ABSTRACT
Background:
Physical exercise delays the sarcopenic process and can reverse the loss of muscle strength, improve quality of life and prognosis in cirrhotic patients.
Objective:
The aim was to verify the effects of face-to-face versus home aerobic exercise on the variables fatigue, respiratory and peripheral muscle strength, functional capacity and quality of life in patients with compensated cirrhosis.
Methods:
Patients were selected by convenience, stratified and randomized into supervised face-to-face exercise (n=13) and home exercise without daily supervision (n=12). Patients were submitted to a program of aerobic physical exercises, with progressive duration of 30 to 50 minutes, twice a week for twelve weeks. Before starting the program and every four weeks, all patients in both groups were assessed for fatigue (fatigue severity scale), respiratory (Pimáx and Pemáx) and peripheral (concentric quadriceps peak torque) muscle strength, functional capacity (6-minute walking distance) and quality of life (Short Form-36 Health Survey questionnaire).
Results:
The face-to-face group showed reduced fatigue (P<0.001), increased inspiratory (P<0.001), expiratory (P<0.001) and peripheral (P<0.001) muscle strength of the 6MWD (P<0.001) and improved quality of life. The home group showed no significant improvement in these variables.
Conclusion:
A face-to-face program of moderate aerobic exercise in patients with compensated cirrhosis reduces fatigue, improves functional capacity and quality of life and increases respiratory and peripheral muscle strength. Home physical exercises do not cause the same adaptive effects in this population.
Keywords:
Physical exercise; sarcopenia; rehabilitation; cirrhosis
RESUMO
Contexto:
O exercício físico retarda o processo sarcopênico e pode reverter a perda de força muscular, melhorar a qualidade de vida e prognóstico em cirróticos.
Objetivo:
O objetivo foi verificar os efeitos do exercício aeróbico presencial versus domiciliar sobre variáveis fadiga, força muscular respiratória e periférica, capacidade funcional e qualidade de vida em pacientes com cirrose compensada.
Métodos:
Os pacientes foram selecionados por conveniência, estratificados e randomizados em exercício presencial supervisionado (n=13) e exercício domiciliar sem supervisão diária (n=12). Os pacientes foram submetidos a um programa de exercícios físicos aeróbicos, com duração progressiva de 30 minutos a 1 hora, duas vezes por semana durante 12 semanas. Antes de iniciar o programa e a cada 4 semanas, todos os pacientes de ambos os grupos foram avaliados quanto à fadiga (escala de gravidade da fadiga), força muscular respiratória (Pimáx e Pemáx) e periférica (pico de torque do quadríceps concêntrico), capacidade funcional (distância caminhada de 6 minutos) e qualidade de vida (questionário Short Form-36 Health Survey).
Resultados:
O grupo presencial apresentou redução da fadiga (P<0,001), aumento da força muscular inspiratória (P<0,001), expiratória (P<0,001), e periférica (P<0,001), da DTC6 (P<0,001) e melhora da qualidade de vida. O grupo domiciliar não apresentou melhora significativa nessas variáveis.
Conclusão:
Um programa presencial de exercícios aeróbicos moderados em pacientes com cirrose compensada reduz a fadiga, melhora a capacidade funcional e qualidade de vida, aumenta força muscular respiratória e periférica. Os exercícios físicos domiciliares não provocam os mesmos efeitos adaptativos nesta população.
Palavras-chave:
Exercício físico; sarcopenia; reabilitação; cirrose
INTRODUCTION
Cirrhosis is the final stage of progressive liver fibrosis and represents the 14th leading cause of death worldwide11. Moon AM, Singal AG, Tapper EB. contemporary epidemiology of chronic liver disease and cirrhosis. Clinic Gastroenterol Hepatol. 2020;18:2650-66.,22. Rowe AI. Lessons from Epidemiology: The Burden of liver Disease. Dig Dis. 2017;35:304-9.. The disease modifies liver function and affects, among others, muscle tissue, causing significant muscle mass loss, which reaches pathological levels characterizing sarcopenia33. Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J Gastroenterol. 2015;21:7637-47..
