Abstracts
Neuromyelitis optica (NMO) is an inflammatory, demyelinating disease of the central nervous system characterized by the association of a serious myelitis and unilateral or bilateral optic neuritis. The present study aimed to analyze the immunological parameters of NMO patients with diagnosis established based on Wingerchuck et al. (1999) criteria. Production of IgG and IgA antibodies to antigens of MBP, PLP 95-116, MOG 92-106, and the cytokines interleukin-4 (IL-4) and interferon-γ (INF-γ) were assessed by Elisa assay. The cohort was formed by 28 NMO patients and a matched healthy control group. NMO patients had significant high levels of IgG to MOG (p<0.0001), PLP (p=0.0002) and MBP (p<0.0001), and solely IgA to MBP (p<0.0001). INF-γ (p=0.61) levels were similar to healthy controls. Increased production of IL-4 (p=0.0084) indicates an important role for this cytokine in the activation of Th2 regulatory cells and of the IgA producers B lymphocyte indicating activation of humoral immunity.
neuromyelitis optica; autoantigens; cytokines; myelin basic protein
A neuromielite óptica (NMO) é doença inflamatória do sistema nervoso central, caracterizada por mielite aguda ou subaguda grave e neurite óptica unilateral ou bilateral. Este estudo objetiva analisar parâmetros imunológicos de pacientes com critérios de Wingerchuck et al. (1999) para NMO. O método de ELISA avaliou a produção de IgG e IgA para antígenos da proteína básica da mielina (MBP), o proteolipídeo (PLP) 95-116, a glicoproteina associada ao oligodendrócito (MOG) 92-106 e as citocinas interleucina-4 (IL-4) e interferon-gama (INF-γ). Foram incluνdos 28 pacientes com NMO pareados com controles saudáveis. Pacientes com NMO apresentaram níveis significativamente elevados de imunoglobulinas reativas dos isotipos IgG para MOG (p<0,0001), PLP (p=0,0002) e MBP (p<0,0001) e IgA somente para MBP (p<0,0001). Os níveis de INF-γ (p=0,61) foram semelhantes aos controles. A produção elevada de IL-4 (p=0,0084) indica papel importante na ativação de células regulatórias Th2 e linfócitos B produtores de IgA e da ativação da imunidade humoral.
neuromielite óptica; autoantígenos; citocinas; proteína básica da mielina
Immune system markers of neuroinflammation in patients with clinical diagnose of neuromyelitis optica
Marcadores imunológicos em pacientes com diagnóstico de neuromielite óptica
Soniza Vieira Alves-LeonI, IV; Maria Lucia Vellutini PimentelII; Gabrielle Sant'AnnaIII; Fabíola Rachid MalfetanoIV; Cláudio Duque EstradaIV; Thereza Quirico- SantosV
INeurology Department/Hospital Universitário Clementino Fraga Filho-Federal University of Rio de Janeiro, Rio de Janeiro RJ, Brazil
IINeurology Department/Santa Casa de Misericórdia do Rio de Janeiro, Rio de Janeiro RJ, Brazil
IIIBiology Department,/Fluminense Federal University, Niterói RJ, Brazil (UFF)
IVPostgraduation Program-Federal University of Rio de Janeiro State, Rio de Janeiro RJ, (UFRJ-UNIRIO)
VBiology Department, UFF, Niterói RJ, Brazil
ABSTRACT
Neuromyelitis optica (NMO) is an inflammatory, demyelinating disease of the central nervous system characterized by the association of a serious myelitis and unilateral or bilateral optic neuritis. The present study aimed to analyze the immunological parameters of NMO patients with diagnosis established based on Wingerchuck et al. (1999) criteria. Production of IgG and IgA antibodies to antigens of MBP, PLP 95116, MOG 92106, and the cytokines interleukin-4 (IL-4) and interferon-γ (INF-γ) were assessed by Elisa assay. The cohort was formed by 28 NMO patients and a matched healthy control group. NMO patients had significant high levels of IgG to MOG (p<0.0001), PLP (p=0.0002) and MBP (p<0.0001), and solely IgA to MBP (p<0.0001). INF-γ (p=0.61) levels were similar to healthy controls. Increased production of IL-4 (p=0.0084) indicates an important role for this cytokine in the activation of Th2 regulatory cells and of the IgA producers B lymphocyte indicating activation of humoral immunity.
