Abstracts
OBJECTIVE: Early brain insults can cause cavitary lesions including porencephaly (POR) and multicystic encephalopathy (MCE). The objective of this study was to investigate clinical and electrographic correlates associated to these types of destructive brain lesions. METHOD: Patients with POR and MCE were selected and submitted to clinical and Video-EEG monitoring. The following variables were analyzed: demographic data, type of lesion, presence of gliosis, perinatal complications, epilepsy, brain atrophy, and presence and frequency of epileptiform discharges. RESULTS: Twenty patients were included, 65% males, 35% females, ages ranging from 1 to 40 years, 14 with MCE and 6 with POR. Eighteen patients had hemiparesis, 19 had epilepsy (current or in the past), seven of them had refractory seizures, and 16 had epileptiform discharges. All patients with MCE had gliosis while only 2 with POR had it. CONCLUSIONS: No correlation was observed between type of lesion and clinical and electrographical outcome. However, a positive correlation was observed between frequency of discharges and presence of brain atrophy, and between MCE and gliosis.
porencephaly; multicystic encephalopathy; epilepsy; brain lesion
OBJETIVO: Insultos cerebrais precoces podem causar lesões cavitárias incluindo porencefalias (POR) e encefalomalacias multicisticas (EMC). O objetivo deste estudo foi investigar correlatos clínicos e eletrográficos associados a estes dois tipos de lesões destrutivas. MÉTODO: Pacientes com POR e EMC foram selecionados e submetidos à avaliação neurológica e monitorização vídeo-eletrencefalográfica, analisando-se as seguintes variáveis: dados demográficos, tipo de lesão, presença de gliose, complicações perinatais, epilepsia, atrofia cerebral, presença e freqüência de descargas epilépticas. RESULTADO: Vinte pacientes foram incluídos, sendo 65% do sexo masculino, 35% do feminino, idades entre 1 e 40 anos, sendo 14 com EMC e 6 com POR. Dezoito pacientes tinham hemiparesia, 19 tinham ou tiveram epilepsia (7 deles refratários ao tratamento medicamentoso) e 16 deles tinham paroxismos epileptiformes. Todos com MCE tinham gliose associada, contra apenas 2 dos pacientes com POR. CONCLUSÃO: Não houve correlação entre tipo de lesão e evolução clínica e eletrográfica. Houve, entretando, correlação positiva entre freqüência de descargas epilépticas e presença de atrofia cerebral, e entre lesão do tipo EMC e presença de gliose.
porencefalia; encefalopatia multicística; epilepsia; lesão cerebral
Early destructive lesions in the developing brain: clinical and electrographic correlates
Lesões destrutivas precoces no cérebro em desenvolvimento: correlatos clínicos e eletrográficos
Cristiane LowI; Eliana GarzonI; Henrique Carrete Jr.II; Luis Celso VilanovaI; Elza Márcia T. YacubianI; Américo C. SakamotoI, III
IUNIPETE - Unidade de Pesquisa e Tratamento das Epilepsias - Departamento de Neurologia e Neurocirurgia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo SP, Brasil
IIDepartamento de Radiologia, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo SP, Brasil
IIIDepartamento de Neurologia, Psiquiatria e Psicologia Médica, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto SP, Brasil
ABSTRACT
OBJECTIVE: Early brain insults can cause cavitary lesions including porencephaly (POR) and multicystic encephalopathy (MCE). The objective of this study was to investigate clinical and electrographic correlates associated to these types of destructive brain lesions.
METHOD: Patients with POR and MCE were selected and submitted to clinical and Video-EEG monitoring. The following variables were analyzed: demographic data, type of lesion, presence of gliosis, perinatal complications, epilepsy, brain atrophy, and presence and frequency of epileptiform discharges.
RESULTS: Twenty patients were included, 65% males, 35% females, ages ranging from 1 to 40 years, 14 with MCE and 6 with POR. Eighteen patients had hemiparesis, 19 had epilepsy (current or in the past), seven of them had refractory seizures, and 16 had epileptiform discharges. All patients with MCE had gliosis while only 2 with POR had it.
CONCLUSIONS: No correlation was observed between type of lesion and clinical and electrographical outcome. However, a positive correlation was observed between frequency of discharges and presence of brain atrophy, and between MCE and gliosis.
Key words: porencephaly, multicystic encephalopathy, epilepsy, brain lesion.
RESUMO
OBJETIVO: Insultos cerebrais precoces podem causar lesões cavitárias incluindo porencefalias (POR) e encefalomalacias multicisticas (EMC). O objetivo deste estudo foi investigar correlatos clínicos e eletrográficos associados a estes dois tipos de lesões destrutivas.
