ABSTRACT
BACKGROUND:
Hospital-based studies recently have shown increases in colorectal cancer survival, and better survival for women, young people, and patients diagnosed at an early disease stage.
OBJECTIVE:
To describe the overall survival and analyze the prognostic factors of patients treated for colorectal cancer at an oncology center.
METHODS:
The analysis included patients diagnosed with colon and rectal adenocarcinoma between 2000 and 2013 and identified in the Hospital Cancer Registry at A.C.Camargo Cancer Center. Overall 5-year survival was estimated using the Kaplan-Meier method, and prognostic factors were evaluated in a Cox regression model. Hazard ratios (HR) are reported with 95% confidence intervals (CI).
RESULTS:
Of 2,279 colorectal cancer cases analyzed, 58.4% were in the colon. The 5-year overall survival rate for colorectal cancer patients was 63.5% (65.6% and 60.6% for colonic and rectal malignancies, respectively). The risk of death was elevated for patients in the 50-74-year (HR=1.24, 95%CI =1.02-1.51) and ≥75-year (HR=3.02, 95%CI =2.42-3.78) age groups, for patients with rectal cancer (HR=1.37, 95%CI =1.11-1.69) and for those whose treatment was started >60 days after diagnosis (HR=1.22, 95%CI =1.04-1.43). The risk decreased for patients diagnosed in recent time periods (2005-2009 HR=0.76, 95%CI =0.63-0.91; 2010-2013 HR=0.69, 95%CI =0.57-0.83).
CONCLUSION:
Better survival of patients with colorectal cancer improves with early stage and started treatment within 60 days of diagnosis. Age over 70 years old was an independent factor predictive of a poor prognosis. The overall survival increased to all patients treated in the period 2000-2004 to 2010-2013.
HEADINGS:
Survival analysis; Colorectal neoplasms; Registries; Prognosis
RESUMO
CONTEXTO:
Estudos hospitalares recentes têm demonstrado aumento da sobrevida do câncer colorretal e melhor sobrevida para mulheres, jovens e pacientes diagnosticados em estágio precoce da doença.
OBJETIVO:
Descrever a sobrevida global e analisar os fatores prognósticos de pacientes tratados para câncer colorretal em um centro de oncologia.
MÉTODOS:
Foram incluídos pacientes com diagnóstico de adenocarcinoma de cólon e reto entre 2000 e 2013, identificados no Registro Hospitalar de Câncer do A.C.Camargo Cancer Center. A sobrevida global aos 5 anos foi estimada pelo método de Kaplan-Meier e os fatores prognósticos foram avaliados pelo modelo de Cox. As razões de risco (HR) são relatadas com intervalos de confiança (IC) de 95%.
RESULTADOS:
Dos 2.279 casos de câncer colorretal analisados, 58,4% eram de cólon. A taxa de sobrevida global aos 5 anos para pacientes com câncer colorretal foi de 63,5% (65,6% e 60,6% para câncer de cólon e retal, respectivamente). O risco de óbito foi elevado para pacientes na faixa etária de 50-74 anos (HR=1,24; IC95% =1,02-1,51) e ≥75 anos (HR=3,02; IC95% =2,42-3,78), para pacientes com câncer retal (HR=1,37; IC95% =1,11-1,69) e para aqueles cujo tratamento foi iniciado >60 dias após o diagnóstico (HR=1,22; IC95% =1,04-1,43). O risco diminuiu para pacientes diagnosticados em períodos recentes (2005-2009 HR=0,76; IC95% =0,63-0,91; 2010-2013 HR=0,69; IC95% =0,57-0,83).
CONCLUSÃO:
A sobrevida dos pacientes com câncer colorretal é maior naqueles em estágio inicial e com início do tratamento antes dos 60 dias.. Idade acima de 70 anos foi fator independente preditivo de mau prognóstico. A sobrevida global aumentou para todos os pacientes tratados no período de 2000-2004 a 2010-2013.