Key words: neuromyelitis optica, autoantigens, cytokines, myelin basic protein.
RESUMO
A neuromielite óptica (NMO) é doença inflamatória do sistema nervoso central, caracterizada por mielite aguda ou subaguda grave e neurite óptica unilateral ou bilateral. Este estudo objetiva analisar parâmetros imunológicos de pacientes com critérios de Wingerchuck et al. (1999) para NMO. O método de ELISA avaliou a produção de IgG e IgA para antígenos da proteína básica da mielina (MBP), o proteolipídeo (PLP) 95116, a glicoproteina associada ao oligodendrócito (MOG) 92106 e as citocinas interleucina-4 (IL-4) e interferon-gama (INF-γ). Foram incluνdos 28 pacientes com NMO pareados com controles saudáveis. Pacientes com NMO apresentaram níveis significativamente elevados de imunoglobulinas reativas dos isotipos IgG para MOG (p<0,0001), PLP (p=0,0002) e MBP (p<0,0001) e IgA somente para MBP (p<0,0001). Os níveis de INF-γ (p=0,61) foram semelhantes aos controles. A produção elevada de IL-4 (p=0,0084) indica papel importante na ativação de células regulatórias Th2 e linfócitos B produtores de IgA e da ativação da imunidade humoral.
Palavras-chave: neuromielite óptica, autoantígenos, citocinas, proteína básica da mielina.
Neuromyelitis optica (NMO) was described, for the first time, in 1894, by Eugène Devic1 and further considered a variant of multiple sclerosis (MS) or a distinct syndrome2,3. Acute transverse myelitis can be the first symptom. One or more episodes of optic neuritis (ON) can occur in combination with myelitis, been more acute and severe in this case. The interval between ON and myelitis is variable; when monophasic the interval is shorter. Optic neuritis can be unilateral or bilateral. The paraclinical diagnose is made with brain MRI, spinal cord MRI and cerebrospinal fluid (CSF) analysis. The brain MRI can be normal at the onset of NMO or show non-specific white-matter lesions that do not satisfy neurological criteria for MS3-5. MRI of the spinal cord shows longitudinally extensive lesions and characteristically span three or more contiguous vertebral segments4-8. Prominent CSF pleocytosis with more than 50 leucocytes with neutrophil predominance is characteristic of NMO and rare in MS. The synthesis of IgG is smaller than seen in MS. Therefore the oligoclonal bands which mean intrathecal synthesis of immunoglobulin are found only in 23% of NMO patients, but in 88% of MS patients4. The supportive diagnose is based on the presence of autoantibodies which are found in 50% of the cases, on the visual evoked potential (subclinical ON) and on brain MRI with gadolinium enhancement of the optic nerves or chiasm. Since its description NMO was considered a variant of MS. The pathologic studies, the CSF and the MRI showed evidences that it should be a distinctive disease. The diagnostic criteria had been established only in 1999, by Wingerchuck et al. 9 and were divided into absolute criteria, major supportive criteria and minor criteria. Recently, Lennon et al.10,11 reported a serum autoantibody to immunoglobulin G, which is a specific marker to NMO, NMO-IgG, to be selectively linked to the acquaporin-4 channels (AQP-4), a component of the protein distroglican complex, localized in the endfeet of astrocytes, processed in the blood-brain barrier10,11. The high prevalence of autoantibodies in NMO and its association to collagen diseases suggest a malfunction of B cells immunity12 and the detection of the autoantibody NMO-IgG corroborate this observation13.