Sarcopenia is a progressive and generalized syndrome of loss of skeletal muscle and muscle strength, and results from the imbalance between protein synthesis and degradation due to nutritional, metabolic and biochemical abnormalities, and increases patient morbidity and mortality44. Cruz - Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Aging. 2019;48:16-31.,55. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-93.. In addition, it causes damage to body composition, aerobic capacity, muscle strength and power production, resulting in impairments in functionality and quality of life. It is an independent mortality factor, which implies a worse clinical outcome66. Evans W. Functional and metabolic consequences of sarcopenia. J Nutri. 1997;127:998S-1003S..
Cirrhotic patients have a high prevalence of sarcopenia and physical inactivity, which, added to aging, malnutrition, decreased hepatic protein synthesis, hypermetabolism, increased inflammatory cytokines, hyperammonemia and low testosterone levels, allow muscle deconditioning, resulting in reduced cardiovascular reserve, increased physical fragility and decreased strength and quality of life77. Sinclair M, Gow PJ, Grossmann M, Shannon A, Hoermann R, Angus PW. Low serum testosterone is associated with adverse outcome in men with cirrhosis independent of the model for end-stage liver disease score. Liver Transpl. 2016;22:1482-90.,88. Mazurak VC, Tandon P, Montano- Loza AJ. Nutrition and the transplant candidate. Liver transpl. 2017;23:1451-64..
There is no effective treatment to reverse cirrhosis and management focuses on treating the primary disease, managing complications and liver transplantation. However, a poor physical condition, even after the transplant, has a negative impact on the success of the procedure, resulting in lower survival99. Carvalho EM, Isern MRM, Lima PA, Machado CS, Biagini AP, Massarollo PCB. Muscle strength and mortality while on a liver transplant waiting list. Rev Brazil Physioter. 2008;12:235-40.
10. Limongi V, Cestaro EJ Veloso-Guedes CA, Rosalen ST, Silva AMO, Bion IFSF. Relationship between respiratory muscle strength and vital capacity in mortality on the waiting list and in the postoperative period of liver transplantation. J Bras transpl. 2011;14:1594-97.-1111. Faustini Pereira JL, Galant LH, Rossi D, Telles da Rosa LH, Garcia E, de Mello Brandão AB, et al. Functional capacity, respiratory muscle strength, and oxygen consumption predict mortality in patients with cirrhosis. Can J Gastroenterol Hepatol. 2016;2016:6940374..
Research on the benefits of physical activity in cirrhotic patients is at an early stage, but suggests that physical exercise is essential as it delays the sarcopenic process1212. Haran PH, Rivas DA, Fieldingauthor RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia sarcopenia Muscle. 2012;3:157-62., increases muscle mass and strength1313. Morkane CM, Kearney O, Bruce DA, Melikian CN, Martin DS. An outpatient hospital-based exercise training program for patients with cirrhotic liver disease awaiting transplantation: a feasibility trial. Transplantation. 2020;104:97-103.
14. Debette-Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, et al. customized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145-50.-1515. Aamann L, Dam G, Borre M, Drljevic -Nielsen A, Overgaard K, Andersen H, et al. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clinic Gastroenterol Hepatol . 2020;18:1179-87.e6., improves functionality1616. Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home exercise training improves exercise capacity in cirrhosis patients: Role of exercise adherence. Sci Rep. 2018;8:99.,1717. Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci. 2020;65:3350-59., reduces the risk of falls1818. Román E, García- Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, et al. effects of an Exercise program on Functional Capacity, Body Composition and risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One. 2016;11:e0151652. and fatigue1919. Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clinic Gastroenterol Hepatol . 2014;12:1920-6.e2., promotes glycemic control2020. Nishida Y, Ide Y, Okada M, Otsuka T, Eguchi Y, Ozaki I, et al. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res. 2017;47:193-200., increases protein synthesis2121. Hasten DL, Pak- Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 years old. Am J Physiol endocrine Metab. 2000;278:620-6. and provides better quality of life1919. Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clinic Gastroenterol Hepatol . 2014;12:1920-6.e2.,2222. Román E, Torrades MT, Nadal MJ, Cárdenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise program and leucine supplementation in patients with cirrhosis. Dig Dis Sci . 2014;59:1966-75. enabling a better prognosis.
