analysis to further estimate the association of TNF-α -308G>A polymorphism with susceptibility to the CD.
METHODS
Search strategy
A comprehensive and systematic computer searches were performed in PubMed, Google Scholar, Web of Science, EMBASE, Google Scholar, Wanfang, China National Knowledge Infrastructure (CNKI), Islamic World Science Citation Center (ISC), and Scientific Information Database (SID) databases up to December 10, 2018 for all studies related to the association between the TNF-α -308G>A polymorphism and celiac disease. Combinations of the following keywords and terms were used in the search: (“Coeliac disease” OR “coeliac” OR “gluten-sensitive enteropathy”) and (“Tumor Necrosis Factor-alpha” OR “TNF-α” OR “Cachexin” OR “Cachectin”) and (“TNF -308G>A” OR “rs1800629”). There was no language restriction on the literature search. Additionally, reference lists of the eligible studies, reviews and previous meta-analysis articles were also manually reviewed to identify potentially relevant studies.
Eligibility criteria
The inclusion criteria of studies in our meta-analysis were as follows: 1) a case-control and/or cohort studies with matching control subjects; 2) studies that evaluated the association between the TNF-α -308G>A polymorphism and celiac disease risk; 3) reported available genotype frequencies dates for both case and control groups; and 4) studies with sufficient info as well as genetic distribution to estimate the odds ratio (OR) with 95% confidence interval (CI). Accordingly, the following exclusion criteria were also used: 1) case only studies or no control group; 2) family based and or linkage studies; 3) no sufficient data reported; 4) case reports, abstracts, comments, letter to editors, reviews, posters; and 5) duplicated and or overlapped data with previous studies.
Data extraction
Two authors independently and carefully extracted the data from eligible studies in accordance with inclusion criteria. For each of included eligible studies the following data were collected: first authors, year of publication, country of origin, ethnicity (Caucasian, Asian, African and Mixed), source of healthy controls (hospital-based studies and population-based studies), number of cases and controls, the numbers of cases and controls for each genotype, Hardy-Weinberg equilibrium (HWE) in controls, and minor allele frequency (MAF). Any disagreement of the included studies and data was resolved by discussion among the authors and if a conflicting evaluation still existed another author was consulted to resolve the dispute.
Statistical analyses
The associations between the TNF-α -308G>A polymorphism and celiac disease risk were measured by odds ratios (OR) with 95% confidence intervals (CI). The Z-test was used to assess the significance of the pooled OR, in which P<0.05 was considered as statistically significant. Five genetic models, i.e., allele (A vs G), homozygote (AA vs GG), heterozygote (AG vs GG), dominant (AA+AG vs GG) and recessive (AA vs AG+GG) were utilized for pooling the ORs. Between-study heterogeneity was analyzed by a chi-squared-based Q-statistic test, in which the P-value <0.05 was considered significant. In addition, the I2 value was used to assess the degree of between-study heterogeneity (I2<25%, no heterogeneity; I2 25%-50%, moderate heterogeneity; I2>50%, large or extreme heterogeneity). Therefore, to test the reliability of the pooled data, the Mantel-Haenszel method (fixed-effects, if P>0.05 or I2<50%) and the DerSimonian-Laird method (random-effects, if P<0.05 or I2>50%) were applied to estimate the pooled ORs, respectively. Hardy-Weinberg equilibrium (HWE) was calculated for control groups by Fisher exact test., and P<0.05 was considered as a departure from HWE. Subgroup analysis was carried out based on ethnicity, source of controls, genotyping methods, and HWE status. The results stability and reliability was evaluated using sensitivity analysis, in which one study was deleted each time and the analyses were repeated. In addition, sensitivity analysis was performed buy excluding those studies departure from HWE. Begg’s funnel plot and Egger’s linear regression asymmetry test were used to test the publication bias; P<0.05 indicated that the result was statistically significant. All analyses were performed with the Comprehensive Meta-Analysis (CMA) 2.0 software (Biostat, USA). Two-sided P-values <0.05 were considered statistically significant.