DESCRITORES:
Análise de sobrevida; Neoplasias colorretais; Sistema de registros; Prognóstico
INTRODUCTION
In 2018, colorectal cancer (CRC) was the third most common cancer among men and the second most among women worldwide. It is the fourth cause of death from cancer among men and the second among women11. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.. In Brazil, the estimates for 2018 show that CRC was the third most common cancer in men (17,380 cases; 8.7%) and the second in women (18,980 cases; 9.4%)22. National Cancer Institute (INCA). Estimate 2018: Cancer incidence in Brazil. [Internet]. [Accessed 2019 June 19]. Available from: https://www.inca.gov.br/search/conteudo/estimativa
https://www.inca.gov.br/search/conteudo/...
.
Cancer incidence and mortality rate have been related to the human development of the countries. CRC incidence and mortality rates have been increasing in low- and middle-income countries, while it is stable or decreasing in highly developed countries33. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691.. Within Brazil, mortality rates and trends have been found to differ across federal units, after adjusting for socioeconomic conditions44. Guimaraes RM, Rocha PGM, Muzi CD, Ramos RS. Increase income and mortality of colorectal cancer in Brazil, 2001-2009. Arq Gastroenterol. 2013;50:64-9.-55. Oliveira MM, Latorre MRDO, Tanaka LF, Rossi BM, Curado MP. Disparities in colorectal cancer mortality across Brazilian States. Rev Bras Epidemiol. 2018;21:e180012..
In terms of relative population survival, data from 296 registers show high variability in relative survival among patients with colon cancer. Israel, South Korea, and Australia have survival rates over 70%. The survival rate from six population-based cancer registries of Brazilian have been stable in fifteen years period (44.5% for 2000-2004, 50.6% for 2005-2009, and 48.3% for 2010-2014). For rectal cancer, relative survival has been more variable across world regions (294 registers), with only Korea and Australia having rates above 70%. In Brazil, the relative survival was 37.7% for 2000-2004, 45.7% for 2005-2009, and 42.4% for 2010-201466. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-75.. Hospital-based studies recently have shown increases in survival, and better survival for women, young people, and patients diagnosed at an early disease stage77. Agüero F, Murta-Nascimento C, Gallén M, Andreu-García M, Pera M, Hernández C, et al. Colorectal cancer survival: results from a hospital-based cancer registry. Rev Esp Enferm Dig. 2012;104:572-7.
8. Jørgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353-60.
9. Quan D, Gallinger S, Nhan C, Auer RA, Biagi JJ, Fletcher GG, et al.; Surgical Oncology Program at Cancer Care Ontario. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: a systematic review. Surgery. 2012;151:860-70.-1010. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J Epidemiol. 2019;29:110-5..
The survival data for patients treated at specialized cancer centers serves as an indicator of how a patient comes to be treated. Very few studies have use data from Brazilian hospital cancer registries to evaluate CRC survival. Thus, the objective this study was to describe the profile of patients treated for CRC at an oncology center and to analyze their survival.
METHODS
We included patients with CRC during the period of 2000 to 2013 who were treated at A.C.Camargo Cancer Center (ACCCC) with follow-up through December 31, 2018: invasive adenocarcinomas (M81403, 82013, 82103, 82113, 82203, 82613, 82623, 82633, 84413, 84703, 84803, 84813, 84903, 85103, 85603); colon cancer (ICD-O3 C18-19); and rectal cancer (ICD-O3 C20). We obtained the cases from the ACCCC’s Hospital Cancer Registry. The ACCCC’s Research Ethics Committee approved the project (n. 2462/17) on May 12, 2017.
Analyses were performed based on patient age group (≤49, 50-74, and ≥75 years of age), gender (male and female), period of diagnosis (2000-2004, 2005-2009, and 2010-2013), site (left colon, right colon, unspecified, and rectal), clinical stage (I, II, III, and IV); delay to start treatment from the date of diagnosis in days (≤60 days and ≥61 days)1111. Presidency of the Republic. Law 12.732/12 - Provides on the first treatment of patients with proven malignant neoplasia and establishes a deadline for its beginning. Official Journal of the Union 2012. Available at: Available at: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm . Accessed on: May 10, 2019.
https://www.planalto.gov.br/ccivil_03/_a...