This study aimed to verify the effects of moderate aerobic exercise in person versus at home on fatigue, respiratory and peripheral muscle strength, functional capacity and quality of life in patients with compensated cirrhosis.
METHODS
This study is a randomized controlled, parallel, open-label, 2-arm clinical trial approved by the Research Ethics Committee (CEP) of the Federal University of Health Sciences of Porto Alegre (UFCSPA) and the Santa Casa de Misericordia Hospital Complex (n° 3805918 and 3938979, respectively) and was registered in the ReBec Clinical Trials registry database (n. RBR-3gtcvjU111112367585). All patients gave their informed consent before being included in the study.
Cirrhotic patients from 18 to 75 years old, of both genders, with cirrhosis of any etiology, in outpatient follow-up, were included. Due to the risk of increased portal pressure and bleeding from esophageal varices, patients with decompensated liver disease, characterized by Class C of the Child-Turcotte-Pugh score, with manifestations of portal hypertension (splenomegaly, grade III esophageal varices, increased portal vein or collateral circulation) were excluded. Patients with dietary supplementation with amino acids, recent hospitalization, neuromuscular diseases or orthopedic alterations that compromised the performance of tests or physical exercises, or with contraindications for the same were also excluded.
Patients were selected by convenience, stratified according to age and gender and randomized by the researchers, using the Microsoft Excel® program, into two groups: Face -to-face group (FG) and home group (HG). A trained researcher (P1) performed all assessments of patients in both groups and a second trained researcher (P2) supervised physical activity in the FG and guided exercises to the HG. The face-to-face assessments and interventions were performed simultaneously by the researchers in the same laboratory. Thus, blinding was only possible for the professional who performed the analysis statistic.
In-person exercise protocol
Patients filled out an evaluation form, prepared by the researchers, with demographic and clinical characteristics. Subsequently, they were evaluated by P1, regarding respiratory and peripheral muscle strength, functional capacity, quality of life and fatigue. In the following session, supervised by P2, they performed aerobic exercises twice a week, for 12 weeks. The first session consisted of 5 minutes of warm-up, followed by 30 minutes of treadmill walk, at the maximum speed tolerated by the patient, according to the Borg scale2323. Borg G. Perceived exertion and pain scales. Champaign, IL: Human Kinetics. 1998.. Two minutes of walking were added to each session, up to a limit of 50 minutes of walking, time maintained until the end of the protocol. All patients were reassessed at the 4th, 8th and 12th weeks of exercise.
Home exercise protocol
Patients were evaluated in the same way as the face-to-face group by P1 and then instructed by P2 to perform a 5-minute warm-up period followed by walking on level ground, twice a week, for 12 weeks. As in the face-to-face group, each day, they added 2 minutes of walking, until reaching 50 minutes, at the maximum tolerated speed. Patients were instructed to discontinue exercise in the event of malaise, arrhythmias, dizziness, shortness of breath and to seek medical attention as soon as possible. All patients were reassessed in person at the 4th, 8th and 12th weeks of exercise.
Variables analyzed
Fatigue was assessed using the Fatigue Severity Scale (FSS), where a score equal to or greater than four indicates severe fatigue2424. Rossi D, Galant LH, Marroni CA. psychometric property of the fatigue severity scale and correlation with depression and quality of life in cirrhotic patients. Arch Gastroenterol. 2017;54:344-8. Respiratory muscle strength was measured through maximal inspiratory (Pimáx) and expiratory (Pemáx) pressure, using the Globalmed® MVD 500 digital manovacuometer. To assess peripheral muscle strength, concentric isokinetic peak torque (PT) values were used, in Newton/Meters (Nm), generated by the knee extensor muscles of the dominant limb in the Biodex System 3 Isokinetic Dynamometer with the Biodex Advantage software version 3.0 (Biodex Medical Systems, Inc., Shirley, New York, USA). We evaluated the functional condition through the distance covered in the 6-minute walk test (6MWD), according to the American Thoracic Society guidelines2525. ATS statement: Guidelines for the six -minute walk test. Am J Breathe Crit Care Med. 2002;166:111-7. and the quality of life through the Medical Outcomes Study, Short Form-36 Health Survey questionnaire (SF-36)2626. Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Translation into Portuguese and validation of the generic SF-36 quality of life assessment questionnaire (Brazil SF-36). Rev Brazil Rheumatol. 1999;39:143-50..
