ABSTRACT
BACKGROUND:
Gastric cancer is known as the fourth most common cancer. Current treatments for cancer have damaged the sensitive tissues of the healthy body, and in many cases, cancer will be recurrent. Therefore, need for treatments that are more effective is well felt. Researchers have recently shifted their attention towards antipsychotic dopamine antagonists to treat cancer. The anticancer activities of aripiprazole remain unknown.
OBJECTIVE:
This study aimed to evaluate the efficacy and safety of aripiprazole on gastric cancer and normal cell lines.
METHODS:
In this regard, the cytotoxicity and genotoxicity of aripiprazole were investigated in MKN45 and NIH3T3 cell lines by methyl tetrazolium assay and on peripheral blood lymphocytes by micronucleus assay. For this purpose, cells were cultured in 96 wells plate. Stock solutions of aripiprazole and cisplatin were prepared. After cell incubation with different concentrations of aripiprazole (1, 10, 25, 50, 100 and 200 μL), methyl tetrazolium solution was added. For micronucleus assay fresh blood was added to RPMI culture medium 1640 supplemented, and different concentrations of aripiprazole (50, 100 and 200 μL) were added.
RESULTS:
The finding of present study showed that the IC50 of aripiprazole in the cancer cell line (21.36 μg/mL) was lower than that in the normal cell line (54.17 μg/mL). Moreover, the micronucleus assay showed that the frequency of micronuclei of aripiprazole at concentrations below 200 μM was much less than cisplatin.
CONCLUSION:
Aripiprazole can be a good cytotoxic compound and good candidate for further studies of cancer therapy.
HEADINGS:
Stomach neoplasms; Dopamine antagonists; Aripiprazole, therapeutic use; Tetrazolium salts; Micronucleus tests; Mutagenicity tests
RESUMO
CONTEXTO:
O câncer gástrico é conhecido como o quarto câncer mais comum. Os tratamentos atuais para o câncer danificaram os tecidos sensíveis do corpo saudável e, em muitos casos, o cancro será recorrente. Portanto, a necessidade de tratamentos que são mais eficazes é desejada. Recentemente, os pesquisadores mudaram sua atenção para os antagonistas antipsicóticos da dopamina para tratar o câncer. As atividades anticâncer de aripiprazol permanecem desconhecidas.
OBJETIVO:
Este estudo objetivou avaliar a eficácia e a segurança do aripiprazol no câncer gástrico e nas linhagens celulares normais.
MÉTODOS:
A este respeito, a citotoxicidade e a genotoxicidade do aripiprazol foram investigadas em linhas celulares MKN45 e NIH3T3 por ensaio de metil tetrazólio e em linfócitos periféricos de sangue por ensaio de micronúcleos. Para este efeito, as células foram cultivadas em 96 placas. As soluções de estoque de aripiprazol e cisplatina foram preparadas. Após incubação celular com diferentes concentrações de aripiprazol (1, 10, 25, 50, 100 e 200 μL), a solução de metil tetrazólio foi adicionada. Para o ensaio do micronúcleo o sangue fresco foi adicionado ao meio de cultura RPMI 1640 suplementado, e as concentrações diferentes de aripiprazole (50, 100 e 200 μL) foram adicionadas.
RESULTADOS:
O presente estudo mostrou que o IC50 de aripiprazol na linhagem celular cancerosa (21,36 μg/mL) foi menor do que na linha celular normal (54,17 μg/ mL). Além disso, o ensaio de micronúcleos demonstrou que a frequência de micronúcleos de aripiprazol em concentrações inferiores a 200 μM foi muito inferior à cisplatina.
CONCLUSÃO:
O aripiprazol pode ser um bom composto citotóxico e bom candidato para estudos adicionais da terapia do câncer.