; type of treatment for the colon (surgery, surgery + chemotherapy, other combination, or none for the colon; surgery; surgery + chemotherapy, radiation therapy + chemotherapy; surgery + chemotherapy, + radiotherapy, other combination, or none for the rectal). Absolute and relative frequencies were calculated. Patients groups were compared with chi-squared tests.
Overall Survival (OS) was calculated as the difference between the date of diagnosis and the date of death (from any cause) or the date of the most recent information in the medical records. Survival curves were estimated by the Kaplan-Meier estimator, and 60-month probabilities were presented according to independent variables. Survival curves were compared with the log-rank test. Hazard ratios (HR) and associated 95% confidence intervals (95%CI) were estimated with a Cox regression model. The assumption of proportional hazards was assessed based on the so-called Schoenfeld residuals and the Grambsch and Therneau global test. There was one independent variable that did not satisfy the proportional hazards assumption (clinical stage) and thus the stratified Cox model by clinical stage was fitted. There was evidence that covariates had a constant effect over time in all cases. The significance level was fixed at 5% for all tests and the analyses performed in Stata SE 15.
RESULTS
Between 2000 and 2013, 2,279 patients with CRC were treated at the ACCCC. The majority of these patients were male (51.3%); 50-74 years old (62.5%), with colon cancer (58.4%), clinical stage III/IV (52.8%), and started treatment within 60 days of diagnosis (70.4%) (all P<0.05) (Table 1). Among colon cancer patients, 43.2% had surgery alone and 40.8% had surgery and chemotherapy. Among rectal cancer patients, 41.5% had surgery, chemotherapy, and radiation therapy, and 20.7% had surgery alone (Table 2).
Characteristics of patients diagnosed with colorectal cancer and treated at A.C.Camargo Cancer Center, between 2000 and 2013.
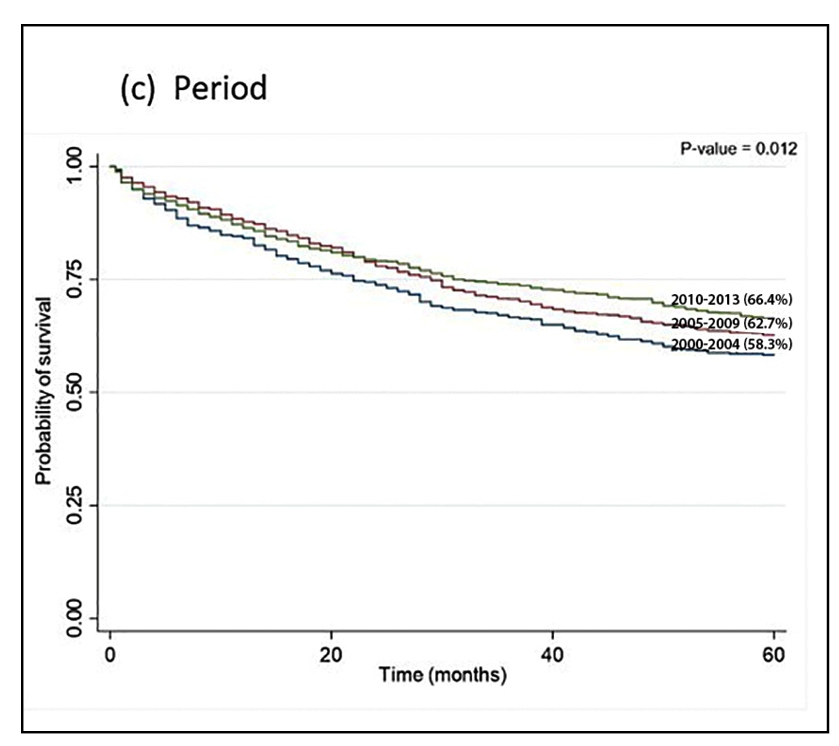
We observed a 5-year OS rate of 63.5% (Figure 1.A), without difference between men and women. Survival was higher in patients younger than 49 years old (70.0%) and worse in those over 75 years old, 43.8% (P<0.001). OS was better for the most recent period (2010-2013, 66.4%) than for prior time periods (P<0.012, Figure 1.C). Patients younger than 49 years old with stage I or II colon cancer had an OS of 100%, while rectal cancer for stage I was 89.8% and 88.0% for stage II (Table 3). In the adjusted model stratified by clinical stage, mortality risk increased with increasing age (50-74 years HR=1.24; and ≥75 years HR=3.02) and was high for those diagnosed with rectal cancer (HR=1.37) and for those who started treatment more than 60 days after diagnosis (HR=1.22). Mortality risk was lowest for the two most recent time periods (2005-2009, HR=0.76; and 2010-2013, HR=0.69) (Table 4).