Data analysis
Quantitative variables were described by average and standard deviation and categorical, by absolute and relative frequencies. The comparison of averages was performed by the t-student test. Comparison of proportions was performed using Pearson’s chi-square or Fisher’s exact tests. Simultaneous intra- and inter-group comparisons were performed using the generalized estimating equations (GEE) model, complemented by the Least Significant Difference Test. The significance level adopted was 5% (P<0.05) and the analyzes were performed using the Statistical Program Package for the Social Sciences (SPSS) version 21.0.
RESULTS
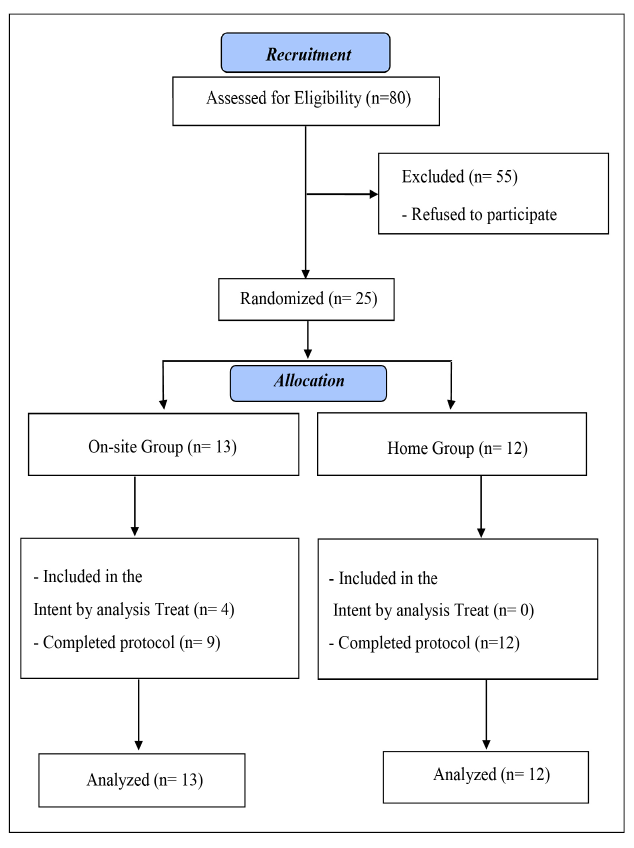
This study was carried out between 2020 and 2021. Figure 1 shows the flow of patients in the study, according to the standards of CONSORT.
Descriptive analysis of demographic characteristics of the patients analyzed in the study is shown in Table 1.
The behavior of the variables Fatigue, Pi and Pemáx, Extension PT and 6MWD between the groups throughout the exercise program is shown in Table 2. Regarding fatigue, in the intragroup comparison, the FG showed a significant reduction at each evaluated moment, with significantly lower values from the 8th week and reduced by 1.86 points, on average, during the total follow-up period, while the HG increased by 0.10 points on average, this difference being statistically significant.
The behavior of quality of life is shown in Table 3. The FG showed a statistically significant increase in the scores of almost all the SF-36 domains in relation to the HG. This increase was not significant in the mental health, social aspects and pain domains.
DISCUSSION
There are still few studies that analyze the benefits and effects of physical exercise in cirrhotic patients, evaluating different patient profiles and physical activity modalities.
The physical exercise protocols proposed for this population are still quite heterogeneous with very restrictive inclusion criteria. In the studies carried out to date, these exercises had different modalities, ranging from 6 weeks to 12 months, with sessions from 1 to 3 times a week, lasting up to 1 hour. Most studies have found positive and significant results with exercise in this population, although little is known about its clinical repercussions on the disease.
In this study, we observed that patients in the FG did not show a significant improvement in the variables studied, when compared to patients in the HG. Professional supervision could contribute to better results2727. Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical clinical outcomes _ process, healthcare utilization and costs associated with telerehabilitation disable. Rehabil. 2009;31:427-47.,2828. Grona SL, Bath B, Busch A, Rotter T, Trask C, Harrison E. Use of videoconferencing for physical therapy in people with musculoskeletal conditions: A systematic Review. J Telemed telecare. 2018;24:341-55..