Lucchinetti et al.14 revealed demyelinated lesions in association to perivascular deposits of immunoglobulin, local activation of the complement cascade and eosinophil infiltration, suggesting a humoral immunity in its pathogenesis14,15. Another mechanisms involved in this humoral reaction would be the production of the antibody anti-myelin oligodendrocyte glycoprotein (anti-MOG) and secretion of interleukin-2 (IL-2), a cytokine related to autoimmunity due to T cells. NMO is the first CSN idiopathic, demyelinated disease to have a specific biological marker and can represent the first example of a new class of an autoimmune channelopathy10. Through the AQP-4 channels, small, but important quantities of peripheral immunoglobulin can reach the CNS at the vulnerable regions of the blood-brain barrier10,12,16.
The goal of this study was to determine the serum levels of IgG and IgA antibodies to antigens of myelin basic protein (MBP), proteolipid (PLP) 95116, myelin oligodendrocyte glycoprotein (MOG) 92106, and the cytokines interleukin-4 (IL-4) and interferon-γ (INF-γ) in NMO patients comparing to healthy controls.
METHOD
Twenty-eight NMO patients were included, considering the 1999 Wingerchuk criteria9, notwithstanding sex, age or ethnic background. They were followed from July 2003 to November 2005. The 1999 Wingerchuk criteria9 considered the diagnose of NMO in patients with optic neuritis and transverse myelitis, with no other neurological manifestation, a normal brain MRI at the onset or a spinal cord MRI with a longitudinal extensive lesion, or a CSF with more than 50 cells/mm3 or bilateral optic neuritis or severe optic neuritis in one eye, plus a severe paresis (strength grade <2) in one limb. The CSF was analyzed in 23 out of 28 patients (78.5%). Optic neuritis and myelitis were considered notwithstanding the interval separating neither disease-defining attacks nor the clinical presentation, monophasic or relapsing. The patients were ethnically classified as Afro-Brazilians, with Niger ancestries until the third generation, and Caucasians, with none afro-descendents on their background. The term Afro-Brazilian was considered taking into account the anthropological concept proposed by Ribeiro17. The neurological impairment was measured by the Expanded Disability Status Scale (EDSS), stablished by Kurtzke18, which evaluates the functional systems (FS) related to various functions of the central nervous system (CNS); pyramidal, cerebellar, brain stem, sensory, bowel and bladder, visual, cerebral or mental and other functions. The score goes from zero, normal neurological exam, to ten, death due to MS. A control group was formed by 28 healthy individuals, notwithstanding sex, age or ethnic background. In this group there were active scientists, interns and employees from the Cellular Pathology Laboratory, Department of Molecular and Cellular Biology, Fluminense Federal University, Rio de Janeiro, Brazil. All of them were evaluated for inflammatory markers and autoimmunity through laboratory exams which had normal results. All patients signed a consent term. The Project was approved by the National Council in Ethic and Research (CONEP), register number 1265, May 29th 2000.
Patients with neurological evidence of optic neuritis and myelitis who did not fulfill the Wingerchuk criteria for NMO were excluded, as were patients with other autoimmune diseases like Sjögren syndrome, Behçet disease or systemic lupus erythematosus. In the control group the individuals with abnormal laboratorial parameters of inflammation and autoimmunity, although clinically healthy, were excluded.
RESULTS
Among 28 NMO patients, 21 were females (75%) and seven males. Taking into account the ethnic group, 14 patients (50%) were Afro-descendents and 14 were Caucasians (50%). The control group was composed of 28 healthy individuals, 15 (58%) were females and 11 (42%) males; 21 (81%) were Caucasians and five (19%) were Afro-Brazilians. Ages ranged from 18 to 43 years old.
Table 1 describes patients characteristics related to sex, ethnic group, age of inclusion in the study, disease duration, age of the first bout, interval between the first and second bouts, total number of bouts and actual EDSS.
