Abstracts
OBJECTIVE: To search for right/left asymmetries in the dendritic trees of the neuronal populations and in the cell-free layer volumes of the human hipoccampal formation. METHOD: In necropsic material obtained from six male individuals we performed a quantitative Golgi study of the dendritic trees of dentate granules, CA3 and CA1 pyramidal neurons and a volumetric analysis of dentate gyrus molecular layer, strata oriens plus alveus and strata lacunosum-moleculare plus radiatum of CA3 and CA1 fields. RESULTS: We found inter-hemispheric asymmetries in the dendrites trees of all neurons, reaching the significant level in the number of granule cells dendritic segments (higher in the left than in the right hemisphere), dendritic branching density of CA3 pyramidal cells and mean dendritic length of CA1 apical terminal segments (higher in the right than in the opposite side). No volumetric differences were observed. CONCLUSION: This study points to different anatomical patterns of connectivity in the hippocampal formations of both hemispheres which may underlie functional asymmetries.
brain asymmetries; hippocampal formation; dendritic asymmetries
OBJETIVO: Pesquisar a existência de assimetrias direita/esquerda nas arborizações dendríticas neuronais e nos volumes das camadas não celulares da formação do hipocampo humano. MÉTODO: Efectuamos estudo quantitativo Golgi das arborizações dendríticas dos grânulos da fascia denteada e das células piramidais de CA3 e CA1, e uma análise estereológica dos volumes da camada molecular da fascia denteada, do strata oriens + alveus e do strata lacunosum-moleculare + radiatum de CA3 e de CA1 em material necrópsico colhido em 6 indivíduos do sexo masculino. RESULTADOS: Encontrámos assimetrias inter-hemisféricas nas arborizações dendríticas de todos os neurónios, significativas no número de segmentos dendríticos das células granulares (maior à esquerda do que à direita) na densidade de ramificação dendrítica das pirâmides de CA3 e no comprimento dendrítico médio dos segmentos apicais terminais das pirâmides de CA1 (maiores à direita do que à esquerda). Não encontramos diferenças volumétricas. CONCLUSÃO: Estes resultados alertam para diferentes padrões anatómicos de conectividade nas formações do hipocampo de ambos os hemisférios que podem fundamentar assimetrias funcionais.
assimetrias cerebrais; formação do hipocampo; assimetrias dendríticas
ARTICLES
Dendritic right/left asymmetries in the neurons of the human hippocampal formation: a quantitative Golgi study
Assimetrias dendríticas direita/esquerda nos neurónios da formação do hipocampo humano: estudo quantitativo Golgi
Maria José SáI; Carlos RuelaII; Maria Dulce MadeiraIII
IInvestigadora do Instituto de Anatomia, Faculdade de Medicina da Universidade do Porto; Assistente Graduada de Neurologia, Directora da Unidade de LCR e da Clínica de Esclerose Múltipla, Serviço de Neurologia, Hospital S. João, Porto, Portugal
IIProfessor Associado de Anatomia, Instituto de Anatomia, Faculdade de Medicina da Universidade do Porto, Portugal
IIIProfessora Catedrática de Anatomia, Instituto de Anatomia, Faculdade de Medicina da Universidade do Porto, Portugal. Supported by Fundação para a Ciência e a Tecnologia, Unit 121/94
ABSTRACT
OBJECTIVE: To search for right/left asymmetries in the dendritic trees of the neuronal populations and in the cell-free layer volumes of the human hipoccampal formation.
METHOD: In necropsic material obtained from six male individuals we performed a quantitative Golgi study of the dendritic trees of dentate granules, CA3 and CA1 pyramidal neurons and a volumetric analysis of dentate gyrus molecular layer, strata oriens plus alveus and strata lacunosum-moleculare plus radiatum of CA3 and CA1 fields.
RESULTS: We found inter-hemispheric asymmetries in the dendrites trees of all neurons, reaching the significant level in the number of granule cells dendritic segments (higher in the left than in the right hemisphere), dendritic branching density of CA3 pyramidal cells and mean dendritic length of CA1 apical terminal segments (higher in the right than in the opposite side). No volumetric differences were observed.
CONCLUSION: This study points to different anatomical patterns of connectivity in the hippocampal formations of both hemispheres which may underlie functional asymmetries.
Key words: brain asymmetries, hippocampal formation, dendritic asymmetries.
