Abstracts
We report on a patient presenting Pick's disease similar to the one reported by Pick in 1892, with ubiquitin-positive and tau-negative inclusions. His diagnosis was made on the basis of clinical (language disturbance and behavioural disorders), neuropsychological (progressive aphasia of the expression type and late mutism), neuroimaging with magnetic resonance (bilateral frontal and temporal lobes atrophy) and brain single photon emission computed tomography (frontal and temporal lobes hypoperfusion) studies. Macroscopic examination showed atrophy on the frontal and temporal lobes. The left hippocampus displayed a major circumscribed atrophy. The diagnostic confirmation was made by the neuropathological findings of the autopsy that showed neuronal loss with gliosis of the adjacent white matter and apearence of status spongiosus in the middle frontal and especially in the upper temporal lobes. There were also neuronal swelling (ballooned cell) and argyrophilic inclusions (Pick's bodies) in the left and right hippocampi. Anti-ubiquitin reaction tested positive and anti-tau tested negative.
Pick's disease; lobar dementia; frontal lobe type degeneration; frontotemporal dementia
Descrevemos um caso de doença de Pick similar ao do relato original de 1892, com inclusões de ubiquitina-positivas e proteína tau negativas. O diagnóstico foi realizado com base no estudo clínico que apresentou como principais características distúrbio de linguagem e de comportamento. Na avaliação neuropsicológica, predominava afasia progressiva, especialmente do tipo expressão, seguida de mutismo. O estudo de neuroimagem por ressonância magnética mostrou atrofia frontal principalmente na região temporal bilateral. A tomografia cerebral por emissão de fóton simples mostru hipoperfusão moderada nos lobos frontal e temporal, bilateralmente. Os achados de necrópsia evidenciaram moderada atrofia do lobo frontal e intensa atrofia circunscrita do lobo temporal, predominantemente à esquerda. Do ponto de vista histopatológico, no lobo frontal medial e principalmente no lobo temporal superior, havia perda neuronal com presença de gliose da substância branca adjacente e status spongiosus. Também foram vistos células baloniformes (células de Pick) e corpúsculos argirofílicos (corpos de PicK) em neurônios das regiões hipocampais. As reações imuno-histoquímicas foram positivas para a ubiquitina e negativas para a proteína tau.
doença de Pick; demência lobar; degeneração do tipo do lobo frontal; demência fronto-temporal
CLASSIC PICK'S DISEASE TYPE WITH UBIQUITIN-POSITIVE AND TAU-NEGATIVE INCLUSIONS
Case report
Paulo Roberto de Brito-Marques1 1 Behavioral Neurology Unit, Department of Neurology, Faculty of Medical Sciences, University of Pernambuco, Brazil (UFPE). 2Department of Pathology, Health Sciences Center, UFPE. , Roberto Vieira de Mello1 1 Behavioral Neurology Unit, Department of Neurology, Faculty of Medical Sciences, University of Pernambuco, Brazil (UFPE). 2Department of Pathology, Health Sciences Center, UFPE. ,2 1 Behavioral Neurology Unit, Department of Neurology, Faculty of Medical Sciences, University of Pernambuco, Brazil (UFPE). 2Department of Pathology, Health Sciences Center, UFPE. , Luciano Montenegro1 1 Behavioral Neurology Unit, Department of Neurology, Faculty of Medical Sciences, University of Pernambuco, Brazil (UFPE). 2Department of Pathology, Health Sciences Center, UFPE. ,2 1 Behavioral Neurology Unit, Department of Neurology, Faculty of Medical Sciences, University of Pernambuco, Brazil (UFPE). 2Department of Pathology, Health Sciences Center, UFPE.