MÉTODO: Pacientes com POR e EMC foram selecionados e submetidos à avaliação neurológica e monitorização vídeo-eletrencefalográfica, analisando-se as seguintes variáveis: dados demográficos, tipo de lesão, presença de gliose, complicações perinatais, epilepsia, atrofia cerebral, presença e freqüência de descargas epilépticas.
RESULTADO: Vinte pacientes foram incluídos, sendo 65% do sexo masculino, 35% do feminino, idades entre 1 e 40 anos, sendo 14 com EMC e 6 com POR. Dezoito pacientes tinham hemiparesia, 19 tinham ou tiveram epilepsia (7 deles refratários ao tratamento medicamentoso) e 16 deles tinham paroxismos epileptiformes. Todos com MCE tinham gliose associada, contra apenas 2 dos pacientes com POR.
CONCLUSÃO: Não houve correlação entre tipo de lesão e evolução clínica e eletrográfica. Houve, entretando, correlação positiva entre freqüência de descargas epilépticas e presença de atrofia cerebral, e entre lesão do tipo EMC e presença de gliose.
Palavras-chave: porencefalia, encefalopatia multicística, epilepsia, lesão cerebral.
Destructive brain lesions result from aggression to the central nervous system (CNS)1 and may be congenital or acquired during the postnatal period2. Several factors may be implicated in their etiology, such as infections, anoxia and exposure to aggressing factors, among others3,4.
When extensive, these lesions produce destruction of brain tissue and cavitations, the major ones being multicystic encephalomalacia (MCE) and porencephaly (POR). Interestingly, these cavitary lesions correspond to differentiated reactions to the same type of brain insult, with these responses depending on the phase of CNS development during which the injury occurs2. POR is caused by very early insults occurring at the end of the second or the beginning of the third trimester of pregnancy, affecting an immature brain still unable to express significant astrocyte reaction. This leads to the formation of a cavity with smooth walls, precise limits and little or no perilesional gliosis. In contrast, MCE results from insults occurring at the end of pregnancy, during delivery or during the first days of life, reaching a brain that is already more mature and able to express differentiated tissue responses, leading to the formation of lesions of imprecise limits containing trabeculae and showing moderate or intense glial reaction3,5-9.
The differences between these two forms of brain cavities prompted us to question whether these different CNS responses to the same type of insult might be related to a better or worse prognosis from a clinical and neurophysiological viewpoint. On this basis, the objective of the present study was to investigate patients with destructive lesions of the POR and MCE types, in an attempt to correlate the clinical and neurophysiological aspects associated to each type of lesion.
METHOD
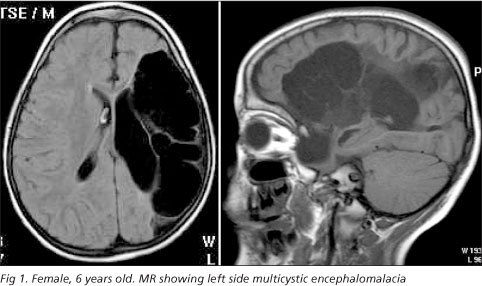
In this study we included 20 consecutive patients with structural lesions of the POR or MCE type diagnosed by brain magnetic resonance (MR) examination (Figs 1 and 2). All patients were selected at the Neurology and Neuropediatric Outpatient Clinics from Hospital São Paulo, Escola Paulista de Medicina, Federal University of São Paulo (UNIFESP). The MRs were independently analyzed by two neuroradiologists using Barkovich's criteria9, as follows: a) POR: an homogeneous cavity with smooth walls and precise limits whose interior is isointense in relation to cerebrospinal fluid (CSF). There is no structure inside the cavity and the intensity of the adjacent brain is normal; b) MCE: one or multiple cavities of varying sizes containing glial septations and CSF that confer a non-homogeneous aspect to the cavity. The adjacent brain may present a glial reaction consisting of astrocyte proliferation, which is one of the pathological characteristics of this lesion.
All selected patients were submitted to clinical neurological evaluation and to continuous video-electroencephalographic monitoring (video-EEG) ranging in duration from a minimum of three hours to a maximum of four days. The more prolonged exams were carried out on patients with refractory epilepsy who needed a more detailed evaluation including the assessment of potential surgical treatment. The data thus obtained were correlated with neuroimaging and video-EEG findings and with neurological evaluation. The following variables were analyzed: a) type of lesion; b) presence of gliosis; c) presence of epilepsy; d) presence of perinatal complications; e) presence of epileptic discharges; f) frequency of discharges on video-EEG; g) presence of atrophy.