RESUMO
OBJETIVO: Pesquisar a existência de assimetrias direita/esquerda nas arborizações dendríticas neuronais e nos volumes das camadas não celulares da formação do hipocampo humano.
MÉTODO: Efectuamos estudo quantitativo Golgi das arborizações dendríticas dos grânulos da fascia denteada e das células piramidais de CA3 e CA1, e uma análise estereológica dos volumes da camada molecular da fascia denteada, do strata oriens + alveus e do strata lacunosum-moleculare + radiatum de CA3 e de CA1 em material necrópsico colhido em 6 indivíduos do sexo masculino.
RESULTADOS: Encontrámos assimetrias inter-hemisféricas nas arborizações dendríticas de todos os neurónios, significativas no número de segmentos dendríticos das células granulares (maior à esquerda do que à direita) na densidade de ramificação dendrítica das pirâmides de CA3 e no comprimento dendrítico médio dos segmentos apicais terminais das pirâmides de CA1 (maiores à direita do que à esquerda). Não encontramos diferenças volumétricas.
CONCLUSÃO: Estes resultados alertam para diferentes padrões anatómicos de conectividade nas formações do hipocampo de ambos os hemisférios que podem fundamentar assimetrias funcionais.
Palavras-chave: assimetrias cerebrais, formação do hipocampo, assimetrias dendríticas.
The existence of anatomical inter-hemispheric asymmetries has since long been described in several regions of the normal human brain, mainly in those related to the processing of language functions1-4. In effect, most studies addressing this issue were centered in the neocortex where right/left differences were observed in macroscopic features, such as the shape and configuration of cerebral sulci and specific cortical areas, as well as in fine aspects of its architecture, namely the number, size and shape of neurons, and the extent and spatial organization of their dendritic arborizations3,5-7. Conversely, few anatomical studies were conducted in the archicortex, namely in the hippocampal formation8-10 despite the clinical and image evidence of laterality in a number of functions related to the processing of specific memory tasks11-20. Actually, a few years ago, we reported the existence of right/left asymmetries in the morphology of the human hippocampal formation, in a stereological study focused on the cell-containing layers of the main hippocampal subdivisions8. Specifically, we have found that the right hippocampal formation contained 20% more granule cells and 14% more CA3-2 pyramidal neurons than the left, and that the volumes of the cell-containing layers and of their constituent neurons did not differ between the right and the left hippocampal formations8. The lack of parallelism between the right/left differences observed in the number of neurons and in the volumes of the hippocampal layers suggested the existence of inter-hemispheric differences in the components of the neuropil in the granular layer of the dentate gyrus and in the CA3-2 pyramidal cell layer.
Because there are descriptions of hemispheric differences in the length of the distal dendritic segments of CA1 pyramidal cells10, we decided to further investigate this subject by performing a quantitative study of the dendritic arborizations of the main neuronal populations of the human hippocampal formation, using Golgi impregnated material. Furthermore, we have estimated, using stereological methods, the volumes of the layers where the dendritic trees of the hippocampal formation neurons are located, such as the molecular layer of the dentate gyrus, the stratum oriens plus stratum alveus and the stratum lacunosum-moleculare plus stratum radiatum of the CA3 and CA1 hippocampal fields.
METHOD
Subjects Brains from six male adult individuals, who died suddenly after traumatic accidents not involving the skull, were collected during autopsies performed at the Medical Legal Institute of Porto, as previously described8,21. The average age was 32 years (range 23-49 years), the mean brain weight was 1426 g (range 1355-1670 g) and the mean postmortem delay was 33 h (range 24-37 h). None of the subjects had shown signs of neurological or psychiatric diseases prior to death neither medical records of alcohol or drug abuse.
Tissue processing In each subject, the hippocampal formations of both hemispheres were dissected and fixed in 4% paraformaldehyde for at least three months after the autopsy. Six right and six left hippocampal formations from the same subjects were analyzed. All the studies were focused on the subdivisions of the hippocampal formation where the constituents of the trisynaptic intrinsic circuit are located, that is, the dentate gyrus and the hippocampus proper22. After dissection, the material was identified by a code number, so that the investigators were not aware of its provenience as regards the side. The hippocampal formations were embedded in a 7% agar solution and sliced in the coronal plane at regular intervals of 3.7 mm, thus originating 12-14 parallel coronal slabs per each hippocampal formation, as previously described8,21. Before starting the histological processing for the stereological analysis, each slab was cut in the coronal plane into 2 slices, one 2 mm-thick and the other 1.7 mm-thick, respectively in its anterior and posterior faces. Thus, 2 subsets of 12-14 parallel coronal slices from each hippocampal formation were obtained, which allowed the collection of material to perform simultaneously the stereological estimates of the volumes of the hippocampal layers and the study of the Golgi impregnated dendritic arborizations.