ABSTRACT - We report on a patient presenting Pick's disease similar to the one reported by Pick in 1892, with ubiquitin-positive and tau-negative inclusions. His diagnosis was made on the basis of clinical (language disturbance and behavioural disorders), neuropsychological (progressive aphasia of the expression type and late mutism), neuroimaging with magnetic resonance (bilateral frontal and temporal lobes atrophy) and brain single photon emission computed tomography (frontal and temporal lobes hypoperfusion) studies. Macroscopic examination showed atrophy on the frontal and temporal lobes. The left hippocampus displayed a major circumscribed atrophy. The diagnostic confirmation was made by the neuropathological findings of the autopsy that showed neuronal loss with gliosis of the adjacent white matter and apearence of status spongiosus in the middle frontal and especially in the upper temporal lobes. There were also neuronal swelling (ballooned cell) and argyrophilic inclusions (Pick's bodies) in the left and right hippocampi. Anti-ubiquitin reaction tested positive and anti-tau tested negative.
KEY WORDS: Pick's disease, lobar dementia, frontal lobe type degeneration, frontotemporal dementia.
Doença de Pick do tipo clássico com inclusão de ubiquitina positiva e proteína tau negativa: relato de caso
RESUMO - Descrevemos um caso de doença de Pick similar ao do relato original de 1892, com inclusões de ubiquitina-positivas e proteína tau negativas. O diagnóstico foi realizado com base no estudo clínico que apresentou como principais características distúrbio de linguagem e de comportamento. Na avaliação neuropsicológica, predominava afasia progressiva, especialmente do tipo expressão, seguida de mutismo. O estudo de neuroimagem por ressonância magnética mostrou atrofia frontal principalmente na região temporal bilateral. A tomografia cerebral por emissão de fóton simples mostru hipoperfusão moderada nos lobos frontal e temporal, bilateralmente. Os achados de necrópsia evidenciaram moderada atrofia do lobo frontal e intensa atrofia circunscrita do lobo temporal, predominantemente à esquerda. Do ponto de vista histopatológico, no lobo frontal medial e principalmente no lobo temporal superior, havia perda neuronal com presença de gliose da substância branca adjacente e status spongiosus. Também foram vistos células baloniformes (células de Pick) e corpúsculos argirofílicos (corpos de PicK) em neurônios das regiões hipocampais. As reações imuno-histoquímicas foram positivas para a ubiquitina e negativas para a proteína tau.
PALAVRAS-CHAVE: doença de Pick, demência lobar, degeneração do tipo do lobo frontal, demência fronto-temporal.
Pick's disease (PD) is believed to occur in cases of personality change (severe frontal lobe pathology), elements of the Klüver-Bucy syndrome (medial temporal lobe pathology, especially affecting the amygdala), and frontotemporal atrophy on neuroimaging. Common behaviors include apathy, decreased initiative, disinhibition and impulsivity1. At the cognitive level, PD is characterized in the first place by negative signs, e.g. apragmatism and mutism, the interpretation of which is difficult 2. During 1892-1904, Arnold Pick of Prague first described a special form of macroscopic cerebral degeneration in which the atrophy is circumscribed on the left temporal lobe (lobar atrophy). It was a study based on some cases from presenile dementia with aphasia involving of both gray and white matter - hence the term lobar rather than cortical. In 1911, Alzheimer presented the first careful study of the microscopic changes characteristic of this disease: 1) ballooned cells (cortical neuronal swellings) are found mainly in the frontal cortex; 2) argyrophilic neuronal inclusions (Pick's bodies) predominate in the medial parts of the temporal lobes, especially in the atrophic hippocampi were also found3.