The age of symptom onset varied from eight to 46 years old (mean age=27 years), 21.4% younger than 20 years old, 32.1% between 20 and 30 years old and 46.5% older than 30 years old. Considering the first bout, optical neuritis was the most common symptom, found in 13 patients (46.4%). Two of them had bilateral optical neuritis (15.4% of all the optical neuritis in the study). Eight patients (28.6%) had transverse myelitis. Optical neuritis and myelitis were present as a first bout in seven patients (25%). The recurrent presentation of NMO was prevalent in our series (92.8%). The interval between the index events varied from three to 228 months and in seven cases they were simultaneously. The mean duration of the disease was 17.5 years, ranging from six to 29 years (Table 2). Three patients (10.7%) patients had it for five years and 25 (89.3%) had it for more than five years. In our series there were no deaths. The average number of bouts in the recurrent forms was 8.5 (2 to 15); seven patients (25%) had more than one bout per year, all Afro-Brazilians. During the bouts all patients were treated with endovenous methylprednisolone, 1 g/day/3 days, repeated during 3 to 5 weeks. Nine patients (32.14%) had an EDSS >4 when entering the study group; 66.66% were Afro-descendents and 33.4% were Caucasians. Tables 3 and 4 synthesized our results.
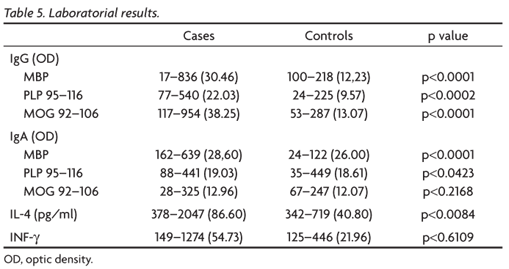
Laboratorial results
The results and statistical significance found in NMO patients and control groups are found in Table 5. The Mann-Whitney test was applied. The plasma of each patient and control were analyzed three times.
We found higher levels of IgG and IgA antibodies to native MBP with statistic significance (p<0.0001) in NMO patients compared to the controls.
The blood levels of IgG to PLP 95-116 were also higher with statistic significance (p=0.0002) in NMO patients compared to controls, although this was not observed in PLP 95-116 IgA (p=0.0423).
NMO patients had an expressive (p<0.001) higher blood levels of IgG antibodies to MOG 92-106 but the IgA antibodies to the same sequence did not show statistic significance (p=0.2168).
The plasmatic levels of IFN-γ did not show statistic significance in the group of NMO patients (p=0.6109) although the IL-4 plasmatic levels were significantly higher (p=0.0084) in this group compared to the control group.
DISCUSSION
In the last years the classification of NMO, a disease considered to be a MS variant until recently, had changed. The description of the first biological marker highly sensitive and specific to NMO10,14, clearly directed the classification of NMO to a distinct nosological condition4,10,12,19,20. The understanding of the possible mechanisms involved in NMO and the search for immunological elements that differentiate it from MS had been the goal of the last studies since Wingerchuk et al. criteria9.
The present study initiated in 2003, and our inclusion criteria for NMO patients was based on Wingerchuk et al. criteria9, considering the symptoms of optical neuritis and transverse myelitis, the lack of compromise of the other functional systems and the vertebral column MRI showing extensive demielinating lesion (more than 3 segments), with a normal brain MRI9. After the finalization of the inclusion of our patients at the end of 2005 a new diagnostic criteria had been proposed in 2006, considering the AQP-4 antibody level12.
In our Neurological Services there were 16.8% NMO patients, which is higher than Pirko et al.21 found (12.5%). They only considered patients with ON who turned to be NMO during five years of follow-up and not the totality of patients with demielinization of the central nervous system (CNS). They also found a higher number of women in their study group.
Among our 28 NMO patients there were 75% of women (3:1), similar to what we found in the literature by Bergamaschi and Ghezzi22, Papais-Alvarenga et al.23; Cree et al.5; O'Riordan et al.3. We found 50% of Afro-descendents, not so different (58%) from the findings of Papais-Alvarenga et al.23. Another authors, Wingerchuk et al.6, Bergamaschi and Gezzi22, Cree5, Wingercuk et al.6 and O'Riordan et al.3 also found similar clinical and demographic characteristics, with a predominance of women and non-Caucasians among their NMO patients. The high number of Afro-descendents in our group should be related to the racial miscegenation in Brazil, especially in the Southeast regions (Alves-Leon et al.)24.