DESCRITORES:
Neoplasias gástricas; Antagonistas de dopamina; Aripiprazol, uso terapêutico; Sais de tetrazólio; Testes para micronúcleos; Testes de mutagenicidade
INTRODUCTION
Cancer is now believed to rank among the three major causes of death and among them gastric cancer is known as the third leading cause of cancer-related death worldwide; however, the incidence of gastric cancer varies greatly and this number can change across populations11. Thun MJ, DeLancey JO, Center MM, Jemal AaW, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2009;31:100-10.,22. Ghassemi-Barghi N, Varshosaz J, Etebari M, Dehkordi AJ. Role of recombinant human erythropoietin loading chitosan-tripolyphosphate nanoparticles in busulfan-induced genotoxicity: Analysis of DNA fragmentation via comet assay in cultured HepG2 cells. Toxicology in Vitro. 2016;36:46-52.. Although there are various treatment options to treat localized gastric cancer, ranging from minimally invasive EMR to aggressive extended lymphadenectomy and perioperative empirical adjuvant chemoradiation, gastric cancer still poses a major clinical challenge. Because most disease is detected at a late stage, where there are limited treatment options for that and patients have a median survival of only 6~9 months33. Rugge M, Fassan M, and Graham DY. Epidemiology of gastric cancer. In Gastric Cancer (pp 23-34). 2015; Springer, Cham.. Recently, important data was published on the potential genotoxic or carcinogenic effects of antipsychotics, as well as their cytotoxic properties on cancer cells. Thus, researchers have shifted their attention towards antipsychotic dopamine antagonists to treat cancers. Aripiprazole, an atypical antipsychotic drug with mixed antagonism and agonism on dopamine D2 and serotonin receptors, is usually prescribed to cure psychosis such as schizophrenia, episodes associated with bipolar disorder, delayed sleep phase syndrome, and irritability in children with autism44. Fond G, Macgregor A, Attal J, Larue A, Brittner M, Ducasse D, Capdevielle D. Antipsychotic drugs: pro-cancer or anti-cancer? A systematic review. Med Hypotheses. 2012;79:38-42.. Aripiprazole has shown growth inhibitions against some cancer cell lines such as the glioma cell lines U251 and LN428, the gastric cancer cell line MKN-1, the breast cancer cell line MDA-MB-231, the colon carcinoma cell line CT26, the human embryonic kidney cell line HEK293, serum-cultured cancer cells and cancer stem cells55. Kim MS, Yoo BC, Yang WS, Han SY, Jeong D, Song JM, et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget. 2018;9:5979.,66. Shokrzadeh M, Ghassemi-Barghi N. Melatonin loading chitosan-tripolyphosphate nanoparticles: Application in attenuating etoposide-induced genotoxicity in HepG2 cells. Pharmacology. 2018;102:74-80.. In this study, anti-cancer effects of aripiprazole on MKN45 cancer cell line and NIH3T3 cell line were carefully examined by using methyl tetrazolium (MTT) assay. This assay is one of the most common employed for the detection of cytotoxicity or cell viability following exposure to toxic substances and is one of the most sensitive cytotoxicity assay. This assay was shown to have a very wide applicability for measuring survival and/or proliferation of various cells and can potentially be applied to any assay in which living cells must be distinguished from dead cells or a lack of cells. Besides, it showed the potential value for quantitative and rapid measurement of cell death77. Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171-7.
8. Shokrzadeh M, Ghassemi-Barghi N. Antioxidant and Genoprotective Effects of Amifostine against Irinotecan Toxicity in Human Hepatoma Cells. Int J Cancer Res Ter. 2018;3(1):1-5.-99. Shokrzadeh M, Mohammadpour A, Hoseini V, Abediankenari S, Ghassemi-Barghi N, Tabari YS. Serum cytokine of IL-2, IL-10 and IL-12 levels in patients with stomach adenocarcinoma. Arq Gastroenterol. 2018;55:385-9.. Moreover, we used micronucleus assay to assess genetic damage of aripiprazole in human peripheral blood lymphocytes. The development of the cytokinesis-block (CB) technique has transformed the human-lymphocyte micronucleus (MN) assay into a reliable and precise method for assessing chromosome damage1010. Lawarence B, Murugan K. Comparison of analgesic and anti-inflammatory activities of purified anthocyanin from Osbeckia aspera (L.) blume and Osbeckia reticulata bedd. Using animal models. Asian J Pharm Clin Res. 2019;12:106-11.,1111. Shokrzadeh M, Ghassemi-Barghi N. Genoprotective Effects of Amifostine against Mitomycin C-induced Toxicity in Human Hepatoma Cells. Int J Cancer Res Ter . 2018;3(1):1-5.. On the other hand, cisplatin based combination chemotherapies have been used since the 1980’s. However, the results of cisplatin-based regimens have not been satisfactory, and they have shown a response rate of less than 50%1212. Kim KH, Jeung KJ, Kim HJ, Bae SB, Kim CK, Lee NS, et al. Phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with recurrent or metastatic gastric cancer. Cancer Res Treat. 2007;39:49.. Such problems along with many side effects still remain for anti-cancer drugs such as Docetaxel, Paclitaxel, Doxorubicin, and etc. as well1313. Schrijvers D, Wanders J, Dirix L, Prove A, Vonck I, Van Oosterom AaK, Kaye S. Coping with toxicities of docetaxel (TaxotereTM). Ann Oncol. 1993;4:610-11.
14. Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, et al. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995;13:645-51.
15. Feng SS, Huang G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol) from nanospheres of biodegradable polymers. J Control Release. 2001;71:53-69.
16. Chen Y, Wan Y, Wang Y, Zhang H, Jiao Z. Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int J Nanomedicine. 2011;6:2321-6.-1717. Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, et al. On the receiving end-patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203-8.. Therefore, the need for new agents remains essential. Actually, a favorable profile of safety and tolerability and good clinical effectiveness are among the advantages of aripiprazole1818. Gründer G, Kungel M, Ebrecht M, Göröcs T, Modell S. Aripiprazole: pharmacodynamics of a dopamine partial agonist for the treatment of schizophrenia. Pharmacopsychiatry. 2006;39:21-5.,1919. Ghassemi-Barghi N, Etebari M, Jafarian-Dehkordi A. Protective effect of amifostine on busulfan induced DNA damage in human hepatoma cells. Toxicol Mech Methods. 2017;27:52-7.. However, the anticancer effects of aripiprazole on gastric cancer MKN45 cell line, and the possible genotoxicity of it in human cells has not been confirmed yet. This study aimed to evaluate the efficacy and safety of aripiprazole on gastric cancer and normal cell lines. In this regard, the cytotoxicity and genotoxicity of aripiprazole were investigated in MKN45 and NIH3T3 cell lines by MTT assay and on peripheral blood lymphocytes by MN assay.
METHODS
Cell culture
NIH3T3 and MKN45 Cell lines (Pasteur Institute, Tehran, Iran) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (GIBCO, Berlin, Germany) with 10% fetal bovine serum (Gibco-BRL, Germany) and 100 μg/mL streptomycin (Gibco-BRL, Germany) and 100 IU/mL penicillin (Gibco-BRL, Germany). Cell cultures were adjusted to allow for exponential growth.
MTT assay
NIH3T3 and MKN45 Cell lines (104 cells) were cultured in 200 μL DMEM/F12 medium containing 10% bovine serum in 96 wells plate and incubated at 37°C for 24h. Stock solutions of aripiprazole and cisplatin were prepared in 1% DMSO and phosphate buffered saline (PBS), respectively. Twenty-μL of MTT solution (5 mg/mL) was added to each well following 48h incubation with different concentrations of aripiprazole (1, 10, 25, 50, 100 and 200 μL). The optical density (OD) of the MTT reaction was measured on a microplate ELISA reader at 570 nm. All experiments were repeated two times and each treatment was run in triplicate. The percentage of cell viability was calculated using the equation: [mean (OD) of treated cells/mean OD of control cells (1%DMSO)] ×100.
Micronucleus assay (CBMN assay)
Fresh blood was collected from 10 healthy, no smoking, no alcoholic, male donors aged between 25-35 years by venepuncture in heparinized falcons. 0.5 mL of whole blood was added to 4.5 mL of Roswell Park Memorial Institute (RPMI) culture medium 1640 supplemented with 10% fetal bovine serum containing L-glutamine, antibiotics, and phytohemagglutinin (PHA), and different doses of duloxetine (1, 10, 25, 50, 100 and 200 μL). The binucleated lymphocytes were harvested 28h after adding Cyt-B (Sigma, Missouri, USA); they were treated by hypotonic KCl (0.075M) to red blood cell (RBC) lysis. Then fixative solution (methanol: acetic acid =6:1) was added to the cells prior to slide preparation and staining. For slide preparation, 2-3 drops of cell suspension were thrown on a clean slide. The slides were stained with Giemsa solution (4%) for 7-10 mins. They were observed at 40× and 100× magnifications using a light microscope to estimate mitotic index (the cells with two or more nuclei per 1000 observed cells) and micronuclei frequency (the number of micronuclei in 1000 binucleated cells) are lymphocytes that were once divided by mitosis. The experiment was performed two times. Mitotic Index has a direct relation with cells’ proliferative activity.