Kaplan-Meier 5-year overall survival rates for patients with colorectal cancer from 2000 to 2013.
Patients whose cancer was in the colon had a 5-year OS of 65.6%, (P<0.001). Subgroups of colon cancer patients with higher OS rates included patients who were ≤49 years old (73.5%) (Table 3). Mortality risk increased with increasing age (50-74 years HR=1.24; and ≥75 years HR=2.85). Mortality risk decreased for the most recent time periods (2005-2009 HR=0.87; and 2010-2013 HR=0.77) (Table 4).
Patients with rectal cancer had a 5-year OS rate of 60.6% (Figure 1.B). Again, subgroups of rectal cancer patients with higher OS rates included patients who were ≤49 years old (66.0%) (P<0.001) (Table 3). Mortality risk was increased among patients aged 75 and over (HR=2.47) (Table 4).
The best survival rates were observed among the subgroup of young patients with early stage (I-II) colon cancer, whose 5-year OS achieved 100%. The worst survival rates were observed among the subgroup of elderly patients with metastatic rectal cancer, whose 5-year OS were 7.1%.
DISCUSSION
The 5-year OS rate obtained for our CRC patient cohort (63.5%) was similar to those found in hospital-based studies in Iran (58.5% for 2005-2010)1212. Zare-Bandamiri M, Khanjani N, Jahani Y, Mohammadianpanah M. Factors Affecting Survival in Patients with Colorectal Cancer in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:159-63., Australia (63.0% for 2005-2010)1313. Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev . 2015;16:2431-40., and Taiwan (68.7% for 2007-2013)1414. Lee CH, Cheng SC, Tung HY, Chang SC, Ching CY, Wu SF. The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran J Public Health. 2018;47:519-30., but notably higher than rates reported in China (range, 28.4-41.7% in the period of 2002-2014)1515. Chen JG, Chen HZ, Zhu J, Yang YL, Zhang YH, Huang PX, et al. Cancer survival in patients from a hospital-based cancer registry, China. J Cancer. 2018;9:851-60.. We did not identify differences based on sex, even after adjusting for other characteristics1212. Zare-Bandamiri M, Khanjani N, Jahani Y, Mohammadianpanah M. Factors Affecting Survival in Patients with Colorectal Cancer in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:159-63.-1313. Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev . 2015;16:2431-40.. Some studies have suggested that survival may be lower for men, due to differences in access to health services77. Agüero F, Murta-Nascimento C, Gallén M, Andreu-García M, Pera M, Hernández C, et al. Colorectal cancer survival: results from a hospital-based cancer registry. Rev Esp Enferm Dig. 2012;104:572-7.,1616. Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401-9..