Poser and Vernant25 analyzed the association among NMO and endocrinopathies in Martinique, and suggested this to be a distinct nosological condition. The actual knowledge of the distribution of acquaporin channels in the CNS and their relation to NMO diagnosis lead us to conclude that NMO patients can have endocrinopathies due to the diencephalon compromise, what may have been seen in the Martinique Afro-descendents patients24,25. Our NMO patients did not have endocrinopathies.
The index event in our patients with NMOR occurred in ages older than 30 years (46.5%), similar to the literature4,9,22,26,27. Among the initial symptoms there were: unilateral optical neuritis (39.3%) or bilateral (7.1%), myelitis (28.6%) and neuritis plus myelitis (25.0%). Another authors also observed unilateral optical neuritis as the main manifestation in their patients9,22. The interval of time between the first and the second event, neuritis or myelitis, in NMOR, was among three and 228 months which are not in concordance to the literature. Wingerchuck et al.19 analyzing 96 NMO patients, 84.4% (81 patients) with recurrent presentation, found this interval to be four to 48 months. Another authors observed similar interval4,5,14,22, although Wingerchuk et al.9 considered even years and Ghezzi et al.28 considered one to 120 months, which is still a smaller interval than ours.
In our study the majority of patients (89.3%) had 17.5 years as a median duration of disease and there were no deaths. These results showed a higher longevity than we found in the literature where the mortality rate was 30% in five years4,9,29. The high morbidity observed by Scolding30 in another study where 50% of the patients had unilateral or bilateral amaurosis, walked with aid after five years were not seen in our patients. In our group, only five (17.8%) had a final EDSS>6.0.
The final EDSS was not related to the gap of time between the first and second bouts, as described by Ghezzi et al.28 who observed an important incapacity (EDSS>6.0) in those patients with a longer gap among the bouts22,24. These authors characterized the variables related to worst prognosis (EDSS>6): residual EDSS >3 at the beginning of the disease, longer interval between the first and second bouts and a high number of relapses during the first two years of disease. In our casuistic we did not find relation among the EDSS, total number of bouts nor the time between the first and second bouts that could suggest distinct evolutional characteristics from other populations.
We found high levels of all autoantibodies analyzed in this study. MOG 92106, PLP 95116 and MBP levels were higher in NMO patients with statistic significance which can be related to polyclonal activation of the humoral system as it was suggested by Hasse and Schmidt31. The participation of MBP in the EAE model is well studied but recent EAE models investigate the participation of another autoantibodies, like MOG and PLP. The search for prognostic markers in MS evolution in the work of Berger et al.23 showed that patients with clinical isolated syndrome (CIS) like optic neuritis, myelitis, medulla or cerebellar syndrome with a positive MOG and MBP autoantibodies had a higher risk of conversion to a second bout, defining the disease in at least 12 months. This study did not mentioned the type of evolution but point out for the possibility of an initial investigation in patients with isolated optic neuritis and/or myelitis with positive MOG or MBP antibodies, observing their participation in the conversion to NMO. These studies would have an strategic implication in therapeutics.
The participation of encephalithogenic autoantibodies in the CNS IIDDA was thought from an EAE animal model for MS. Another models noticed that EAE could be induced through the immunization of susceptible animals with myelin antigens like MOG, MBP e PLP33. Betelli et al.33 described an EAE model where B and T cells were specific to the same MOG autoantigen, developing spontaneous and severe EAE with inflammatory lesions mainly in the optic nerves and vertebral column of affected mice, a typical pattern of human NMO. The different EAE models and the immuno-pathologic studies of MS suggested that the immune-mediated response by T cells should be crucial in the deflagration of the inflammatory process and that the auto-reactivity reaction of these cells should be insufficient to explain the selective destruction of myelin34. Lucchinetti et al.14 emphasized the importance of humoral immune mechanisms in EAE showing that the extensive demyelinization is without T cells specificity. Therefore, indicate that T cells response against any CNS protein is potentially pathogenic, considering that it is followed by an adequate B cells response35. In our study we investigated the presence of antibodies to MOG, MBP and PLP in relation to the high levels of IL-4 and IFN-γ, molecules Th1/Th2 mediated.