Statistical analysis
One way analysis of variance and Tukey’s honestly significant differences (HSD) test were used for multiple comparisons of data. A P value less than 0.05 was considered as significant. The IC50 (half maximal inhibitory concentration) values were calculated by PRISM software using nonlinear regression. Standard deviations represent average results of double experiments. The IC50 values were compared using the Student’s t-test measuring the effectiveness of a substance to cause cell death or inhibit cell growth. Therefore, the lower amount of IC50 represents a higher toxicity of a compound, which leads to death or inhibition of cell growth.
RESULTS
MTT assay
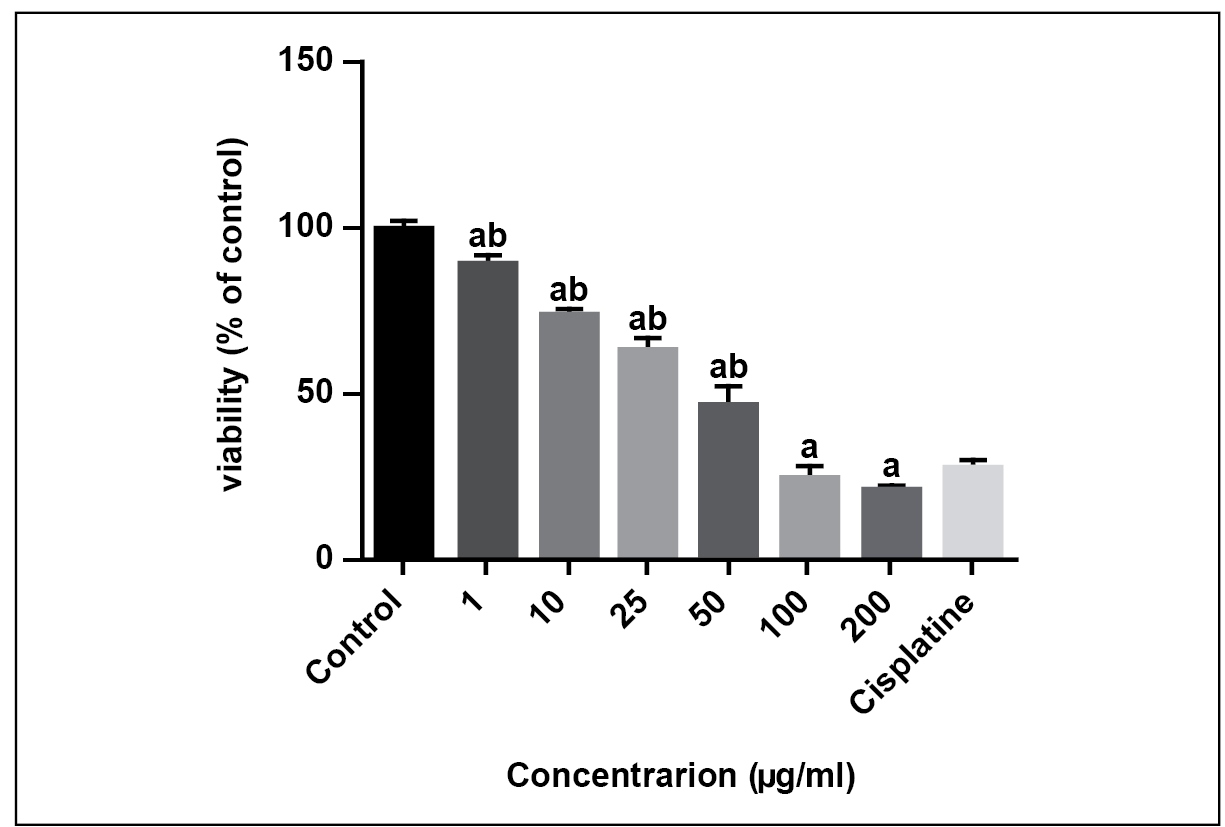
The IC50 of aripiprazole on MKN45 and NIH3T3 cell lines were examined using MTT assay. The IC50 of aripiprazole on MKN45 cell line was 21.36 μg/mL and on NIH3T3 cell line was 54.17 μg/mL. The lower IC50 value is representative of the higher ability of a cytotoxic compound to cause cell death or inhibit cell growth2020. Saravi SS, Shokrzadeh MS, Shirazi FH. Cytotoxicity of Sambucus ebulus on cancer cell lines and protective effects of vitamins C and E against its cytotoxicity on normal cell lines. Afr. J. Biotechnol. 2013;12:3360-5.. The results of the MKN45 cancer cell line (Table 1 and Figure 1) showed that aripiprazole compared to the negative control group had more cytotoxic effects in every concentration. Compared to the positive control group; cisplatin (the common anticancer drug) it had no significant difference at 100 and 200 μM, but at lower concentrations: 50, 25, 10 and 1 μM, it showed less cytotoxic efffects (P<0.05). Furthermore, the results of the NIH3T3 cell line (Table 1 and Figure 2) showed that aripiprazole in comparison with the negative control group had more cytotoxic effects at 10, 25, 50, 100, 200 μM and no significant difference at 1 μM. Moreover, in comparison with the positive control group (cisplatin), it showed less cytotoxic effects in every concentration but 100 μM (P<0.05).