Statistical analysis for the determination of correlations was carried out through Fisher's exact test. The study was approved by the Ethics and Research Committee of UNIFESP and patients or persons responsible for them gave written informed consent agreeing to participate.
RESULTS
Twenty patients (13 males and 8 females) with destructive brain lesions of the MCE and POR type were included in the study. Patient age ranged from 1 to 40 years (mean: 23 years). Six of these patients had POR and 14 received a diagnosis of MCE. Regarding motor function, all patients but two had motor deficit, with two patients having double hemiparesis (cases 2 and 7), while the remaining ones had hemiparesis contralateral to the lesion. Regarding gestational and neonatal history, four patients had a history of complications during delivery. Detailed clinical data are presented in Table 1.
Nineteen of the 20 patients had current or previous epilepsy at some time in life, with only six of them being controlled with antiepileptic drugs and seizure free for more than one year, while seven patients were considered refractory to treatment with antiepileptic drugs.
Video-EEG data showed that all patients had asymmetry of background rhythms. Two types of involvement were observed: asymmetry due to increased slow activity and asymmetry due to depression of background rhythms (Table 2). Nineteen patients had increased slow activity in the injured hemisphere, with nine having depression of background rhythms ipsilateral to the lesion. Regarding non-epileptiform abnormalities, 10 patients had bursts of slow waves which were ipsilateral to the lesion in five patients, contralateral to the lesion in three, and bilateral in the remaining two patients. Epileptiform abnormalities such as spikes, polyspikes, sharp waves and sharp-slow wave complexes were detected in 16 patients, 12 of them had discharges ipsilateral to the lesion, three had bilateral discharges, and only one patient presented epileptiform activity in the hemisphere contralateral to the cavitary lesion (case 10). Epileptic discharges were visually assessed and ranged from very frequent to absent, as shown in Table 2.
According to the criteria proposed by Barkovich, 14 patients had MCE and six POR. Of the 14 patients with MCE, 12 presented moderate or intense gliosis, while two had only discrete gliosis. Four of the six patients with POR did not present gliosis, whereas the remaining two had only discrete gliosis. The corpus callosum was abnormal and thin in all patients except one. The brainstem presented atrophy ipsilateral to the lesion in 16 patients and was normal in four. No malformations associated with the destructive lesions were observed. Fifteen patients presented atrophy of the cerebral parenchyma ipsilateral to the lesion. MR data are presented in Table 3.
We observed no significant correlations between type of lesion and presence of perinatal complications, or between the occurrence of epilepsy and frequency of epileptic discharges in the video-EEG and presence of gliosis. The p-value obtained for these correlations was higher than 0.05. On the other hand, a positive correlation was observed between gliosis and type of lesion (Table 4) and between frequency of discharges and presence of atrophy (Table 5). In the former case, all 14 patients with MCE presented gliosis ranging from discrete to intense, while it was absent in four of the six patients with POR. Regarding the frequency of discharges and atrophy, we observed that 14 out of the 15 patients with atrophy presented epileptic discharges, while no discharges were detected in three of the five patients without atrophy.
DISCUSSION
In the present study we analyzed the clinical, electrographic and imaging characteristics of patients with lesions of the POR and MCE type. Regarding clinical aspects, there was predominance of male patients (65%) and of motor deficit (80%). Congenital hemiparesis is the most common form of cerebral paralysis among term newborns10. Of the 16 patients with hemiparesis, 11 had it on the right, indicating predominant involvement of the dominant hemisphere. Similar data were previously reported in a study involving 51 patients and showing greater involvement of males and predominance of right-side hemiparesis11. The possibility of greater vulnerability of the male brain and slower maturation of the dominant hemisphere were previously proposed by Taylor12.
Regarding pre and perinatal history, four of the 20 patients had suffered complications at the time of delivery, including pre-eclampsia, hemorrhagic gestosis, prematurity and post-term delivery with severe neonatal anoxia. These patients presented motor deficit and epilepsy. The brain lesions were associated with some degree of physical or mental impairment13. We observed that motor alterations or epilepsy were also present in the group of patients who had not suffered perinatal complications.