Although fatigue is frequently reported by patients with cirrhosis, few studies have evaluated the symptom as an outcome with physical activity as an intervention. In our study, fatigue decreased in the FG and increased in the HG. A randomized clinical trial by Zenith et al showed that patients who performed supervised home exercise 3 times a week for 8 weeks also had symptom reduction1919. Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clinic Gastroenterol Hepatol . 2014;12:1920-6.e2..
In the FG, Pi and Pemáx reached an increase of 23 cmH2O and 28.5 cmH2O, respectively. We did not find studies that evaluated respiratory muscle strength as an outcome after exercise or physical activity in this population.
We observed an increase in the 6MWD by 97 meters in the FG patients, a result corroborated by other studies1414. Debette-Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, et al. customized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145-50.
15. Aamann L, Dam G, Borre M, Drljevic -Nielsen A, Overgaard K, Andersen H, et al. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clinic Gastroenterol Hepatol . 2020;18:1179-87.e6.
16. Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home exercise training improves exercise capacity in cirrhosis patients: Role of exercise adherence. Sci Rep. 2018;8:99.-1717. Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci. 2020;65:3350-59.. The literature shows increments of 34 to 80 meters in patients who performed supervised face-to-face exercise similar to that performed in this study. A 30.5 m increase in 6MWD has been suggested as the minimum improvement needed to confer any clinical benefit, but this has not yet been validated in patients with cirrhosis2828. Grona SL, Bath B, Busch A, Rotter T, Trask C, Harrison E. Use of videoconferencing for physical therapy in people with musculoskeletal conditions: A systematic Review. J Telemed telecare. 2018;24:341-55. The greatest increase in 6MWD was reported by Chen et al.1717. Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci. 2020;65:3350-59., of 151 meters, after intervention guided by a pedometer (P=0.03).
FG patients showed a significant increase in PT. Aamann et al. obtained similar results when cirrhotic patients performed strength physical exercise three times a week1515. Aamann L, Dam G, Borre M, Drljevic -Nielsen A, Overgaard K, Andersen H, et al. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clinic Gastroenterol Hepatol . 2020;18:1179-87.e6.. Hiraoka et al. demonstrated a 5.6% increase in muscle strength after 12 weeks of aerobic exercise with daily step count goals (P<0.01). An 11% increase in lower limb strength was also reported over the same time period (P<0.01). However, patients received amino acid supplementation concurrently with exercise2929. Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416-23..
Despite being recommended, the application of physical exercise in cirrhotic patients is far behind other chronic diseases, possibly due to the chance of increased portal pressure. Garcia-Pagan et al. demonstrated that moderate exercise increases portal pressure in patients with portal hypertension and therefore, theoretically increases the risk of variceal bleeding3030. García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300-6.. Other barriers are the lack of specific supervised exercise programs available for this group of patients and the lack of evidence for safely and effectively prescribing and evaluating exercise3131. Ney M, Gramlich L, Mathiesen V, Bailey RJ, Haykowsky M, Ma M, et al. Patient-perceived barriers to lifestyle interventions in cirrhosis. Saudi J Gastroenterol. 2017;23:97-104.. Therefore, the results of current studies may not be easily generalizable to patients with more advanced liver disease, Child-Turcotte-Pugh C or very high MELD. However, recent controlled studies have shown safety, improvements in physical fitness, muscle mass and QOL3232. Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18:146-51.
33. Brustia R, Savier E, Scatton O. Physical exercise in cirrhotic patients: Towards prehabilitation on waiting list for live transplantation. The systematic review and meta- analysis. Clin Res Hepatol Gastroenterol. 2018;42:205-15.-3434. Duarte-Rojo A, Ruiz- Margain A, Montano - Loza AJ, Macias-Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl . 2018;24:122-39..