Micronucleus assay
The genotoxic effects of aripiprazole were studied based on the number of MN produced in peripheral blood lymphocytes following treatment with different concentrations of aripiprazole (Table 1 and Figure 3). Results showed that the MN number was relatively increased based on the increase in the aripiprazole concentration. Fifty-μM concentration of aripiprazole did not produce any significant difference in MN number relative to the control group. While, 100 and 200 μM concentrations of aripiprazole significantly increased the number of MN. Comparison of different concentrations of aripiprazole and cisplatin showed that treatment of lymphocytes with 50 and 100 μM of aripiprazole significantly increased the number of MN. Two hundred-μM concentration of aripiprazole did not produce any significant difference in MN number relative to the cisplatin (P<0.05).
Micronuclei frequency in different concentrations of aripiprazole {a: significant difference compared to the negative control group b: significant difference compared to the positive control group (P<0.05)}.
DISCUSSION
In this study, the cytotoxic effect of Duloxetine on MKN45 gastric cancer cell line was investigated and compared to NIH3T3 normal cell line by MTT assay. Moreover, the genetic damage caused by this drug was also evaluated using MN assay. The IC50 of aripiprazole on MKN45 cell line and NIH3T3 cell line was calculated 21.36 μg/mL and 54.17 μg/mL respectively. However, the IC50 of cisplatin on MKN45 was 12.49 μg/mL and on NIH3T3 cell line was 24.9 μg/mL. The lower IC50 value is representative of the higher ability of a cytotoxic compound to cause cell death or inhibit cell growth. Taken together, it seems that this drug can be mentioned as a good cytotoxic compound. As reported here, the cytotoxicity of aripiprazole on MKN45 cancer cell line is consistent with some other researches55. Kim MS, Yoo BC, Yang WS, Han SY, Jeong D, Song JM, et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget. 2018;9:5979.,2121. Suzuki S, Okada M, Kuramoto K, Takeda H, Sakaki H, Watarai H, et al. Aripiprazole, an antipsychotic and partial dopamine agonist, inhibits cancer stem cells and reverses chemoresistance. Anticancer Res. 2016;36:5153-61.. Anti-cancer effects of aripiprazole on various malignant tumor cells and its molecular mechanism were examined by using cell proliferation assay, xenograft mouse model, immunoblotting analysis, migration assay, luciferase reporter gene assay, kinase assay, and overexpression strategy. Treatment with aripiprazole induced cytotoxicity in U251 glioma cells, MKN-1 gastric adenosquamous carcinoma cells, and CT26 colon carcinoma cells55. Kim MS, Yoo BC, Yang WS, Han SY, Jeong D, Song JM, et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget. 2018;9:5979.. Moreover, the effects of aripiprazole alone and in combination with chemotherapeutic agents in order to test growth ability, the ability to form stem cells/differentiation/chemical resistance, the markers of cancer stem cells, cancer cells and normalized cells was investigated. According to their study, the growth of cancer cells and cancer stem cells was inhibited by aripiprazole in non-toxic concentrations of normal cells. Generally, it could be concluded that aripiprazole could be a good candidate as an anticancer stem cell2121. Suzuki S, Okada M, Kuramoto K, Takeda H, Sakaki H, Watarai H, et al. Aripiprazole, an antipsychotic and partial dopamine agonist, inhibits cancer stem cells and reverses chemoresistance. Anticancer Res. 2016;36:5153-61..