Blocks of tissue comprising the above-mentioned hippocampal subdivisions were collected from the 1.7 mm-thick slices and Golgi impregnated using a modification of the rapid Golgi method23, as previously reported24. Briefly, the blocks were fixed during 5 days in a solution containing 0.2% osmium and 2.4% potassium dichromate in 100 mL of distilled water, and afterwards immersed for 24 h in the dark in a 3% solution of potassium dichromate. The specimens were then transferred into 0.75% silver nitrate and stored in the dark for 3 days, changed once to an equal solution and kept in the dark for a further 3 days. Afterwards, the blocks were briefly immersed in absolute alcohol and terpineol, wrapped in a paraffin wax shell and sliced in the coronal plane at a nominal thickness of 100 µm. The tissue slices were dehydrated, cleared in terpineol and mounted on slides under a synthetic resin (Caedax), without coverslip.
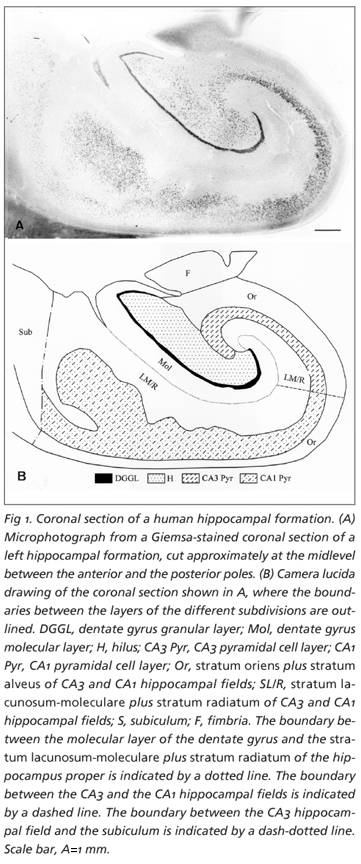
For the stereological study, the 2 mm-thick slices of each hippocampal formation were dehydrated through a graded series of ethanol solutions and embedded in glycolmethacrylate, and, from each embedded slab, one 50 mm-thick section was cut with a microtome. The sections were serially mounted and stained with a Giemsa solution modified for use in glycolmethacrylate-embedded material25 (Fig 1).
Quantitative study of the Golgi-impregnated dendritic trees
Cell selection - The dendritic arborizations of the dentate granule cells and of the pyramidal cells of the CA3 and CA1 hippocampal fields (basal and apical domains) were analyzed. The morphological criteria employed for the selection of the neurons were similar to those described by De Ruiter and Uylings26: integrity and dark homogeneous impregnation throughout the extent of the dendrites, cell bodies located in the middle part of the section thickness to minimize the number of branch segments cut off at the plane of the section and relative isolation from other impregnated cells, blood vessels and silver deposits placed nearby. The existence of cut terminal segments on a neuron was not considered as a criterion for its exclusion from the estimations, because this type of elimination of neurons would have biased the sample toward smaller neurons27. Following these criteria, ten granule cells, eight CA3 pyramidal cells and eight CA1 pyramidal cells were selected from each case, both in the right and in the left hippocampal formations (Fig 2).
Morphometric analysis - The dendritic arborizations of the selected neurons were traced by hand with the aid of a camera lucida, at final magnification of x450 for granule cells and x425 for pyramidal neurons. The parameters evaluated and the analyses performed, which were fairly similar to a prior study of our group24, included:
Number of dendritic segments per cell: The dendritic segments were classified in two major groups - terminal and intermediate segments27. The total number of segments per cell was calculated by summing up the number of terminal and of intermediate dendritic segments.
Metric analysis of dendritic segments: The individual length of the different types of segments was measured with the aid of a MOP-Videoplan, allowing the calculation of the total dendritic length after their respective sum. The total and the mean lengths of the terminal and of the intermediate segments were also obtained.