Survival decreased with increasing age, as previously described77. Agüero F, Murta-Nascimento C, Gallén M, Andreu-García M, Pera M, Hernández C, et al. Colorectal cancer survival: results from a hospital-based cancer registry. Rev Esp Enferm Dig. 2012;104:572-7.,1212. Zare-Bandamiri M, Khanjani N, Jahani Y, Mohammadianpanah M. Factors Affecting Survival in Patients with Colorectal Cancer in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:159-63.,1616. Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401-9.. More than 80% of the patients were 50 years old or older. Increased age is a predictive factor for death in cancer patients1010. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J Epidemiol. 2019;29:110-5.,1717. Lee M, Cronin KA, Gail MH, Feuer EJ. Predicting the absolute risk of dying from colorectal cancer and from other causes using population-based cancer registry data. Stat Med. 2012;31:489-500. and has been associated with a higher risk of comorbidities, which reduce patient survival1818. Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290-314.,1919. Yancik R, Ganz PA, Varricchio CG, Conley B. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol . 2001;19:1147-51.. In a study in Japan, 20% of 792 patients had a Charlson Comorbidity Index (CCI) higher than or equal to 1, with a 1.20 increment of risk of overall mortality for each CCI point1010. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J Epidemiol. 2019;29:110-5.. Meanwhile, in a Danish study of patients 70 years old or older, a 1.41 increase in the risk of overall mortality per CCI increment was observed88. Jørgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353-60.. These data reinforce the importance of the multidisciplinary team in treatment decision, as oncologic diagnosis is not the only risk.
We identified an improvement in survival in recent time periods. Similar results have previously been described77. Agüero F, Murta-Nascimento C, Gallén M, Andreu-García M, Pera M, Hernández C, et al. Colorectal cancer survival: results from a hospital-based cancer registry. Rev Esp Enferm Dig. 2012;104:572-7.,1313. Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev . 2015;16:2431-40.,1616. Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401-9.. Improved survival over time may be related to the implementation of treatment guidelines and new technologies to treat CRC.
OS was five percentage points higher for colon cancer patients (65.6%) than for rectal cancer patients (60.6%), consistent with prior studies reporting a poorer prognosis for rectal cancer than colon cancer66. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-75.,1212. Zare-Bandamiri M, Khanjani N, Jahani Y, Mohammadianpanah M. Factors Affecting Survival in Patients with Colorectal Cancer in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:159-63.-1313. Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev . 2015;16:2431-40.,2020. Yang J, Du XL, Li ST, Wang BY, Wu YY, Chen ZL, et al. Characteristics of Differently Located Colorectal Cancers Support Proximal and Distal Classification: A Population-Based Study of 57,847 Patients. PLoS One. 2016;11:e0167540.. In addition, our findings suggest that this lower of survival may be associated with the percentage of patients who began treatment more than 60 days after diagnosis (21.5% for colon cancer versus 40.9% for rectal cancer). As the treatment of rectal cancer is more complex than colon cancer, involving more exams for staging as well as the need of multimodality treatment, maybe these patients are more susceptible for delaying the start of treatment.
We did not identify any differences based on laterality of colon cancer (right vs left colon), contrasting with prior studies reporting lower survival rates for cancers located in the right colon1616. Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401-9.,2121. Warschkow R, Sulz MC, Marti L, Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer. 2016;16:554.,2222. Buchwald P, Hall C, Davidson C, Dixon L, Dobbs B, Robinson B, et al. Improved survival for rectal cancer compared to colon cancer: the four-cohort study. ANZ J Surg. 2018;88:E114-117.. In comparing survival rates across colon sites, we did not find differences in a combined analysis of patients with stage I, II, or III. However, we did identify a difference according to stage, in the right colon having a better prognosis when diagnosed at stage II (HR=0.92, 95%CI =0.87-0.97) than at stage III (HR=1.12, 95%CI =1.06-1.18)1616. Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401-9..
In terms of the type of treatment patients with stage I or II received only surgery, whereas patients with stage III or IV colon cancer received surgery combined with chemotherapy. For patients with rectal cancer stage II or III the treatment was surgery combined with both radiotherapy and chemotherapy. These findings were similar to those of other studies1313. Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev . 2015;16:2431-40.,2323. Abu Hassan MR, Ismail I, Mohd Suan MA, Ahmad F, Wan Khazim WK, Othman Z, et al. Incidence and mortality rates of colorectal cancer in Malaysia. Epidemiol Health. 2016;38:e2016007.