For a long time, there was a clinical confusion between the semiology of PD and Alzheimer's disease (AD). In classical psychological terms, one could say that if in AD the intellectual faculties are disturbed, in PD their utilization is faulty. Ultrastructurally, the Pick's bodies are made up of straight fibrils, thus differing from the paired helical filaments that characterize AD3. However, isolating PD as he described from the large number of presenile and senile dementias has been difficult for a number of reasons2. The Lund and Manchester Groups have believed that future studies may indicate that there is a spectrum of non-specific histology involving both, or alternatively, that each may reflect a distinct process governed by different genetic or molecular mechanisms4. Constantinidis et al.5 after studying 32 cases of Pick's lobar atrophy offer evidence that one must distinguish some particular histopathological forms, characterized by the presence or absence of argyrophilic inclusions and/or neuronal swellings. They classify PD into three variants. Type A, which is classic PD, has frontotemporal and limbic degeneration characterized by neuronal swellings and argyrophilic inclusions. Type B, has frontal atrophy and cortical degeneration characterized by ballooned neurons, but no Pick's bodies. Many type B patients have extrapyramidal signs, asymmetrical motor syndromes and substantia nigra degeneration and other deep nuclei in addition. Type C is heterogeneous from clinical and pathological perspectives. Such cases have neither Pick's bodies nor bollooned neurons. There is circumscribed cortical atrophy besides varying involvement with the deep gray matter. According to Kertesz6, the PD is used either to designate clinically defined cases of progressive frontotemporal degeneration, as described by Pick, or a pathologic entity defined histologically by the presence of argyrophilic globular inclusions (Pick's bodies) and swollen achromatic neurons (Pick's cells). The so-called Pick's complex, described in 1999 by Kertesz et al.7, contain the clinical variants of PD including a substantive overlap between the clinical syndromes of frontal lobe dementia (FLD), frontotemporal dementia (FTD), primary progressive aphasia (PPA), corticobasal degeneration (CBD) picture, an amyotrophic lateral sclerosis associated with severe cortical degeneration and subcortical gliosis of temporal lobes 8,and progressive anomia (semantic variant) 1. What is Pick's disease after all ?
Because we consider classic PD to be a rare disorder, we present our experience in this case and review the relevant literature.
METHOD
A diagnosis of Pick's disease was made in a male patient according to Neary et al.9. Neurophychological assessment was made on the basis of ANAD10, Token-Test11, and the Trail Making Test12. He was also submitted to neuroimaging studies such as computed tomography (CT) and magnetic resonance (MR), and to brain single photon emission computed tomography (SPECT) and to electro- encephalography (EEG), and to apolipoprotein E (APOE) analysis. The neuropathological examination was performed acording to European Concerted Action on Pick's Disease13. The brain was fixed in 10% formalin for 4 weeks and cut into coronal sections. Pieces of the middle frontal, upper temporal, middle temporal, parietal lobes, hippocampus and para-hippocampus gyri, amygdaloid and Meynert nuclei of the brain were routinely processed and stained with hematoxylin-eosin, Masson, Wölcke's myelin and Bielschowsky methods. Immunohystochemical reaction with antibodies against tau and ubiquitin proteins were carried out.
CASE
A 72-year-old right-handed bussinessman with 5 years of education, was admitted to out-patient in June 1994. He had developed slight forgetfulness and a progressive change in his personality and social conduct about 6 years prior to presentation. At the age of 66 years a previously efficient and independent businessman had become progressively more disorganised and irrascible. His mood was reported to be aggravated; he was irritable, negativist, authoritarian, and uncooperative. He was a very jealous person toward his wife and daughter, characterized by outbursts of rage which were socially embarrassing. He was hypochondriacly obsessed with pain in the legs, which had been extensively investigated, but he kept insisting on his complains. He denied any change in personality. He used to drive his car with less care, but without getting lost. There was emotional lability with easy crying. He did not have any personal history of psychiatric illness. He was forgetful, disclaimed previous events, and made false claims for non-existent happenings. His spontaneous speech disappeared with progressive reduction (spontaneity and economy utterance), stereotypy of speech (repetition of limited repertoire of words, phrases, or themes), echolalia, perseveration and late mutism. Within a few years he had developed severe verbal inertia, and stereotyped expression appeared, reducing communication to a minimum. Motor activity was generally slow, although paradoxically, impulsive responses also occurred. In her everyday life carelessness, inertia and apathy were occasionally interrupted by episodes of impulsivity. He had become progressively more dependent, making no attempt to dress or wash himself.