Dendritic branching density: The branching density of the dendritic trees was evaluated by applying a variant of the method of concentric circles28 without correction for reducing the three-dimensional branching pattern to two dimensions29. The concentric circles were calibrated at intervals of 20 µm for the granule cells, 15 µm for the basal trees of the CA3 and CA1 neurons, and 30 µm for the apical domains of the CA3 and CA1 pyramids. The dendritic intersections at circle 15 beyond the cell body were included in the circle " equal or greater than 15" .
Volume estimation Volumes were estimated, based on the Principle of Cavalieri30, from all 50 µm-thick sections of each hippocampal formation. The estimates were independently obtained in the molecular layer of the dentate gyrus, and in the stratum oriens plus stratum alveus and stratum lacunosum-moleculare plus stratum radiatum of the CA3 and CA1 hippocampal fields (Fig 1). For the sake of simplicity, these layers were collectively designated as cell-free layers, as we did elsewhere21,24, because they contain relatively few neurons when compared to the layers where the cell bodies of the main neuronal populations of the hippocampal formation are located. The CA2 field was included in the CA3 hippocampal subdivision. The boundaries of the layers were defined on the basis of their cytoarchitectonic organization31,32 and outlined with the help of a camera lucida attachment and a 3x objective lens, as previously described21. The area of the sectional profiles of each layer was estimated by point counting using a grid of test points in which the area per point, a(p), was 0.56 mm2 for the molecular layer of the dentate gyrus, 0.27 mm2 for the stratum oriens plus stratum alveus and stratum lacunosum-moleculare plus stratum radiatum of the CA3 field, 0.56 mm2 for the CA1 stratum oriens plus stratum alveus and 1.09 mm2 for the CA1 stratum lacunosum-moleculare plus stratum radiatum. The volumes of the layers were then calculated from the total number of points that fell on each layer, SP, and the distance between the systematically sampled sections t (3.7 mm)30.
On average, the SP counted on the molecular layer of the dentate gyrus was 167, on the CA3-2 stratum oriens plus stratum alveus 251, on the CA3-2 stratum lacunosum-moleculare plus stratum radiatum 182, on the CA1 stratum oriens plus stratum alveus 218 and on the CA1 stratum lacunosum-moleculare plus stratum radiatum 192.
The magnitude of the shrinkage/swelling induced by tissue processing was estimated as described in detail elsewhere31-34. Because the tissue shrinkage factor (SFv) was negligible (0.98), no correction of the volume estimates was performed.
Statistical analysis The precision of the individual estimates of the volume of the layers, evaluated as the coefficient of error (CE), was obtained as a function of the " Nugget effect" and the variance due to the sampling between systematically random sampled sections35. The mean CE was calculated from the estimates for an individual using the relationship: Mean CE = Ö mean CE2 25. The observed variance among individuals was estimated using the coefficient of variation (CV=S.D./mean). A paired Student's t-test was performed to evaluate the effect of right/left side. Differences were considered to be significant if p<0.05.
RESULTS
Qualitative observations The observation of the hippocampal formations and of the sections selected for the stereological estimations showed that their morphology (Fig 1), structural organization and interindividual variability were quite similar to the expected, considering a previous study21. As well, the quality of the Golgi impregnation of the hippocampal formations was good, allowing an easy identification of the fine dendritic structures (Fig 2), and the morphological characteristics of the dendritic arborizations of the different neuronal populations fit the classical descriptions36-39. Yet, the selection of complete dendritic arborizations in the pyramidal cells of the CA3 and CA1 fields was easier for the basal than for the apical trees, due to the length and larger incidence of incomplete branches in the latter, as we have previously reported24.
Quantitative results
Study of the dendritic arborizations:
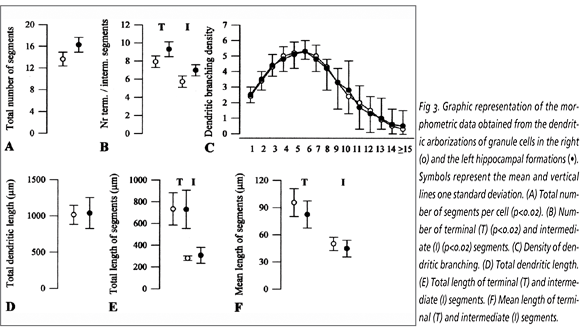
Granule cells: Significant right/left differences were noticed in the total number of dendritic segments and in the number of terminal and intermediate segments, which were 14.3% (p<0.02), 12% (p<0.02), 17.1% (p<0.02), respectively, higher in the left than in the right hippocampal formations (Fig 3). Conversely, no significant differences were found between both hemispheres as regards the dendritic branching density and the dendritic extent, assessed by the total dendritic length and by the length of both the terminal and intermediate dendritic segments. Although no significant right/left differences have been detected in the mean length of the terminals and intermediate segments, their values were 11.3% and 9.5% greater in the right than in the left side, respectively.