RESULTS
Study characteristics
As shown in Figure 1, 108 published studies were identified on the basis of the criteria, of which 43 irrelevant publications were initially excluded. Then, ten studies were excluded because were review, case report, meta-analysis, and focused on other polymorphism rather than -308G>A polymorphism were discarded. Finally, a total number of eleven studies22. Hermann C, Krikovszky D, Vásárhelyi B, Dezsofi A, Madácsy L. Polymorphisms of the TNF-alpha gene and risk of celiac disease in T1DM children. Pediatr Diabetes. 2007;8:138-41.,66. Capilla A, Donat E, Planelles D, Espinós C, Ribes-Koninckx C, Palau F. Genetic analyses of celiac disease in a Spanish population confirm association with CELIAC3 but not with CELIAC4. Tissue Antigens. 2007;70:324-9.,99. Rossi E, Basso D, Zambon CF, Navaglia F, Greco E, Pelloso M, et al. TNFA Haplotype Genetic Testing Improves HLA in Estimating the Risk of Celiac Disease in Children. PLoS One. 2015;10:e0123244.,1414. Garrote JA, Arranz E, Tellería JJ, Castro J, Calvo C, Blanco-Quirós A. TNF alpha and LT alpha gene polymorphisms as additional markers of celiac disease susceptibility in a DQ2-positive population. Immunogenetics. 2002;54:551-5.
15. Cataldo F, Lio D, Marino V, Scola L, Crivello A, Mulè AM, et al. Cytokine genotyping (TNF and IL-10) in patients with celiac disease and selective IgA deficiency. Am J Gastroenterol . 2003;98:850-6.
16. Hahn-Zoric M, Hytönen AM, Hanson LA, Nilsson LA, Padyukov L. Association of -1087 IL10 and -308 TNFA gene polymorphisms with serological markers of coeliac disease. J Clin Immunol. 2003;23:291-6.
17. Garrote JA, Arranz E, Gómez-González E, León AJ, Farré C, Calvo C, et al. IL6, IL10 and TGFB1 gene polymorphisms in coeliac disease: differences between DQ2 positive and negative patients. Allergol Immunopathol (Madr). 2005;33:245-9.
18. Lio D, Scola L, Forte GI, Accomando S, Giacalone A, Crivello A, Cataldo F. TNFα, IFNγ and IL-10 gene polymorphisms in a sample of Sicilian patients with coeliac disease. Dig Liver Dis. 2005;37:756-60.
19. Barisani D, Ceroni S, Meneveri R, Cesana BM, Bardella MT. IL-10 polymorphisms are associated with early-onset celiac disease and severe mucosal damage in patients of Caucasian origin. Genet Med. 2006;8:169-74.
20. Kekik C, Oguz FS, Karahn G, Seyhun YAE. IL-10 and TNF-alpha Gene Polymorphisms in Patients with Celiac Disease. Turkish J Immunol. 2011;16:11-6.-2121. de Albuquerque Maranhão RM, Martins Esteves FA, Crovella S, Segat L, Eleutério Souza PR. Tumor necrosis factor-α and interleukin-6 gene polymorphism association with susceptibility to celiac disease in Italian patients. Genet Mol Res. 2015;14:16343-52. with 1147 cases and 1774 controls were selected for this meta-analysis. The characteristics of selected studies are presented in Table 1. All of the selected studies were written in English and published from 2002 to 2015. Among them, ten studies were based on Caucasian (Spain, Italy, Sweden, Hungary, and Turkey) and one was based on Mixed (Brazil). TNF-α -308G>A polymorphism was determined by PCR-RFLP, PCR Hybridization, PCR-SSP, and ARMS-PCR. The genetic distributions of the control groups in all studies were in accordance with HWE except for two studies (Table 1).