His mother and eldest sister had suffered from a similar illness. No necropsy had been performed. Eccentric behaviour had been observed also in other family members.
The findings of the neurological examination 6 years after the onset of symptoms revealed a right-handed man with muscle weakness in the four limbs, especially in the legs, severe dysarthria and dementia. There was an underutilization of (without pyramidal sign) the contralateral side regarding the more atrophied hemisphere. He felt during the walk. He was in a wheelchair. All the deep tendon reflexes were increased and the Babinski's sign was absent bilaterally.
Neuropsycological examination was limited by poor cooperation. However, the positive findings showed a behaviour that was negativist and irritable, impulsive and inappropiate, with an exaggerated and melodramatic emotional display. His executive-functions such as planning, sequencing, and judgement were compromised. The patient did not initiate conversation, and his responses were brief and often elicited only by continual encouragement. He was fully disoriented regarding both time and space. The clinical findings suggested that he did not have true amnesia, but there was relative preservation of recognition memory compared to free recall. This refleted a general retrieval deficit with difficulty retrieving established memories without a temporal gradient, that is, there was equal impairment across all life periods. He had retention of concrete material in the face of short-term memory deficits. Lexical retrieval was also profoundly impaired. Language showed spontaneous speech with stereotyped phrases. He did not show very formal difficulty in comprehension, although his interpretation of the Token Test indicated severe aphasia of the global type. There was many word finding difficulty and severe anomia. He could not read or write. He had great difficulty carrying out mental calculations. Perceptual and spatial skills were not intact, as measured by his poor ability to identify successive groups of objects and block constructions.
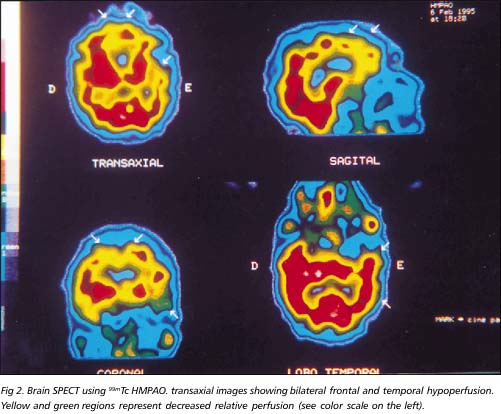
Routine biochemical and hematological were normal, and routine immunological investigations were negative. APOE was e3/e3 alelles. EEG was normal. CT obtained when he was 65 years old showed bilateral frontal hypodensity. MRI performed when he was 72 years old revealed severe bilateral atrophy of the frontotemporal lobes, especially the left temporal lobe. There was an inter-uncal distance of four centimeters (Fig 1). Brain SPECT showed moderate bilateral frontal and temporal hypoperfusion (Fig 2).
For about one year preceding his death he remained beddridden and all personal needs were cared for by nursing personnal at his house. He died at age of 74, after a total clinical course of 8 years. His death in December 1996 was attributed to a respiratory infection. The clinical diagnosis was frontotemporal dementia.
Neuropathological findings - the weight of the brain was 1100g before fixation. Macroscopic examination revealed bilateral frontotemporal atrophy, mainly on the left side (Fig 3). There was a marked lobar atrophy on the atrophic hippocampi and parahippocampi gyri with temporo-insulo-orbito-frontal involvement (Fig 4). There was a moderate enlargement of the ventricles. On microscopic examination, the middle frontal and mainly upper temporal lobes showed neuronal loss with gliosis of the adjacent white matter and statusspongiosus. Neuronal swelling (ballooned cell) and argyrophilic inclusions (Pick's bodies) were seen on the left and right hippocampi (Figs 5a and 5b). The hippocampi did not show neurofibrillary tangles or neuritic plaques. Immunohistochemical staining for anti-tau tested negative and anti-ubiquitin tested positive in the Pick's bodies (Fig 5c). No focal lesions were observed.