CA3 pyramidal cells: The results obtained in the basal trees are shown in Figure 4. No significant differences were found between the right and the left sides concerning the total number of dendritic segments, and the number of terminal and intermediate segments, although those values were slightly higher in the right than in the left hippocampus. Concerning dendritic branching density, significant differences were detected in the circles 8, 10 and 13 (p<0.03), since higher values were found in the right than in the left side. The metric analysis did not reveal significant differences, even though the total dendritic length, the length of the terminal and intermediate segments and the mean length of the terminal and the intermediate segments were 13.8%, 14.2%, 12%, 10.5% and 8.1%, respectively, greater in the right than in the left side. With respect to the apical dendritic arborizations of the CA3 pyramids (Fig 5), no significant right/left differences were observed in the number of segments. The sole parameter displaying significant differences was the dendritic branching density, whose values found in the circles 14 (p< 0.05) and 15 (p<0.03) were higher in the right than in the left side (p<0.05). The dendritic extent was similar in both sides, although the total dendritic length and the length of the terminal and intermediate segments were 17.6%, 18.4% and 17.4%, respectively, higher in the right than in the left side; in addition, the mean length of the terminal and intermediate segments was 13.6% and 12%, respectively, greater in the right than in the left side.
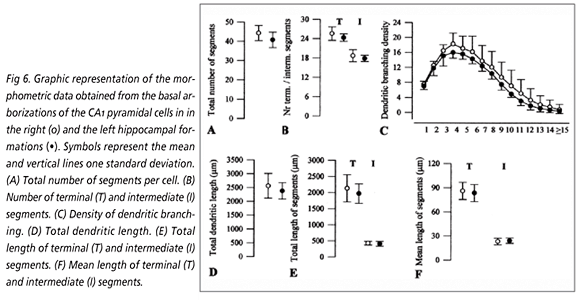
CA1 pyramidal cells: No significant differences were observed between both sides in the basal arborizations of these neurons (Fig 6), despite the slightly higher values found in the right than in the left side in all, but one, of the evaluated parameters. In effect, the total number of dendritic segments, the dendritic branching density, the total dendritic length and the total length of the terminals were higher in the right than in the left hemisphere, whereas the mean length of the intermediate segments was slightly higher in the left than in the right side. Conversely, several dendritic parameters studied in the apical trees of the CA1 pyramids (Fig 7) presented larger values in the left than in the right hippocampus total number of segments, number of terminal and intermediate segments, extent of the intermediate segments but none of these differences reached the significant level. In contrast, the total dendritic extent and the length of the terminal segments displayed higher values in the right than in the opposite side, but only the right/left differences in mean length of the terminal segments reached the significant level (11.2%, p<0.03). The dendritic branching density did not significantly differ between both groups.
Volumetric study of the cell-free layers of the hippocampal formation The results obtained in this study are shown in Table.
Dentate gyrus: No significant differences were observed in the volume of the molecular layer of the dentate gyrus between both hemispheres, although its mean value was 8.3% higher in the right than in the left side.
CA3 hippocampal field: The volumes of the CA3 stratum oriens plus stratum alveus and stratum lacunosum-moleculare plus stratum radiatum were 8.9% and 11.4% greater in the right than in the opposite side, respectively. However, none of these differences reached the significant level.
CA1 hippocampal field: The mean volumes of the CA1 stratum oriens plus stratum alveus and the stratum lacunosum-moleculare plus stratum radiatum were quite similar in both hemispheres.
DISCUSSION
In this study we present evidence for the existence of right/left asymmetries in the dendritic trees of the main neuronal populations of the human hippocampal formation, i.e., the granule cells of the dentate gyrus and the pyramidal cells of the CA3 and CA1 hippocampal fields. However, no significant inter-hemispheric differences were noticed in the volumes of the hippocampal layers that contain the dendritic arborizations of those neurons.