Quantitative synthesis
The result of meta-analysis of the association between TNF-α -308G>A polymorphism and CD risk is shown in Table 1. The pooled results indicated that TNF-α -308G>A polymorphism was associated with increased risk of CD under four genetic models i.e., allele (A vs G: OR=2.077, 95% CI=1.468-2.939, P≤0.001, Figure 2A); homozygote (AA vs GG: OR=8.512, 95% CI=3.740-19.373, P≤0.001); dominate (AA+AG vs GG: OR=1.869, 95% CI=1.161-3.008, P=0.010); and recessive (AA+AG vs GG: OR=4.773, 95% CI=3.181-7.162, P≤0.001), but not under heterozygote model (Figure 2B). Additional subgroup analyses based on ethnicity, countries and genotyping methods were performed. We performed the subgroup analysis by ethnicity only in Caucasian populations due to the limited number of studies in other ethnicities. The pooled analysis showed that there was a significant association between TNF-α -308G>A polymorphism and CD risk in Caucasians four genetic models i.e., allele (A vs G: OR=1.957, 95% CI=1.691-2.830, P≤0.001); homozygote (AA vs GG: OR=8.134, 95% CI=3.255-20.330, P≤0.001); dominant (AA+AG vs GG: OR=1.710, 95% CI=1.043-2.774, P=0.033); and recessive (AA+AG vs GG: OR=4.489, 95% CI=2.920-6.901, P≤0.001).
Forest plot for the association between TNF-α -308G>A polymorphism and CD risk. A. Allele model (A vs G). B. Heterozygote model (AG vs GG).
When stratified by countries, there was a significant association between TNF-α -308G>A polymorphism and CD risk in Italy (A vs G: OR=1.957, 95% CI=1.198-3.744, P=0.010; AA vs GG: OR=8.134, 95% CI=1.851-34.212, P=0.005; AA+AG vs GG: OR=1.710, 95% CI=0.903-2.826, P=0.108; and AA+AG vs GG: OR=4.489, 95% CI=2.147-25.413, P=0.002) and Spain (A vs G: OR=1.957, 95% CI=1.452-2.615, P≤0.001; AA vs GG: OR=8.134, 95% CI=1.851-34.212, P=0.003; AG vs GG: OR=1.710, 95% CI=1.311-2.667, P=0.001; AA+AG vs GG: OR=1.710, 95% CI=1.444-2.864, P≤0.001; and AA+AG vs GG: OR=4.489, 95% CI=1.417-9.779, P=0.008). Furthermore, stratified analyses by genotyping methods showed that TNF-α -308G>A polymorphism was significantly associated with increased risk of CD in the PCR-RFLP group (A vs G: OR=2.286, 95% CI=1.236-4.233, P=0.009; AA vs GG: OR=10.161, 95% CI=1.676-61.642, P=0.012; AA+AG vs GG: OR=2.299, 95% CI=1.027-5.147, P=0.043; and AA+AG vs GG: OR=3.542, 95% CI=2.045-6.135, P≤0.001), but not in the PCR-SSCP group.
Sensitivity analysis and heterogeneity test
We have performed sensitivity analysis to evaluate the robustness of our pooled data by sequentially removing each eligible study and observing the changes in the pooled ORs. However, the significance of the pooled ORs did not changed by any single study under allele five genetic models, indicating that our results were statistically robust and reliable. As seen in Table 2, heterogeneity was observed under five genetic models but not in the heterozygote model in overall population. Thus, subgroup analysis was performed to identify the possible source of heterogeneity by testing the ethnicity, countries, genotyping methods, and HWE status. As seen in Table 2, the results showed that ethnicity, genotyping method, HWE status did not contributed to the heterogeneity. However, the subgroup analysis showed that the between studies heterogeneity was absent in those studies performed among Spanish people.