DISCUSSION
Pick's disease is a rare demential disorder that is sometimes familial. The cardinal features are circumscribed cortical atrophy most often affecting the frontal and temporal poles. There are neuronal swellings and argyrophilic intraneuronal inclusions. Clinical manifestations reflect the distribution of cortical degeneration, and personality deterioration and memory deficits are often more severe than visuospatial and apraxic disorders that are common in AD. These clinical overlap with other non-Alzheimer degenerative disorders is increasingly recognized14. Our patient presented clinical symptoms of bipolar mood-swings, subsequent apragmatism, short-term memory deficits, blood pressure lability, progressive aphasic signs (telegrammatic style), and late mutism and frontotemporal dementia9. There was also a very slight right pyramidal syndrome as the probable cause of the severe atrophy on the left temporal lobe12. Speech disturbances of motor or expressive type are an early sign in PD; with unspontaneous, few-word stereotyped speech, not infrequently with features of echolalia. Later on, mutism and amimia (lack of facial expressions) often develop, as it did in this case15. Reports of patients with a slowly progressive aphasic disorder in association with focal cerebral atrophy have proliferated and it has become evident that a variety of language presentations may occur16. Usually, elementary visual perception, spatial orientation and praxis are normal in most cases of PD12, but in this case the patient presented spatial disoriented and praxic disorder after a clinical course of 6 years. In some frontotemporal dementia, examination discloses the presence of apraxia, parkinsonism, primitive reflexes or a history of urinary or fecal incontinence1.
Frontotemporal dementia is believed to be normal in EEG and abnormal in AD. In a prospective study of 24 patients with presenile dementia associated with cerebral atrophy, clinical and psychological characteristics of the patients' disorders were examined in relation to pathological and chemical findings obtained from tissue analysis following cerebral biopsy. All AD patients had an abnormal EEG, while in non-Alzheimer's disease patients the EEG findings were normal17, as it happened in this case. A circumscribed cortical atrophy, most often involving the frontal and temporal lobes, is a cardinal feature in the presentation of PD, as it has been shown by neuroimaging16. The frontal lobes, the anterior poles and the inferior gyri of the temporal lobes were severely atrophic in our case. It showed a circumscribed cortical atrophy especially on the left side. However, his parietal and occipital lobes revealed mild atrophy on the RMI performed when he was 72 years old. A brain SPECT showed bilateral frontal and temporal moderate hypoperfusion. According to Brito-Marques and Vieira de Mello18, the brain SPECT should be performed in amyotrophic lateral sclerosis and other diseases in patients suspected of having frontotemporal dementia. Usually it showed a bilateral hypoperfusion on frontotemporal lobes and spared parietal and occipital lobes. It is currently the great hope in the study of primary degenerative diseases of the brain.
On macroscopic examination there was, in our case, mainly on the hippocampus and parahippocampus gyri, a cirscumscribed lobar atrophy with temporo-insulo-orbito-frontal diffusion. The frontal, temporal and parietal lobes are all affected in 75 percent of patients by the time the so-called "frontotemporal diseases" terminates, as it was shown by the clinical point of view of the praxic disorders2. Histopathological findings of our patient showed a neuronal loss, most marked in the first three layers in the middle frontal lobe and particularly in the left upper temporal girus. These alterations also were clearly seen in the insulo-orbital areas. There were also an important gliosis in the atrophic cortex and corresponding white matter with proportional demyelination and status spongiosus. Argyrophilic inclusions (Pick's bodies) were found within the cytoplasm and a neuronal achromatic swelling (ballooned cell) in the left and right hippocampi gyri2. The pathological features found in our case were the same as those found by Pick in 1892, however, without immunohistochemical study. He drew attention to the existence of circumscribed atrophies and in 1911 Alzheimer described the histological lesions characteristic of this disease - ballooned achromatic cells (neuronal swellings) and argyrophilic neuronal inclusions5. Post-mortem examination of 12 patients with clinical diagnosis and immunohistochemistry of frontal lobe dementia showed two neuronal inclusions cases in the frontal cortex and hippocampus, typical of Pick's bodies, such patients were considered as having classic PD19. It is showed that classic PD is a rare form of frontotemporal dementia. According to The Lund and Manchester Groups the main characteristics found in PD are the same as those of frontal lobe degeneration, but with intense involvement of all cortical layers and inflated neurons and Pick's bodies, which are silver stained and were tested positive. There is also more intensive white matter involvement. Patients with intense astrocytosis but without inflated neurons or inclusions, or either of them, may for the present be included4. The term FLD type is used to refer to the microvacuolar form in which there is nerve cell loss and spongiform change (microvacuolation), with relatively mild astrocytic gliosis affecting only the outer cortical layers and inflated neurones and inclusion bodies are absent. Thus, the true diagnosis of PD occurs when there are additional Pick's bodies with or without more intensive and widespread cortical gliosis. Morever, the "Pick's type" is adopted to refer to the gliotic form histology in which there is intensive astrocytic gliosis involving all cortical layers and inflated neurones and inclusions may be present20.