We found expressive high levels of IL-4 (p=0.0084) in our patients different from the IFN-γ levels. The IFN-γ effects are partially blocked by IL-4 in the infectious diseases, a cytokine product by Th2 cells which stimulate B cells and the auto-immunity of the specific myelin B cells36,37. Indeed, the high production of IgA to the myelin basic protein suggests higher participation of Th2 regulatory cells and B lymphocytes than Th1, in our patients. We have to consider the Afro-descendents in our study group who have a known Th2 pattern of immunity (regulatory), maybe inducted by MBP. In this context it would be interesting to sequentially analyze, in patients with different ethnics, a possible change in the profile of the neuro-inflammatory response during NMO clinical evolution, which means the change of the isotype IgM to IgG and/or IgA, configuring a more inflammatory (IgM, IgG; complement activation, IFN-g) or regulatory (IgG, IgA, IL-4, TGF beta) response.
Our results can contribute to the search and determination of biologic markers related to NMO and its evolution. A Th2 humoral response mediated by B lymphocites was more prominent than Th1. Considering this profile of auto-reactivity, the levels of IFN-γ and IL-4 could represent the instrument of measure of the therapeutic response to the monoclonal antibodies, like Rituximab, during the treatment of NMO patients. Also, the high expression of MOG 92106, PLP 95116 and MBP autoantibodies in NMO patients could be a marker of prognosis, recurrence or severity of the disease in patients treated with plasmapheresis.
As a limitation of our study we point out the relative small number of patients which did not allowed us to correlate demographic and clinical parameters of morbidity in NMO with the plasmatic levels of IL-4, IFN-γ.
Received 4 June 2008, received in final form 21 July 2008. Accepted 1 August 2008.
Dra. Soniza Vieira Alves-Leon Neurology Department / Inflammatory Demyelinating Diseases Research Unit Hospital Universitário Clementino Fraga Filho / Universidade Federal do Rio de Janeiro - Rua Prof. Rodolpho Paulo Rocco 255 / 8º andar / sala 8E06 - 21941-913 Rio de Janeiro RJ - Brasil. E-mail: sonizavleon@globo.com
- 1. Fillipi M, Rocca MA, Moiola L et al. MRI and magnetization transfer imaging changes in the brain and cervical cord of patients with Devic's neuromyelitis optica. Neurology 1999;53:1705-1710.
- 2. Mandler RN, Davis LE, Jeffery DR et al. Devic's neuromyelitis optica: a clinicopathological study of 8 patients. Ann Neurol 1993;34:162-168.
- 3. O'Riordan JI, Gallagher HL, Thompson AJ et al. Clinical, CSF, and MRI findings in Devic's neuromyelitis optica. J Neurol Neurosurg Psychiatry 1996;60:382-387.
- 4. De Seze J. Neuromyelitis optica. Arch Neurol 2003;60:1336-1338.
- 5. Cree BAC, Goodin DS, Hauser SL. Neuromyelitis optica. Semin Neurol 2002;22:105-122.
- 6. Wingerchuk DM, Scottsdale AZ, Pittock SJ, et al. Neuromyelitis optica diagnostic criteria revised validation and incorporation of the NMO-IgG serum autoantibody. Neurology 2005;64(Suppl 1):A38.
- 7. Weinshenker BG. Neuromyelitis optica: what it is and what it might be. Lancet 2003;361:889-890.
- 8. Lana-Peixoto MA. Devic's neuromyelitis optica: a critical review. Arq Neuropsiquiatr 2008;66:120-138.
- 9. Wingerchuk DM, Hogancamp WF, O'Brien PC, et al. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999;53: 1107-1114.
- 10. Lennon VA, Wingerchuck DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106-2112.
- 11. Lennon VA, Kryzer TJ, Pittock SJ, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. JEM 2005;202: 473-477.
- 12. Wingerchuck DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485-1489.
- 13. Wingerchuck DM, Weinchenker BG. Neuromyelitis optica. Clinical predictors of a relapsing course and survival. Neurology 2003;60:848-853.
- 14. Lucchinetti CF, Mandler RN, McGavern D, et al. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 2002;125:1450-1461.