A recent review showed that several studies investigated the effect of exercise on quality of life using different instruments with positive results3535. West J, Gow PJ, Testro A, Chapman B, Sinclair M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: The review. J Gastroenterol Hepatol. 2021;36:2687-705.. We observed that the HG achieved lower scores in relation to the FG in all domains. Studies report that the lack of direct supervision can result in a loss of exercise effectiveness. This could be an explanation for our result3636. Hwang R, Marwick T. Efficacy of home- based exercise programs for people with chronic heart failure: the meta- analysis. European journal of cardiovascular prevention and reauthenticate: official journal of the European society of Cardiology. Eur J Cardiovasc Prev Rehabil. 2009;16:527-35.,3737. Fokkenrood HJ, Bendermacher BLW, Lauret GJ, Willigendael EM, Prins MH, Teijink JAW. Supervised exercise therapy versus non- supervised exercise therapy for intermittent claudication. Cochrane Database System Rev. 2013;8:CD005263..
The limitations of this study are mainly related to the lack of nutritional control of patients in both groups. In addition, restrictions imposed by the pandemic, such as the reduction in the number of professionals and patients with access to the institutions involved in this study and transport difficulties, lack of supplies for the acquisition of technologies, led to the impossibility of four patients from the FG to continue participating in the study. This scenario only made it possible to supervise the HG in the 4th, 8th and 12th weeks and not on a daily basis like the HG. Many of our patients did not have access to technology that supported applications that would allow us to do this follow-up, even virtually.
However, this study produced statistically significant results, which makes us suppose that moderate aerobic physical exercise cannot be underestimated. In addition, it reinforces the importance of a trained professional supervision for this population. The challenge will be the development of studies with a representative sample, which may involve patients with decompensated disease, to assess the benefits of aerobic physical exercise, minimizing the risks imposed by physical activity with load. In the end, these activities could clarify the effects of exercise on the clinical evolution of these patients.
CONCLUSION
This study showed that 12 weeks of face-to-face sessions of moderate-intensity aerobic exercise, were able to reduce fatigue, strengthen respiratory and peripheral muscles, improve functional capacity and quality of life in cirrhotic patients with compensated disease. Physical activity at home did not have the same effects in these patients.
REFERENCES
-
1Moon AM, Singal AG, Tapper EB. contemporary epidemiology of chronic liver disease and cirrhosis. Clinic Gastroenterol Hepatol. 2020;18:2650-66.
-
2Rowe AI. Lessons from Epidemiology: The Burden of liver Disease. Dig Dis. 2017;35:304-9.
-
3Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J Gastroenterol. 2015;21:7637-47.
-
4Cruz - Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Aging. 2019;48:16-31.
-
5European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-93.
-
6Evans W. Functional and metabolic consequences of sarcopenia. J Nutri. 1997;127:998S-1003S.
-
7Sinclair M, Gow PJ, Grossmann M, Shannon A, Hoermann R, Angus PW. Low serum testosterone is associated with adverse outcome in men with cirrhosis independent of the model for end-stage liver disease score. Liver Transpl. 2016;22:1482-90.
-
8Mazurak VC, Tandon P, Montano- Loza AJ. Nutrition and the transplant candidate. Liver transpl. 2017;23:1451-64.
-
9Carvalho EM, Isern MRM, Lima PA, Machado CS, Biagini AP, Massarollo PCB. Muscle strength and mortality while on a liver transplant waiting list. Rev Brazil Physioter. 2008;12:235-40.
-
10Limongi V, Cestaro EJ Veloso-Guedes CA, Rosalen ST, Silva AMO, Bion IFSF. Relationship between respiratory muscle strength and vital capacity in mortality on the waiting list and in the postoperative period of liver transplantation. J Bras transpl. 2011;14:1594-97.
-
11Faustini Pereira JL, Galant LH, Rossi D, Telles da Rosa LH, Garcia E, de Mello Brandão AB, et al. Functional capacity, respiratory muscle strength, and oxygen consumption predict mortality in patients with cirrhosis. Can J Gastroenterol Hepatol. 2016;2016:6940374.
-
12Haran PH, Rivas DA, Fieldingauthor RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia sarcopenia Muscle. 2012;3:157-62.
-
13Morkane CM, Kearney O, Bruce DA, Melikian CN, Martin DS. An outpatient hospital-based exercise training program for patients with cirrhotic liver disease awaiting transplantation: a feasibility trial. Transplantation. 2020;104:97-103.
-
14Debette-Gratien M, Tabouret T, Antonini MT, Dalmay F, Carrier P, Legros R, et al. customized adapted physical activity before liver transplantation: acceptability and results. Transplantation. 2015;99:145-50.