According to Mann et al.19 the clinical syndrome of frontal lobe dementia is pathologically heterogeneous. The recognition of the relationship between FTD lacking distinctive histology, FTD and parkinsonism linked to chromosome 17, PPA, CBD and motor neuron disease has important implications with the differential diagnosis and treatment of dementias7,21. In the not too distant past it was widely accepted that patients with cerebral atrophy could be confidently assumed to have Alzheimer's disease, once cerebrovascular disease, trauma and alcohol had been excluded, particularly during the presenium. However, it is now becoming increasingly recognised that certain non-Alzheimer forms of cerebral atrophy are more common than it has been generally supposed and these may represent a significant proportion of all cases of dementia19. From the immunohistochemistry point of view with ubiquitin-positive, tau-negative inclusions in FTD with and without motor neuron disease, and in the familial form have been previously shown22. The involvement of glial in neurodegenerative disorders has been increasingly recognized over the last decade, but we do not observe tau inclusions in frontotemporal cortical areas nor the presence ramified astrocytes and small Pick body-like inclusions in this case, as was described in PD23. However, in classic PD the phosphorylated tau from Pick's bodies, the electrophoretic profile, are made of two major components (tau 55, 64 kD) and a minor 69 kD resulting from the lack of tau isoforms with isoforms with the translated exon 1023. It is possible that in our case some biological factors had contributed to the absence of tau protein in the neuron or actually there was not it. The absence of tau protein in this case put aside the possibility of sporadic tauopathies. Nevertheless, the nosological relationships within these pathological variants, where similar histopathological and biochemical changes are present, remain uncertain.
In conclusion: 1- brain SPECT should be performed in patients suspected of having frontotemporal dementia because it showed a bilateral hypoperfusion on frontotemporal lobes and spared parietal and occipital lobes; 2- on neuroimaging, the anatomical atrophy supports the view that clinical manifestations of lobar atrophy are dictated by the topographical distribution of a common underlying pathology, such as Pick's disease, linking the syndromes of progressive aphasia to dementia of the frontotemporal lobes type; 3- at this time, we believe that it is prudent to diagnose early behavioural disorders, progressive aphasia of the expressive type with late mutism and walking troubles as clinical features of PD of the lobar type (lobar frontal or temporal or frontotemporal atrophy), and with balooned cell and Pick's bodies, which present ubiquitin-positive and tau-negative inclusions as classic PD type. However, several types of FTD will be identified as PD and further continuous and long-term clinico-pathological studies are clearly needed.
Received 23 July 2000, received in final form 31 August 2000. Accepted 1 September 2000.
Dr. Paulo Roberto de Brito Marques Rua Santa Terezinha, 58 - 53140-170 Olinda PE - Brasil. E-mail: pbrito@truenet.com.br
- 1. Mendez MF. Pick's disease. In Feinberg TE, Farah MJ. Behavioural neurology and neuropsychology. New York: McGraw-Hill,1997:571-578.