Additionally, we used lymphocytes in our in vitro studies to assess the potential genotoxic effect of aripiprazole. Results from the micronucleus assay confirmed the ability of aripiprazole to induce the formation of micronuclei. The induction of micronuclei is commonly used to evaluate the chromosomal damage. The cellular and tissue toxicity was observed in the increased therapeutic concentrations of aripiprazole. Aripiprazole and its metabolites can bind DNA, causing damage that can result in chromosome breaks, micronucleus formation, and cell death. As evident, the frequency of micronuclei was increased with aripiprazole in comparison with the negative control group but in comparison with the positive control group, it caused less DNA damage at 100 and 50 μM concentrations. The genetic damage caused by aripiprazole at concentrations below 200 μM was much less than cisplatin, indicating that it could be studied more widely due to its low genotoxicity. The results of our findings in this regard coincided with the work of other researchers2222. Powroźnik B, Słoczyńska K, Marciniec K, Zajdel P, Pękala E. Preliminary Safety Assessment of New Azinesulfonamide Analogs of Aripiprazole using Prokaryotic Models. Adv Pharm Bull. 2016;6:377.,2323. Snyder RD. An update on the genotoxicity and carcinogenicity of marketed pharmaceuticals with reference to in silico predictivity. Environ Mol Mutagen. 2009;50:435-50.. The mutagenic and antimutagenic effects of some quinoline- and isoquinolinesulfonamide analogs of aripiprazole were evaluated and their results showed that newly synthesized azinesulfonamide analogs of aripiprazole might be considered as genotoxically safe as they do not display mutagenic activity on the tester strains2222. Powroźnik B, Słoczyńska K, Marciniec K, Zajdel P, Pękala E. Preliminary Safety Assessment of New Azinesulfonamide Analogs of Aripiprazole using Prokaryotic Models. Adv Pharm Bull. 2016;6:377.. In addition, genetic toxicity of 545 medications including aripiprazole were studied by using information from 1999 to 2008. The data also contained anti-cancer and antiviral drugs, nucleosides (all with known mechanistic genotoxicity), steroids with class-specific genotoxicity and biologicals or peptide-based drugs. Their results discussed the link between positive genetic toxicity findings, rodent carcinogenesis, and silica predictions and found that there was supporting evidence for the idea that just the presence of an N-dialkyl group or piperidine aryl ketone may somehow be associated with genotoxicity2323. Snyder RD. An update on the genotoxicity and carcinogenicity of marketed pharmaceuticals with reference to in silico predictivity. Environ Mol Mutagen. 2009;50:435-50..
CONCLUSION
Aripiprazole can be a great cytotoxic compound and beneficial candidate for further studies of cancer therapy. This study in comparison to other studies showed that aripiprazole can be considered as one of the candidates for the synthesis of a safe anticancer drug. Further studies are needed.
ACKNOWLEDGEMENT
This research was supported/partially supported by Sana institute of high education. We thank our colleagues from [mehdi abbasi roshan] who provided insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations/conclusions of this paper.
REFERENCES
-
1Thun MJ, DeLancey JO, Center MM, Jemal AaW, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2009;31:100-10.
-
2Ghassemi-Barghi N, Varshosaz J, Etebari M, Dehkordi AJ. Role of recombinant human erythropoietin loading chitosan-tripolyphosphate nanoparticles in busulfan-induced genotoxicity: Analysis of DNA fragmentation via comet assay in cultured HepG2 cells. Toxicology in Vitro. 2016;36:46-52.
-
3Rugge M, Fassan M, and Graham DY. Epidemiology of gastric cancer. In Gastric Cancer (pp 23-34). 2015; Springer, Cham.
-
4Fond G, Macgregor A, Attal J, Larue A, Brittner M, Ducasse D, Capdevielle D. Antipsychotic drugs: pro-cancer or anti-cancer? A systematic review. Med Hypotheses. 2012;79:38-42.