Analysis of the video-EEG revealed asymmetry of background activity in all patients, characterized by depression ipsilateral to the lesion in 10 cases (50%) and disorganization of background activity ipsilateral to the lesion in 19 (95%). Of the 16 patients with epileptiform abnormalities in the EEG, only one had discharges originating in the hemisphere contralateral to the lesion (case 10). The EEG of this patient showed disorganization and depression of background activity ipsilateral to the lesion, and presence of epileptic discharges in the contralateral hemisphere, a fact that may be explained by the severe atrophy in the hemisphere containing the destructive lesion. Among the patients who did not present epileptiform abnormalities (cases 8,13,15,19), patient 8 had a deeper lesion involving the white matter, patient 13 had a preserved remaining parenchyma with no evidence of atrophy, and patients 15 and 19 showed no evidence of gliosis. The EEG is usually abnormal in the presence of POR14 and as previously reported, it shows background depression ipsilateral to the cavitary lesion15. Background abnormalities are more common in patients with hemispheric lesions16. A small proportion of these patients with extensive hemispheric damage show discordant lateralization of epileptiform discharges and structural lesion16. Although a compensatory skull thickening is usually found, the false lateralization of the epileptiform discharges seems to be more related to the EEG background depression and to the extension of cerebral lesion16.
In the structural imaging exams, other abnormalities were observed in addition to the presence of the destructive lesion. The corpus callosum was thin in 19 patients. Although the corpus callosum is formed during an earlier phase of development, the presence of a formed, but thin corpus callosum may be the result of hypoxic-ischemic insults to the cortex or to the white matter after the complete formation of the corpus callosum (18 to 20 weeks)17.
In our series, 17 patients additionally presented atrophy of the brain stem, Wallerian degeneration (WD) ipsilateral to the lesion and associated with the presence of motor deficit. In the MRC group the degree of WD was correlated with the distribution and severity of the congenital hemiplegia18, and an important association between motor impairment and the degree of asymmetry of the brain stem was also observed. In the present study, we detected brain atrophy ipsilateral to the destructive lesion in 15 patients (75%). Some previous studies have correlated the presence of cortical and subcortical atrophy with the occurrence of epilepsy, the severity of motor impairment and low intellectual quotient6,8,19. Hippocampal atrophy was also observed in patients with congenital destructive hemispheric lesion20. Independently of the lesion type, POR or MRC, approximately two third of the patients had hippocampal atrophy without correlation with duration of epilepsy or seizure frequency20 .
When we tried to correlate the frequency of epileptic discharges with the presence of atrophy and gliosis, we observed a correlation between the frequency of discharges and the presence of atrophy (p< 0.05) but not between the frequency of discharges and the presence of gliosis (cases 15,16 and 17).
The response of the brain to an aggression is known to change according to the maturity of the organ at the time of the insult3,6,8, and the presence of gliosis is related to a better astrocyte response, a fact that theoretically occurs in more mature brains. In the present study we detected a correlation (p< 0.05) between the type of lesion and gliosis, i.e., gliosis was more evident in the presence of MCE. This finding agrees with other literature reports demonstrating that MCE is a lesion that occurs later and consequently involves more extensive gliosis5,7. In contrast, POR occurs earlier and is associated with a less intense glial reaction2,9,20,21.
Previous studies employing magnetic resonance angiography (MRA) have proposed a classification based on the topographic distribution of the lesions22. Hemispheric lesions would correspond to an homogeneous atrophy of an entire hemisphere and in this series could be related to prolonged episodes of unilateral status epilepticus. Lesions limited to a main arterial territory would provoke cavitations or localized retractions of the cerebral tissue, leading to heterogeneous lesions corresponding to extensive gliotic scars. Lesions located between main cerebral arterial territories would be classified as arterial borderzone lesions22. Interestingly the MRA studies corroborated the hypothesis of a vascular nature for these insults and for most patients the degree of cerebral blood flow impairment was proportional to the size of the MRI lesion22. In those cases of borderzone lesions MRA was consistently normal and all of them had history of perinatal complications suggesting that hypoxia or other insults in infants could lead to global hypoperfusion 22. Our cases fit in arterial territory lesions or combination of arterial territory lesions and borderzone lesions. In fact, a vascular etiology sometimes associated to perinatal complications seem to be the probable etiology of these cases.
In conclusion, this study demonstrated that early destructive lesions in the developing brain have poor correlation with clinical and eletrencephalographic outcome. The EEG abnormalities are nonspecific, only pointing to the presence of a focal brain lesion. However, there is a correlation between the frequency of epileptic discharges and the presence of atrophy, and between a MCE lesion and the presence of gliosis. Whether a causal relationship between the high frequency of discharges and the degree of brain atrophy exists this remains open and needs to be addressed in further studies.