- 2. Tissot J, Constantinidis J, Richard J. Pick's disease. In Vinken PJ, Bruyn GW, Klawans HL. Handbook of clinical neurology Vol 2. (46): Neurobehavioural disorders. Amsterdam: Elsevier, 1985:233-246.
- 3. Adams RD, Victor M. Principles of Neurology. 5 Ed. New York: McGraw Hill, 1993:966-968.
- 4. The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994;57:416-418.
- 5. Contantinidis J, Richard J, Tissot R. Pick's disease: histological and clinical correlations. Eur Neurol 1974;11:208-217.
- 6. Kertesz A. Frontotemporal dementia, Pick's disease, and corticobasal degeneration. Arch Neurol 1996;54:1427-1429.
- 7. Kertesz A, Davidson W, Munoz DG. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: the Pick complex. Dement Geriatr Cogn Disord 1999;10(Suppl. 1):46-49.
- 8. Niizato K, Tsuchiya K, Tominaga I, Kato Y, Ikeda K. Pick's disease with amyotrophic lateral sclerosis: report of two autopsy cases and literature review. J Neurol Sci,1997;148:107-112.
- 9. Neary D, Snowden JS, Northen B, Goulding P. Dementia of frontal lobe type. J Neurol Neurosurg Psychiatry 1988;58:353-361.
- 10. Joanette Y, Arlette P, Bernadette S, Brito-Marques PR. Avaliaçăo neuro- psicológica adequada ŕs demęncias. Arq Neuropsiquiatr 1995;53:147-152.
- 11. DeRenzi E, Faglioni P. Normative data and screening power of a shortened version of the Token Test. Cortex 1978,14: 41.
- 12. Reitain R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958;5:152.
- 13. European Concerted Action on Pick's Disease (ECAPD) Consortium. Provisional clinical and neurophatological criteria for the diagnosis of Pick's disease. Eur Neurol 1998;5:519-520.
- 14. Dickson DW. Pick's disease: a modern approach. Brain Pathol 1998;8:339- 354.
- 15. Wallin A, Brun A, Gustafson L. Swedish consensus on dementia disease. Acta Neurol Scand 1994; Suppl 157: 8-18.
- 16. Neary D, Snowden JS, Mann DMA. Familial progressive aphasia: its relationship to other forms of lobar atrophy. J Neurol Neurosurg Psychiatry 1993;56:1122-1125.
- 17. Neary D, Snowden JS, Bowen DM, et al. Neuropsychological syndromes in presenile dementia due to cerebral atrophy. J Neurol Neurosurg Psychiatry1986;49:163-174.
- 18. Brito-Marques PR, Mello RV. Amyotrophic lateral sclerosis with dementia. Arq Neuropsiquiatr 1999;57:277-283.
- 19. Mann DMA, South PW, Snowden JS, Neary D. Dementia of frontal type: neuropathology and immunohistochemistry. J Neurol Neurosurg Psychiatry 1993;56:605-614.
- 20. Neary D. Frontal lobe dementia and motor neuron disease. Dement Geriatr Cogn Disord 1999;10(Supl 1):6-9.
- 21. Spillantini MG, Bird TD, Ghetti B. Frontotemporal dementia and parkinsonism linked to chromosome 17: a new group of taupathies. Brain Pathol 1998;8: 387-402.
- 22. Kertesz A, Kawarai T, Rogaeva E, et al. Familial frontotemporal dementia with ubiquiin-positive, tau-negative inclusions. Neurology 2000;54:818-827.
- 23. Delacourte A. Biochemical and molecular characterization of neurofibrillary degeneration in frontotemporal dementias. Dement Geriatr Cogn Disord 1999;10(Suppl 1):75-79.
Publication Dates
-
Publication in this collection
06 Apr 2001 -
Date of issue
Mar 2001
History
-
Accepted
01 Sept 2000 -
Reviewed
31 Aug 2000 -
Received
23 July 2000