24. Ghazali AK, Musa KI, Naing NN, Mahmood Z. Prognostic factors in patients with colorectal cancer at Hospital Universiti Sains Malaysia. Asian J Surg. 2010;33:127-133.-2525. Yeole BB, Sunny L, Swaminathan R, Sankaranarayanan R, Parkin DM. Population-based survival from colorectal cancer in Mumbai, (Bombay) India. Eur J Cancer . 2001;37:1402-8.. Advanced age may be an independent predictor to preclude adjuvant therapies2626. Adelson P, Fusco K, Karapetis C, Wattchow D, Joshi R, Price T, Sharplin G, Roder D. Use of guideline-recommended adjuvant therapies and survival outcomes for people with colorectal cancer at tertiary referral hospitals in South Australia. J Eval Clin Pract. 2018;24:135-44..
Starting treatment more than 60 days after diagnosis was a predictor of poorer survival, which shows the need for implementing policies that enable universal access to early treatment1111. Presidency of the Republic. Law 12.732/12 - Provides on the first treatment of patients with proven malignant neoplasia and establishes a deadline for its beginning. Official Journal of the Union 2012. Available at: Available at: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm . Accessed on: May 10, 2019.
https://www.planalto.gov.br/ccivil_03/_a...
.
Given than survival rates are better among patients in an early clinical stage, the CRC patient treated at the ACCCC had advanced-stage cancer (colorectal, 52-53%) underscores a need for the implementation of CRC screening programs2727. Ghazali AK, Keegan TJ, Hassan MRA, Taylor BM. The effect of individual-level factors in survival prognosis for colorectal cancer in Malaysia. Int J Community Med Public Health. 2019;6:2313-20.
28. Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K; International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med. 2018;378:1734-40.-2929. Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7-11.. It is notable that survival rates of patients with CRC showed an improvement over time. However, early stage remains critical and to ensuring that treatment starts as soon as possible following diagnosis.
REFERENCES
-
1Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
-
2National Cancer Institute (INCA). Estimate 2018: Cancer incidence in Brazil. [Internet]. [Accessed 2019 June 19]. Available from: https://www.inca.gov.br/search/conteudo/estimativa
» https://www.inca.gov.br/search/conteudo/estimativa -
3Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691.
-
4Guimaraes RM, Rocha PGM, Muzi CD, Ramos RS. Increase income and mortality of colorectal cancer in Brazil, 2001-2009. Arq Gastroenterol. 2013;50:64-9.
-
5Oliveira MM, Latorre MRDO, Tanaka LF, Rossi BM, Curado MP. Disparities in colorectal cancer mortality across Brazilian States. Rev Bras Epidemiol. 2018;21:e180012.
-
6Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-75.
-
7Agüero F, Murta-Nascimento C, Gallén M, Andreu-García M, Pera M, Hernández C, et al. Colorectal cancer survival: results from a hospital-based cancer registry. Rev Esp Enferm Dig. 2012;104:572-7.
-
8Jørgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353-60.
-
9Quan D, Gallinger S, Nhan C, Auer RA, Biagi JJ, Fletcher GG, et al.; Surgical Oncology Program at Cancer Care Ontario. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: a systematic review. Surgery. 2012;151:860-70.
-
10Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of Comorbidities on Survival in Gastric, Colorectal, and Lung Cancer Patients. J Epidemiol. 2019;29:110-5.
-
11Presidency of the Republic. Law 12.732/12 - Provides on the first treatment of patients with proven malignant neoplasia and establishes a deadline for its beginning. Official Journal of the Union 2012. Available at: Available at: https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm Accessed on: May 10, 2019.
» https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12732.htm -
12Zare-Bandamiri M, Khanjani N, Jahani Y, Mohammadianpanah M. Factors Affecting Survival in Patients with Colorectal Cancer in Shiraz, Iran. Asian Pac J Cancer Prev. 2016;17:159-63.