Received 13 July 2006, received in final form 29 November 2006. Accepted 9 February 2007.
Dr. Américo C. Sakamoto - Departamento de Neurologia, Psiquiatria e Psicologia Médica / Faculdade de Medicina de Ribeirão Preto - USP / Campus Universitário - 14048-900 Ribeirão Preto SP - Brasil. E-mail: sakamoto@fmrp.usp.br
- 1. Acosta M, Gallo V, Batshaw ML. Brain development and the ontogeny of developmental disabilities. Adv Pediatr 2002;49:1-57.
- 2. Raybaud C. Destructive lesions of the brain. Neuroradiology 1983;25: 265-291.
- 3. Volpe JJ. Neurology of the newborn. 3.Ed. Philadelphia: Saunders, 1995.
- 4. Weidenheim KM, Bodhireddy SR, Nuovo GJ, Nelson SJ, Dickson WD. Multicystic encephalopathy: review of eight cases with etiologic considerations. J Neuropathol Exp Neurol 1995;54:268-275.
- 5. Ferrer I, Navarro C. Multicystic encephalomalacia of infancy: clinico-pathological report of 7 cases. J Neurol Sci 1978;38:179-189.
- 6. Kotlarek F, Rodewig R, Brull D, Zeumer H. Computed tomographic findings in congenital hemiparesis in childhood and their relation to etiology and prognosis. Neuropediatrics 1981;12:101-109.
- 7. Erasmus C, Blackwood W, Wilson J. Infantile multicyistic encephalomalacia after maternal bee sting anaphylaxis during pregnancy. Arch Dis Child 1982;57:785-787.
- 8. Wiklund LM, Uvebrant P, Flodmark O. Morphology of cerebral lesions in children with congenital hemiplegia: a study with computed tomography. Neuroradiology 1990;32:179-186.
- 9. Barkovich AJ. Pediatric neuroimaging. 3.Ed. Lippincott Williams & Wilkins, 2000.
- 10. Steinlin M, Good M, Martin E, Bänziger O, Largo RH, Boltshauser E. Congenital hemiplegia: morphology of cerebral lesions and pathogenetic aspects from MRI. Neuropediatrics 1993;24:224-229.
- 11. Süssova J, Seidl Z, Faber J. Hemiparetic forms of cerebral palsy in relation to epilepsy and mental retardation. Dev Med Child Neurol 1990; 32:792-795.
- 12. Taylor DC. Differential rates of cerebral maturation between sexes and between hemispheres. Lancet 1969;2:140-142.
- 13. Myers RE, Valerio MG, Martin DP, Nelson KB. Perinatal brain damage: porencephaly in a cynomologous monkey. Biol Neonate 1973;22:253-273.
- 14. Niedermeyer E, Silva FL. Electroencephalography: basic principles, clinical applications, and related fields. 4.Ed. Baltimore: Lippincott 1998.
- 15. Castroviejo IP. Diagnóstico clínico-radiológico em neurologia infantil. Barcelona: Ed. Científico Médico; 1971
- 16. Teixeira RA, Li ML, Santos SLM, et al. Lateralization of epileptiform discharges in patients with epilepsy and precocious destructive brain insults. Arq Neuropsiquiatr 2004;62:1-18.
- 17. Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. Am J Neuroradiol 1992;13:959-972.
- 18. Bouza H, Dubowitz LM, Rutherford M, Pennock JM. Prediction of outcome in children with congenital hemiplegia: a magnetic resonance imaging study. Neuropediatrics 1994;25:60-66.
- 19. Uvebrant P. Hemiplegic cerebral palsy: aetiology and outcome. Acta Paediatr Scand Suppl 1988;345(Suppl):S1-S100.
- 20. Teixeira RA, Leone AAA, Honorato DC, Damasceno BP, Guerreiro CAM, Cendes F. Congenital destructive hemispheric lesions and epilepsy: clinical features and relevance of associated hippocampal atrophy. Arq Neuropsiquiatr 2000;58:990-1001.
- 21. Norman RM, Urich H, Woods GE. The relationship between prenatal porencephaly and encephalomalacias of early life. J Ment Sci 1958;104: 758-771.
- 22. Teixeira RA, Zanardi VA, Li LM, Santos SLM, Cendes F. Epilepsy and destructive brain insults in early life: a topographical classification on the basis of MRI findings. Seizure 2004;13:383-391.
Publication Dates
-
Publication in this collection
24 July 2007 -
Date of issue
June 2007
History
-
Reviewed
29 Nov 2006 -
Received
13 July 2006 -
Accepted
09 Feb 2007