- 15. Correale J, Fiol M. Activation of humoral immunity and eosinophils in neuromyelitis optica. Neurology 2004;63:2363-2370.
- 16. Pittock SC, Weinshenker BG, Lucchinetti CF, et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4-expression. Arch Neurol 2006;63:964-968.
- 17. Ribeiro D. O povo brasileiro: a formação e o sentido do Brasil. São Paulo: Companhia das Letras, 1995/1996.
- 18. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-1452.
- 19. Wingerchuk DM. Neuromyelitis optica. Intern MS J 2006;13:42-50.
- 20. Kikuchi S, Fukazawa T."OSMS is NMO, but not MS": confirmed by NMO-IgG?. Lancet Neurol 2005;4:594-595.
- 21. Pirko I, Blauwet LR, Lesnick LR, Weinshenker BC. The natural hystory of recurrent neuromyelitis optica. Arch Neurol 2004;61:1401-1405.
- 22. Bergamaschi R, Ghezzi A. Devic's neuromyelitis optica: clinical features and prognostic factors. Neurol Sci 2004;25(Suppl):S364-S367.
- 23. Papais-Alvarenga RM, Miranda-Santos CM, Puccioni-Sohler M, et al. Optic neuromyelitis syndrome in Braziian patients. J Neurol Neurosurg Psychiatry 2002;73:429-435.
- 24. Alves-Leon SV, Papais-Alvarenga R, Magalhães M, et al. Ethnicity-dependent association of HLA DRB1-DQA1-DQB1 alleles in Brazilian multiple sclerosis patients. Acta Neurol Scand 2007;111:306-311.
- 25. Poser C, Vernant J. La sclérose en plaques dans la race noire. Bull Soc Pathol Exot 1993;86:428-432.
- 26. Vernant JC, Cabre P, Smadja D, et al. Recurrent optic neuromyelitis with endocrinopathies: a new syndrome. Neurology 1997;48:58-64.
- 27. Rubiera M, Rio J, Tintore M, et al. Neuromyelitis optica diagnosis in clinically isolated syndromes suggestive of multiple sclerosis. Neurology 2006;66:1568-1570.
- 28. Ghezzi A, Bergamaschi R, Martinelli V, et al. Clinical characteristics, course and prognosis of relapsing Devic's neuromyelitis optica. J Neurol 2004;251:47-52.
- 29. Weinstock-Guttmann B, Ramanathan M, Lincoff N, et al. Study of mitoxantrone for the treatment of recurrent neuromyelitis optica (Devic disease). Arch Neurol 2006;63:957-963.
- 30. Scolding N. Devic's disease and autoantibodies. Lancet 2005;4:136-137.
- 31. Haase CG, Schimdt S. Detection of brain-autoantibodies to myelin oligodendrocyte glycoprotein, S100beta and myelin basic protein in patients with Devic's neuromyelitis optica. Neurosci Lett 2001;307: 131-133.
- 32. Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med 2003;349:139-145.
- 33. Bettelli E, Baeten D, Jäger A, et al. Myelin oligodrendocyte glycoprotein-specific T an B cells cooperate to induce a Devic-like disease in mice. J Clin Invest 2006;116:2393-2402.
- 34. Schmidt S. Candidate autoantigens in multiple sclerosis. Mult Scler 1999;5:147-160.
- 35. Lassmann H. Neuropathology in multiple sclerosis: new concepts. Mult Scler 1998;4:93-98.
- 36. Myers KJ, Sprent J, Dougherty JP, Ron Y. Synergy between encephalitogenic T cells and myelin basic protein-specific antibodies in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol 1992;41:1-8.
- 37. Sobel RA, Kuchroo VK. The immunopathology of acute experimental allergic encephalomyelitis induced with myelin proteolipid protein. T cell receptors in inflammatory lesions. J Immunol 1992;149:1444- 1451.
Publication Dates
-
Publication in this collection
15 Oct 2008 -
Date of issue
2008
History
-
Accepted
01 Aug 2008 -
Reviewed
21 July 2008 -
Received
04 June 2008