Publication bias
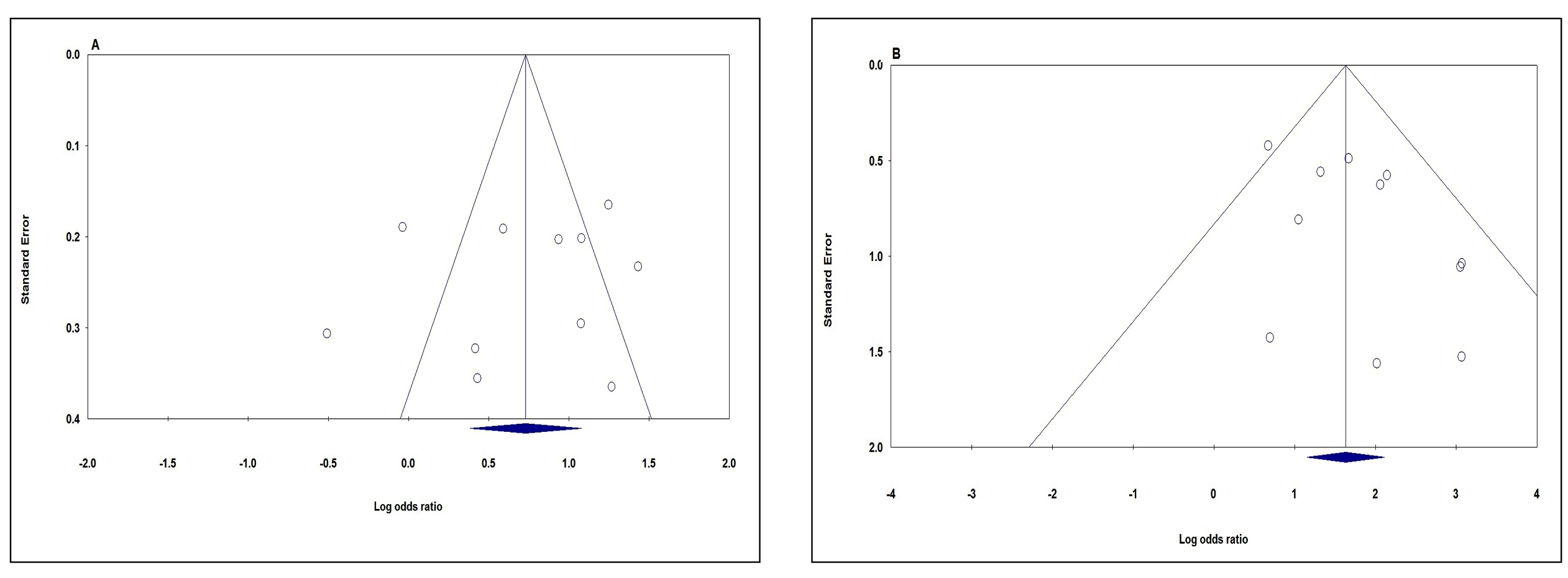
We utilized the Begg’s funnel plot and Egger’s test to assess publication bias in the present meta-analysis. The funnel plots’ shape under all five genetic models did not provide obvious evidence of asymmetry. Moreover, the P values of Egger’s test were greater than 0.05 under all five genetic models i.e., allele (A vs G: P Beggs=0.876, P Eggers=0.568, Figure 3A); homozygote (AA vs GG P Beggs=0.436, P Eggers=0.240); heterozygote (AG vs GG: P Beggs=0.212, P Eggers=0.301); dominate (AA+AG vs GG: P Beggs=0.350, P Eggers=0.365); and recessive (AA+AG vs GG: P Beggs=0.350, P Eggers=0.109, Figure 3B), which providing statistical evidence for the funnel plots’ symmetry (Table 2). Therefore, the Begg’s funnel plot and Egger’s test results suggest that publication bias was not evident in current meta-analysis.
Funnel plot for publication bias in the meta-analysis of TNF-α -308G>A polymorphism and CD risk. A. Allele model (A vs G). B. Recessive model (AG vs GG).
DISCUSSION
CD is a T-cell-mediated an intestinal disorder which Genetic factors, including HLA class II genes, are the most important factors in the development of CD88. de la Concha EG, Fernández-Arquero M, Vigil P, Rubio A, Maluenda C, Polanco I, et al. Celiac disease and TNF promoter polymorphisms. Hum Immunol. 2000;61:513-7.. In the past few years, several case-control studies have evaluated the association of TNF-α -308G>A polymorphism with susceptibility to the CD55. Khan S, Mandal RK, Jawed A, Dar SA, Wahid M, Panda AK, et al. TNF-α -308 G > A (rs1800629) Polymorphism is Associated with Celiac Disease: A Meta-analysis of 11 Case-Control Studies. Sci Rep. 2016;6:32677.. However, some of those studies are not regarded as strong markers of CD. The present meta-analysis of eleven studies, involving a total of 1147 cases and 1774 controls provides the most comprehensive assessment so far of the association of TNF-α -308G>A polymorphism with CD risk.