With respect to the granule cells, the existence of a higher number of dendritic segments in the left side, when compared to the opposite one, nicely fits the slightly greater mean nuclear volume previously found in the granule cells located in the left dentate gyrus8. That finding is in accordance with the hemispheric asymmetries recently described in the human entorhinal cortex, a brain region that projects to the hippocampal formation, where leftward size predominance was found40. Besides, as the left dentate gyrus contains fewer granule cells than the right one8, we may hypothesize that the higher number of dendritic segments that we found in the left side might somewhat compensate for this difference. On the other hand, the absence of significant right/left asymmetries in the dendritic extent and branching pattern of the granule cell dendritic arborizations parallels the lack of inter-hemispheric asymmetries in the volumes of the dentate gyrus molecular layer, and of the granule cell layer itself that we have previously reported8. Curiously, the variations found in the just mentioned parameters were all of the same type, with slightly higher values in the right than in the left dentate gyrus8. Thus, as a whole, and taking into account the previous stereological study8, we may summarize the right/left asymmetries found in the dentate gyrus as follows: in the left side, granule cells have more dendritic segments and somewhat larger nuclei; in the opposite side, the molecular and the granular layers have fairly higher volumes, and there are more granule cells, which, in turn, have slightly longer dendritic trees.
With respect to the CA3 hippocampal field, we found that all dendritic parameters assessed both in the basal and in the apical arborizations of the pyramidal cells had higher values in the right than in the opposite hemisphere. This asymmetry might be ascribed to the larger size of the mossy fiber system in the right dentate gyrus, expected on the basis of the presence of significantly more granule cells in this hemisphere than in the left8 and of a one-way unidirectional projections in this brain region41. In addition, the inter-hemispheric differences in the dendritic extent and branching pattern of the whole CA3 pyramidal cell dendritic trees are in agreement with the results obtained in the volumes of the CA3 cell-free layers, where we have observed slightly higher values in the right than in the left side. As well, these findings may also be correlated to the slightly larger volume of the CA3 pyramidal cell layer and to the significantly higher number of its constituent neurons in the right hippocampal formation compared to the left one, that we have previously described8. Even being aware that significant right/left asymmetries were only detected in the dendritic branching density of the distal portions of the basal and apical dendritic trees of CA3 pyramidal cells, the target of the commissural fibers and of the perforant pathway, respectively, we found it useful to highlight the main characteristics of CA3 field in each side. Thus, and assembling the prior stereological study8, we may advance that the right CA3 hippocampal field has a slightly larger volume and contains more pyramidal cells that display larger dendritic trees than the corresponding field of the opposite hemisphere.
As regards the CA1 pyramidal cells, the absence of right/left asymmetries in the architecture of their basal dendritic trees does not fit the study of Barrera et al.10 in which it was found that the distal total dendritic length of the CA1 basal arborizations was larger in the left than in the right hemisphere. This discrepancy may be due in part to differences in the composition of the samples analyzed in both studies, since our subjects were, on average, 20 years younger than the male individuals studied by Barrera et al.10. On the contrary, we noticed that the values of most parameters assessed in the basal dendritic trees of the CA1 pyramidal cells were slightly higher in the right than in the left side. These results may be in part related to the greater number of Schaffer collaterals originating from the CA3 pyramidal cells of the right hemisphere, as the number of these neurons is significantly higher than in the opposite side8. In effect, the Schaffer collaterals establish synapses in the stratum radiatum and also in the stratum oriens of the CA1 field, where the basal arborizations are located42. In addition, the mean length of the terminal segments of the apical dendritic trees was significantly larger in the right than in the left side, and this was the sole parameter that showed important inter-hemispheric variations in the CA1 pyramidal cells. Thus, we recognize that the structural organization of the dendritic trees of the CA1 pyramidal cells is quite similar in both hemispheres, which is in accordance with the presence of similar neuronal numbers and neuronal size8, as well as with the analogous volumes found in the cell-free layers in this hippocampal subdivision of both hemispheres. Even so, we underline that in the CA1 hippocampal field of the right hemisphere there are slightly more neurons of smaller size8, that display longer dendritic segments and more basal, but less apical, dendritic segments, than in the left side.