-
5Kim MS, Yoo BC, Yang WS, Han SY, Jeong D, Song JM, et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget. 2018;9:5979.
-
6Shokrzadeh M, Ghassemi-Barghi N. Melatonin loading chitosan-tripolyphosphate nanoparticles: Application in attenuating etoposide-induced genotoxicity in HepG2 cells. Pharmacology. 2018;102:74-80.
-
7Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171-7.
-
8Shokrzadeh M, Ghassemi-Barghi N. Antioxidant and Genoprotective Effects of Amifostine against Irinotecan Toxicity in Human Hepatoma Cells. Int J Cancer Res Ter. 2018;3(1):1-5.
-
9Shokrzadeh M, Mohammadpour A, Hoseini V, Abediankenari S, Ghassemi-Barghi N, Tabari YS. Serum cytokine of IL-2, IL-10 and IL-12 levels in patients with stomach adenocarcinoma. Arq Gastroenterol. 2018;55:385-9.
-
10Lawarence B, Murugan K. Comparison of analgesic and anti-inflammatory activities of purified anthocyanin from Osbeckia aspera (L.) blume and Osbeckia reticulata bedd. Using animal models. Asian J Pharm Clin Res. 2019;12:106-11.
-
11Shokrzadeh M, Ghassemi-Barghi N. Genoprotective Effects of Amifostine against Mitomycin C-induced Toxicity in Human Hepatoma Cells. Int J Cancer Res Ter . 2018;3(1):1-5.
-
12Kim KH, Jeung KJ, Kim HJ, Bae SB, Kim CK, Lee NS, et al. Phase II study of docetaxel and cisplatin as first-line chemotherapy in patients with recurrent or metastatic gastric cancer. Cancer Res Treat. 2007;39:49.
-
13Schrijvers D, Wanders J, Dirix L, Prove A, Vonck I, Van Oosterom AaK, Kaye S. Coping with toxicities of docetaxel (TaxotereTM). Ann Oncol. 1993;4:610-11.
-
14Fossella FV, Lee JS, Shin DM, Calayag M, Huber M, Perez-Soler R, et al. Phase II study of docetaxel for advanced or metastatic platinum-refractory non-small-cell lung cancer. J Clin Oncol. 1995;13:645-51.
-
15Feng SS, Huang G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol) from nanospheres of biodegradable polymers. J Control Release. 2001;71:53-69.
-
16Chen Y, Wan Y, Wang Y, Zhang H, Jiao Z. Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int J Nanomedicine. 2011;6:2321-6.
-
17Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, et al. On the receiving end-patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983;19:203-8.
-
18Gründer G, Kungel M, Ebrecht M, Göröcs T, Modell S. Aripiprazole: pharmacodynamics of a dopamine partial agonist for the treatment of schizophrenia. Pharmacopsychiatry. 2006;39:21-5.
-
19Ghassemi-Barghi N, Etebari M, Jafarian-Dehkordi A. Protective effect of amifostine on busulfan induced DNA damage in human hepatoma cells. Toxicol Mech Methods. 2017;27:52-7.
-
20Saravi SS, Shokrzadeh MS, Shirazi FH. Cytotoxicity of Sambucus ebulus on cancer cell lines and protective effects of vitamins C and E against its cytotoxicity on normal cell lines. Afr. J. Biotechnol. 2013;12:3360-5.
-
21Suzuki S, Okada M, Kuramoto K, Takeda H, Sakaki H, Watarai H, et al. Aripiprazole, an antipsychotic and partial dopamine agonist, inhibits cancer stem cells and reverses chemoresistance. Anticancer Res. 2016;36:5153-61.
-
22Powroźnik B, Słoczyńska K, Marciniec K, Zajdel P, Pękala E. Preliminary Safety Assessment of New Azinesulfonamide Analogs of Aripiprazole using Prokaryotic Models. Adv Pharm Bull. 2016;6:377.
-
23Snyder RD. An update on the genotoxicity and carcinogenicity of marketed pharmaceuticals with reference to in silico predictivity. Environ Mol Mutagen. 2009;50:435-50.
-
Disclosure of funding: no funding received
Publication Dates
-
Publication in this collection
26 Aug 2019 -
Date of issue
Apr-Jun 2019
History
-
Received
14 Jan 2019 -
Accepted
11 Mar 2019