In this study, we included eleven case-control studies with specific information about the association of TNF-α -308G>A polymorphism with CD risk which composed of only two ethnicities from six countries, in a meta-analysis. Although the previous meta-analysis by Khan et al., found that TNF-α -308G>A polymorphism was associated with increased risk of CD, they have not evaluated the association by ethnicity, countries and genotyping methods 5. Our pooled results suggest that there is an association between TNF-α -308G>A polymorphism and CD risk. In addition, the subgroup analysis showed that that the TNF-α -308G>A polymorphism is associated with CD in Caucasians and RFLP-PCR group of studies. We have performed subgroup analyses by ethnicity and genotyping method since they might be important variables in examination association between TNF-α -308G>A polymorphism and CD risk. However, the comprehensive searches were not funded studies among Asians and we performed the subgroup analysis by ethnicity only among Caucasians. Therefore, the results of the current meta-analysis should be applied just for Caucasians. Moreover, subgroup analysis by genotyping methods showed that there was significant association between TNF-α -308G>A polymorphism and CD risk in group of studies used RFLP-PCR, but not PCR-SSP group.
There are several limitations to our meta-analysis. First, the number of included studies was insufficient for comprehensive analysis. Second, we have included only published studies in this meta-analysis, and there may still be some unpublished studies in line with the conditions. Thus, publication bias may exist; even no statistical evidence suggests publication bias in the meta-analysis. Third, among the included studies, almost all of the included studies are from Caucasians, just one study on mixed population (Brazil), and no study reported the association of TNF-α -308G>A polymorphism with CD risk and there was no any studies among Asians, which may influence the reliability of our results about association of TNF-α -308G>A polymorphism and CD in overall and by ethnicity. Thus, the discrepancy of association among different ethnicities should be interpreted carefully and additional studies with larger sample size in different ethnicities especially among Asians are necessary. Finally, multiple factors can influence the development of CD, especially the gene-gene and gene-environment interactions, which were not analyzed due to lack of sufficient data. In addition, for lack of all individual raw data, we could not assess the cancer risk stratified by other covariates, including age, sex, life style, and other risk factors.
In conclusion, the current meta-analysis supports the association between TNF-α -308G>A polymorphism with CD risk. However, for lack of data in Asian population, the results of the current meta-analysis should be applied just for Caucasians. Thus, Large-scale case-controls in multiple ethnicity groups need to be performed in the future to confirm and improve these findings.
ACKNOWLEDGEMENTS
The authors would like to thank Sahel Khajeh-Noori from Mother and Newborn Health Research Center for assisting in the revision of this paper.
REFERENCES
-
1Zupin L, Polesello V, Catamo E, Crovella S, Segat L. Interleukin-10 gene promoter polymorphisms in celiac patients from north-eastern Italy. Hum Immunol. 2014;75:656-61.
-
2Hermann C, Krikovszky D, Vásárhelyi B, Dezsofi A, Madácsy L. Polymorphisms of the TNF-alpha gene and risk of celiac disease in T1DM children. Pediatr Diabetes. 2007;8:138-41.
-
3Dieli-Crimi R, Cénit MC, Núñez C. The genetics of celiac disease: A comprehensive review of clinical implications. J Autoimmun. 2015;64:26-41.
-
4Rubio-Tapia A, Rahim MW, See JA, Lahr BD, Wu TT, Murray JA. Mucosal recovery and mortality in adults with celiac disease after treatment with a gluten-free diet. Am J Gastroenterol. 2010;105:1412-20.
-
5Khan S, Mandal RK, Jawed A, Dar SA, Wahid M, Panda AK, et al. TNF-α -308 G > A (rs1800629) Polymorphism is Associated with Celiac Disease: A Meta-analysis of 11 Case-Control Studies. Sci Rep. 2016;6:32677.
-
6Capilla A, Donat E, Planelles D, Espinós C, Ribes-Koninckx C, Palau F. Genetic analyses of celiac disease in a Spanish population confirm association with CELIAC3 but not with CELIAC4. Tissue Antigens. 2007;70:324-9.