-
13Roder D, Karapetis CS, Wattchow D, Moore J, Singhal N, Joshi R, et al. Colorectal cancer treatment and survival: the experience of major public hospitals in south Australia over three decades. Asian Pac J Cancer Prev . 2015;16:2431-40.
-
14Lee CH, Cheng SC, Tung HY, Chang SC, Ching CY, Wu SF. The Risk Factors Affecting Survival in Colorectal Cancer in Taiwan. Iran J Public Health. 2018;47:519-30.
-
15Chen JG, Chen HZ, Zhu J, Yang YL, Zhang YH, Huang PX, et al. Cancer survival in patients from a hospital-based cancer registry, China. J Cancer. 2018;9:851-60.
-
16Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401-9.
-
17Lee M, Cronin KA, Gail MH, Feuer EJ. Predicting the absolute risk of dying from colorectal cancer and from other causes using population-based cancer registry data. Stat Med. 2012;31:489-500.
-
18Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290-314.
-
19Yancik R, Ganz PA, Varricchio CG, Conley B. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol . 2001;19:1147-51.
-
20Yang J, Du XL, Li ST, Wang BY, Wu YY, Chen ZL, et al. Characteristics of Differently Located Colorectal Cancers Support Proximal and Distal Classification: A Population-Based Study of 57,847 Patients. PLoS One. 2016;11:e0167540.
-
21Warschkow R, Sulz MC, Marti L, Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer. 2016;16:554.
-
22Buchwald P, Hall C, Davidson C, Dixon L, Dobbs B, Robinson B, et al. Improved survival for rectal cancer compared to colon cancer: the four-cohort study. ANZ J Surg. 2018;88:E114-117.
-
23Abu Hassan MR, Ismail I, Mohd Suan MA, Ahmad F, Wan Khazim WK, Othman Z, et al. Incidence and mortality rates of colorectal cancer in Malaysia. Epidemiol Health. 2016;38:e2016007.
-
24Ghazali AK, Musa KI, Naing NN, Mahmood Z. Prognostic factors in patients with colorectal cancer at Hospital Universiti Sains Malaysia. Asian J Surg. 2010;33:127-133.
-
25Yeole BB, Sunny L, Swaminathan R, Sankaranarayanan R, Parkin DM. Population-based survival from colorectal cancer in Mumbai, (Bombay) India. Eur J Cancer . 2001;37:1402-8.
-
26Adelson P, Fusco K, Karapetis C, Wattchow D, Joshi R, Price T, Sharplin G, Roder D. Use of guideline-recommended adjuvant therapies and survival outcomes for people with colorectal cancer at tertiary referral hospitals in South Australia. J Eval Clin Pract. 2018;24:135-44.
-
27Ghazali AK, Keegan TJ, Hassan MRA, Taylor BM. The effect of individual-level factors in survival prognosis for colorectal cancer in Malaysia. Int J Community Med Public Health. 2019;6:2313-20.
-
28Lauby-Secretan B, Vilahur N, Bianchini F, Guha N, Straif K; International Agency for Research on Cancer Handbook Working Group. The IARC Perspective on Colorectal Cancer Screening. N Engl J Med. 2018;378:1734-40.
-
29Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7-11.
-
Disclosure of funding: no funding received
-
ERRATUM
In article Survival of patients with colorectal cancer in a Cancer Center, doi.org/10.1590/S0004-2803.202000000-32, published in journal Arq Gastroenterol. 2020;57(2):172-7, in page 173, FIGURE 1 (C Period):Which was read2000-2004 (58.3%)2005-2009 (62.7%)2010-2013 (58.3%)Read2010-2013 (66.4%)2005-2009 (62.7%)2000-2004 (58.3%)
Publication Dates
-
Publication in this collection
01 July 2020 -
Date of issue
Apr-Jun 2020
History
-
Received
17 Dec 2019 -
Accepted
27 Jan 2020