-
15Aamann L, Dam G, Borre M, Drljevic -Nielsen A, Overgaard K, Andersen H, et al. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clinic Gastroenterol Hepatol . 2020;18:1179-87.e6.
-
16Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, et al. Home exercise training improves exercise capacity in cirrhosis patients: Role of exercise adherence. Sci Rep. 2018;8:99.
-
17Chen HW, Ferrando A, White MG, Dennis RA, Xie J, Pauly M, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci. 2020;65:3350-59.
-
18Román E, García- Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, et al. effects of an Exercise program on Functional Capacity, Body Composition and risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One. 2016;11:e0151652.
-
19Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clinic Gastroenterol Hepatol . 2014;12:1920-6.e2.
-
20Nishida Y, Ide Y, Okada M, Otsuka T, Eguchi Y, Ozaki I, et al. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res. 2017;47:193-200.
-
21Hasten DL, Pak- Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 years old. Am J Physiol endocrine Metab. 2000;278:620-6.
-
22Román E, Torrades MT, Nadal MJ, Cárdenas G, Nieto JC, Vidal S, et al. Randomized pilot study: effects of an exercise program and leucine supplementation in patients with cirrhosis. Dig Dis Sci . 2014;59:1966-75.
-
23Borg G. Perceived exertion and pain scales. Champaign, IL: Human Kinetics. 1998.
-
24Rossi D, Galant LH, Marroni CA. psychometric property of the fatigue severity scale and correlation with depression and quality of life in cirrhotic patients. Arch Gastroenterol. 2017;54:344-8.
-
25ATS statement: Guidelines for the six -minute walk test. Am J Breathe Crit Care Med. 2002;166:111-7.
-
26Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Translation into Portuguese and validation of the generic SF-36 quality of life assessment questionnaire (Brazil SF-36). Rev Brazil Rheumatol. 1999;39:143-50.
-
27Kairy D, Lehoux P, Vincent C, Visintin M. A systematic review of clinical clinical outcomes _ process, healthcare utilization and costs associated with telerehabilitation disable. Rehabil. 2009;31:427-47.
-
28Grona SL, Bath B, Busch A, Rotter T, Trask C, Harrison E. Use of videoconferencing for physical therapy in people with musculoskeletal conditions: A systematic Review. J Telemed telecare. 2018;24:341-55.
-
29Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416-23.
-
30García-Pagàn JC, Santos C, Barberá JA, Luca A, Roca J, Rodriguez-Roisin R, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300-6.
-
31Ney M, Gramlich L, Mathiesen V, Bailey RJ, Haykowsky M, Ma M, et al. Patient-perceived barriers to lifestyle interventions in cirrhosis. Saudi J Gastroenterol. 2017;23:97-104.
-
32Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18:146-51.
-
33Brustia R, Savier E, Scatton O. Physical exercise in cirrhotic patients: Towards prehabilitation on waiting list for live transplantation. The systematic review and meta- analysis. Clin Res Hepatol Gastroenterol. 2018;42:205-15.
-
34Duarte-Rojo A, Ruiz- Margain A, Montano - Loza AJ, Macias-Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl . 2018;24:122-39.
-
35West J, Gow PJ, Testro A, Chapman B, Sinclair M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: The review. J Gastroenterol Hepatol. 2021;36:2687-705.
-
36Hwang R, Marwick T. Efficacy of home- based exercise programs for people with chronic heart failure: the meta- analysis. European journal of cardiovascular prevention and reauthenticate: official journal of the European society of Cardiology. Eur J Cardiovasc Prev Rehabil. 2009;16:527-35.
-
37Fokkenrood HJ, Bendermacher BLW, Lauret GJ, Willigendael EM, Prins MH, Teijink JAW. Supervised exercise therapy versus non- supervised exercise therapy for intermittent claudication. Cochrane Database System Rev. 2013;8:CD005263.
-
Disclosure of funding: Coordination for the Improvement of Higher Education Personnel (CAPES).
Publication Dates
-
Publication in this collection
09 Sept 2022 -
Date of issue
Jul-Sep 2022
History
-
Received
27 Feb 2022 -
Accepted
13 Apr 2022