Therefore, the existence of right/left differences in the dendritic arborizations of the neuronal populations of the main subdivisions of the human hippocampal formation suggests that the morphology of its excitatory trisynaptic circuit is different in both hemispheres, which might finally lead to functional asymmetries. Actually, as regards the first synapse, the dendritic trees of the granule cells were somewhat more developed in the left than in the right dentate gyrus. This is interesting in view of the increasing evidence that the dentate gyrus is involved in the early processing of the information43 and that the left hippocampal formation is implicated in verbal memory tasks20. Conversely, the pyramidal cells of the CA3 and CA1 hippocampal fields, which are regions of the hippocampal circuitry involved in the later processing of the information, displayed higher values in the right hemisphere, in most of the evaluated dendritic parameters. Yet, the right hippocampus has been classically associated with the processing of the visual memory11. In addition, we have reasons to believe that the components of the second synapse, that is, the mossy fibers and the apical dendritic trees of the CA3 pyramidal cells, as well as the constituents of third synapse of the circuit, i.e. the Schaffer collaterals and the CA1 apical dendritic segments, are morphologically enriched in the right hemisphere. However, as the granule cells of the left side display more dendritic segments than in the right, which suggests that they have a larger receptor surface, one may speculate that our findings give strength to our previous assumption that the organization of the neural circuits that include the hippocampal formation is more complex in the left than in the right hemisphere8.
In conclusion, this study presents evidence for the existence of different anatomical patterns in the connectivity of the hippocampal formation in both hemispheres, which may underlie right/left asymmetries in its functional activity. In addition, it provides a morphological substrate for the clinical evidence of laterality in this brain region, namely in those functions related to the processing of specific memory tasks.
Acknowledgements We thank Professor J. Pinto da Costa for providing the hippocampal material and Dr. Marta Carvalho for technical assistance.
Received 30 March 2007. Accepted 24 September 2007.
Dra. Maria José Sá - Instituto de Anatomia / Faculdade de Medicina da Universidade do Porto - Alameda Professor Hernâni Monteiro 4200-319 Porto - Portugal. E-mail: mjsa@med.up.pt
Institute of Anatomy, Porto Medical School, Portugal, Department of Neurology, Hospital São João, Porto, Portugal
- 1. Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right-left asymmetries in the brain. Structural differences between the hemispheres may underlie cerebral dominance. Science 1978;199:852-856.
- 2. Steinmetz H, Volkmann J, Jancke L, Freund HJ. Anatomical left right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol 1991;29:315-319.
- 3. Jacobs B, Schall M, Scheibel AB. A quantitative dendritic analysis of Wernickes area in humans: II. Gender, hemispheric, and environmental factors. J Comp Neurol 1993;327:97-111.
- 4. Hutzler JJ. The specialized structure of human language cortex: pyramidal cell size asymmetries within auditory and language-associated regions of the temporal lobes. Brain Lang 2003;86:226-242.
- 5. Hellige JB. Hemispheric asymmetry and components of perception, cognition and action. In Kosslyn SM (Ed). Hemispheric asymmetry: whats right and whats left. Ch. 3.Ed. Cambridge: Harvard University Press, 1993:65-113.
- 6. Paus T, Okay N, Caramanos Z, et al. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol 1996;376:664-673.
- 7. Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 1997;384:312-320.
- 8. Sá MJ, Pereira A, Paula-Barbosa MM, Madeira MD. Anatomical asymmetries in the human hippocampal formation. Acta Stereol 1999;18:161-176.
- 9. Zaidel DW. Regional differentiation of neuron morphology in human left and right hippocampus: comparing normal to schizophrenia. Int J Psychophysiol 1999;34:187-196.
- 10. Barrera A, Jiménez L, González GM, Montiel J, Aboitiz F. Dendritic structure of single hippocampal neurons according to sex and hemisphere of origin in middle-aged and elderly human subjects. Brain Res 2001;906:31-37.
- 11. Kimura D. Right temporal-lobe damage: perception of unfamiliar stimuli after damage. Arch Neurol 1963;8:264-271.
- 12. Lencz T, McCarthy G, Bronen RA, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological functions. Ann Neurol 1992;31:629-637.
- 13. Hellige JB. Biological asymmetries in the human brain. In Kosslyn SM (Ed). Hemispheric asymmetry: whats right and whats left. Ch. 4.Ed. Cambridge: Harvard University Press, 1993:116-135.
- 14. Goldberg TE, Torrey EF, Berman F, Weinberger DR. Relations between neuropsychological performance and brain morphological and physiological measures in monozygotic twins discordant for schizophrenia. Psychiatr Res 1994;55:51-61.
- 15. Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: Activation of the right hippocampus in taxi drivers J Neurosci 1997;17:7103-7110.
- 16. Brockway JP. Deep language structures: memory for connected discourse produced unilateral (L) hippocampal activation observed by functional magnetic resonance imaging. Brain Cogn 1999;40:57-60.