-
7Huang S-Q, Zhang N, Zhou Z-X, Huang CC, Zeng CL, Xiao D, et al. Association of LPP and TAGAP Polymorphisms with Celiac Disease Risk: A Meta-Analysis. Int J Environ Res Public Health. 2017;14(2).
-
8de la Concha EG, Fernández-Arquero M, Vigil P, Rubio A, Maluenda C, Polanco I, et al. Celiac disease and TNF promoter polymorphisms. Hum Immunol. 2000;61:513-7.
-
9Rossi E, Basso D, Zambon CF, Navaglia F, Greco E, Pelloso M, et al. TNFA Haplotype Genetic Testing Improves HLA in Estimating the Risk of Celiac Disease in Children. PLoS One. 2015;10:e0123244.
-
10Lis K, Kuzawińska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors - state of knowledge. Arch Med Sci. 2014;10:1175-85.
-
11Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R, Neamatzadeh H. A survey on the role of interleukin-10 in breast cancer: A narrative. Reports Biochem Mol Biol. 2017;7(1).
-
12Azarpira MR, Ghilian MM, Sobhan MR, Mehdinezhad-Yazdi M, Aghili K, Miresmaeili SM4, Neamatzadeh H. Association of MTHFR and TNF-α genes polymorphisms with susceptibility to Legg-Calve-Perthes disease in Iranian children: a case-control study. J Orthop. 2018;15:984-7.
-
13Aslebahar F, Neamatzadeh H, Meibodi B, Karimi-Zarchi M, Tabatabaei RS, Noori-Shadkam M, et al. Association of Tumor Necrosis Factor-α (TNF-α) -308G>A and -238G>A Polymorphisms with Recurrent Pregnancy Loss Risk: A Meta-Analysis. Int J Fertil Steril. 2019;12:284-92.
-
14Garrote JA, Arranz E, Tellería JJ, Castro J, Calvo C, Blanco-Quirós A. TNF alpha and LT alpha gene polymorphisms as additional markers of celiac disease susceptibility in a DQ2-positive population. Immunogenetics. 2002;54:551-5.
-
15Cataldo F, Lio D, Marino V, Scola L, Crivello A, Mulè AM, et al. Cytokine genotyping (TNF and IL-10) in patients with celiac disease and selective IgA deficiency. Am J Gastroenterol . 2003;98:850-6.
-
16Hahn-Zoric M, Hytönen AM, Hanson LA, Nilsson LA, Padyukov L. Association of -1087 IL10 and -308 TNFA gene polymorphisms with serological markers of coeliac disease. J Clin Immunol. 2003;23:291-6.
-
17Garrote JA, Arranz E, Gómez-González E, León AJ, Farré C, Calvo C, et al. IL6, IL10 and TGFB1 gene polymorphisms in coeliac disease: differences between DQ2 positive and negative patients. Allergol Immunopathol (Madr). 2005;33:245-9.
-
18Lio D, Scola L, Forte GI, Accomando S, Giacalone A, Crivello A, Cataldo F. TNFα, IFNγ and IL-10 gene polymorphisms in a sample of Sicilian patients with coeliac disease. Dig Liver Dis. 2005;37:756-60.
-
19Barisani D, Ceroni S, Meneveri R, Cesana BM, Bardella MT. IL-10 polymorphisms are associated with early-onset celiac disease and severe mucosal damage in patients of Caucasian origin. Genet Med. 2006;8:169-74.
-
20Kekik C, Oguz FS, Karahn G, Seyhun YAE. IL-10 and TNF-alpha Gene Polymorphisms in Patients with Celiac Disease. Turkish J Immunol. 2011;16:11-6.
-
21de Albuquerque Maranhão RM, Martins Esteves FA, Crovella S, Segat L, Eleutério Souza PR. Tumor necrosis factor-α and interleukin-6 gene polymorphism association with susceptibility to celiac disease in Italian patients. Genet Mol Res. 2015;14:16343-52.
-
Disclosure of funding: no funding received
Publication Dates
-
Publication in this collection
20 May 2019 -
Date of issue
Jan-Mar 2019
History
-
Received
07 Jan 2019 -
Accepted
27 Feb 2019