- 17. Nolte J. Drives, emotion, and memories: the hypothalamus and limbic system. In Shreiner J (Ed). The human brain: an introduction to its functional anatomy. 5.Ed. St Louis: Mosby Inc, 2002:559-585.
- 18. Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J Int Neuropsychol Soc 2004;10:664-678.
- 19. Platel H. Functional neuroimaging of semantic and episodic musical memory. Ann N Y Acad Sci 2005;1060:136-147.
- 20. Alessio A, Bonilha L, Rorden C, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav 2006;8:593-600.
- 21. Sá MJ, Madeira MD, Ruela C, et al. AIDS does not alter the total number of neurons in the hippocampal formation but induces cell atrophy: a stereological study. Acta Neuropathol 2000;99:643-653.
- 22. Lopes da Silva FH, Witter MP, Boeijinga PH, Lohman AHM. Anatomical organization and physiology of the limbic cortex. Physiol Rev 1990;70:453-511.
- 23. Ramón y Cajal S, Castro F. Métodos para la demonstración de la morfologia de las neuronas/procederes de Golgi y sus variantes. In Salvat SA (Ed). Elementos de tecnica micrografica del sistema nervioso. 2.Ed. Barcelona: Mallorca, 1972:63-80.
- 24. Sá MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol 2004;107:97-110.
- 25. West MJ, Slomianska L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 1991;231:482-497.
- 26. De Ruiter JP, Uylings HBM. Morphometric and dendritic analysis of fascia dentata granule cells in human aging and senile dementia. Brain Res 1987;402:217-229.
- 27. Uylings HBM, Ruiz-Marcos A, Van Pelt J. The metric analysis of three-dimensional dendritic tree patterns: a methodological review. J Neurosci Methods 1986; 18:127-151.
- 28. Eayrs TJ. The cerebral cortex of normal and hypothyroid rats. Acta Anat 1955;25:160-183.
- 29. De Voogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science 1981;214:202-204.
- 30. Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc 1987;147:229-263.
- 31. West MJ, Gundersen HJG. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol 1990;296:1-22.
- 32. West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimers disease. Lancet. 1994;344:769-772.
- 33. Uylings HBM, Van Eden CG, Hofman MA. Morphometry of size/volume variables and comparison of their bivariate relations in the nervous system under different conditions. J Neurosci Methods 1986;18:19-37.
- 34. Madeira MD, Cadete-Leite A, Andrade JP, Paula-Barbosa MM. Effects of hypothyroidism upon the granular layer of the dentate gyrus in male and female adult rats: a morphometric study. J Comp Neurol 1991;314:171-186.
- 35. West MJ, Østergaard K, Andreassen OA, Finsen B. Estimation of the number of somatostatin neurons in the striatum: an in situ hybridization study using the optical fractionator method. J Comp Neurol 1996;370:11-22.
- 36. Ramón y Cajal S. Histologie du Systéme Nerveux de lHomme et des Vertébrés. Vol. II. Paris:Maloine, 1911.
- 37. Lorente de Nó R. Studies on the structure of cerebral cortex. II Continuation of the study of the ammonic system. J Psychol Neurol 1934;46:113-177.
- 38. Lindsay RD, Scheibel AB. Quantitative analysis of dendritic branching pattern of granular cells from human dentate gyrus. Exp Neurol 1976;52:295-310.
- 39. Desmond NL, Levy WB. Granule cell dendritic spine density in the rat hippocampus varies with spine shape and location. Neurosci Lett 1985;54:219-224.
- 40. Simic G, Bexheti S, Kelovic Z, et al. Hemispheric asymmetry, modular variability and age-related changes in the human entorhinal cortex. Neuroscience 2005;130:911-925.
- 41. Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 1989;31:571-591.
- 42. Amaral DG, Witter MP. Hippocampal formation: in the rat nervous system. Ed. Paxinos G. Academic Press, 1995:443-493.
- 43. Flood DG, Coleman PD. Hippocampal plasticity in normal aging and decreased plasticity in Alzheimers disease. In Storm-Mathisen J, Zimmer J, Ottersen OP (Eds). Understanding the brain through the hippocampus. Progress in brain research. Vol.83. Amsterdam: Elsevier, 1990:435-444.
Publication Dates
-
Publication in this collection
07 Mar 2008 -
Date of issue
Dec 2007
History
-
Accepted
24 Sept 2007 -
Received
30 Mar 2007