Abstracts
We evaluated the frequency, demographic, clinical, disability evolution and genetic association of HLA DRB1*1501, DRB1*1503, DQA1*0102, DQB1*0602 and DPA1*0301 alleles in patients diagnosed as acute disseminated encephalomyelitis (ADEM) among a population of CNS demyelinating diseases. Fifteen patients (8.4%) of our series were diagnosed as ADEM. The mean age onset was 35.23 years (range 12 to 77), 53.3% were male and follow-up range was 8.5 to 16 years. Two cases (13.3%) had a preceding infection before neurological symptoms, one presented a parainfectious demyelinating, and one case had been submitted to hepatitis B vaccination four weeks before the clinical onset. The EDSS range was 3.0 to 9.5. Eight patients (53.3%) presented MRI with multiple large lesions. CSF was normal in 73.3%. The severe disability observed at EDSS onset improved in 86.66% patients. The genetic susceptibility for ADEM was significantly associated with the HLA DQB1*0602, DRB1*1501 and DRB1*1503 alleles (<0.05) in monophasic ADEM.
ADEM; HLA class II; demyelinating disease
Avaliamos as frequencia, características demográficas, clínicas e de associação genética dos alelos HLA DRB1*1501, DRB1*1503, DQA1*0102, DQB1*0602 e DPA1*0301 em pacientes com diagnóstico de encefalomielite aguda disseminada (ADEM) em população com doença desmielinizante do SNC. Quinze (8,4%) pacientes de nossa série foram diagnosticados como ADEM. A média de idade foi 35,23 anos (variando entre 12 e 77), 53,3% eram homens e o tempo de acompanhamento variou entre 8,5 e 16 anos. Dois casos (13,3%) apresentaram infecção prévia, um apresentou processo desmielinizante para infeccioso e outro havia se submetido a vacinação para hepatite B quatro semanas antes. O EDSS variou entre 3,0 e 9,5. Oito pacientes (53,3%) apresentaram grandes lesões na RM. O LCR foi normal em 73,3%. A incapacidade grave quantificada pelo EDSS foi seguida de melhora importante em 86,6% dos pacientes. A susceptibilidade genética na ADEM foi significativamente associada com os alelos HLA DQB1*0602, DRB1*1501 e DRB1*1503 (p<0,05) nos pacientes com quadro monofásico.
ADEM; HLA classe II; doenças desmielinizantes
Acute disseminated encephalomyelitis: clinical features, HLA DRB1*1501, HLA DRB1*1503, HLA DQA1*0102, HLA DQB1*0602, and HLA DPA1*0301 allelic association study
Encefalomielite aguda disseminada: características clínicas e estudo de associação com os alelos HLA DRB1*1501, HLA DRB1*1503, HLA DQA1*0102, HLA DQB1*0602 e DPA1*0301
Soniza Vieira Alves-LeonI,II,III; Maria Lucia Veluttini-PimentelIII,IV; Maria Emmerick GouveiaV; Fabíola Rachid MalfetanoIII,V; Emerson L. GasparetoVI; Marcos P. AlvarengaII; Izabel FrugulhettiVI; Thereza Quirico-SantosVII
INeurologist Associate Professor, Universidade Federal do Estado do Rio de Janeiro (UNIRIO), Rio de Janeiro RJ, Brazil
IINeurology Postgraduation Program UNIRIO
IIIUniversidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro RJ, Brazil
IVNeurologist, Serviço de Neurologia da Santa Casa da Misericórdia do Rio de Janeiro, Rio de Janeiro RJ, Brazil
VLaboratory of Molecular Biology, Universidade Federal Fluminense (UFF), Neurologist, Hospital Naval Marcílio Dias, Rio de Janeiro RJ, Brazil
VIAssistant Professor of Radiology Department, UFRJ
VIIFull Professor, UFF
ABSTRACT
We evaluated the frequency, demographic, clinical, disability evolution and genetic association of HLA DRB1*1501, DRB1*1503, DQA1*0102, DQB1*0602 and DPA1*0301 alleles in patients diagnosed as acute disseminated encephalomyelitis (ADEM) among a population of CNS demyelinating diseases. Fifteen patients (8.4%) of our series were diagnosed as ADEM. The mean age onset was 35.23 years (range 12 to 77), 53.3% were male and follow-up range was 8.5 to 16 years. Two cases (13.3%) had a preceding infection before neurological symptoms, one presented a parainfectious demyelinating, and one case had been submitted to hepatitis B vaccination four weeks before the clinical onset. The EDSS range was 3.0 to 9.5. Eight patients (53.3%) presented MRI with multiple large lesions. CSF was normal in 73.3%. The severe disability observed at EDSS onset improved in 86.66% patients. The genetic susceptibility for ADEM was significantly associated with the HLA DQB1*0602, DRB1*1501 and DRB1*1503 alleles (<0.05) in monophasic ADEM.
Key words: ADEM, HLA class II, demyelinating disease.
RESUMO
Avaliamos as frequencia, características demográficas, clínicas e de associação genética dos alelos HLA DRB1*1501, DRB1*1503, DQA1*0102, DQB1*0602 e DPA1*0301 em pacientes com diagnóstico de encefalomielite aguda disseminada (ADEM) em população com doença desmielinizante do SNC. Quinze (8,4%) pacientes de nossa série foram diagnosticados como ADEM. A média de idade foi 35,23 anos (variando entre 12 e 77), 53,3% eram homens e o tempo de acompanhamento variou entre 8,5 e 16 anos. Dois casos (13,3%) apresentaram infecção prévia, um apresentou processo desmielinizante para infeccioso e outro havia se submetido a vacinação para hepatite B quatro semanas antes. O EDSS variou entre 3,0 e 9,5. Oito pacientes (53,3%) apresentaram grandes lesões na RM. O LCR foi normal em 73,3%. A incapacidade grave quantificada pelo EDSS foi seguida de melhora importante em 86,6% dos pacientes. A susceptibilidade genética na ADEM foi significativamente associada com os alelos HLA DQB1*0602, DRB1*1501 e DRB1*1503 (p<0,05) nos pacientes com quadro monofásico.
Palavras-chave: ADEM, HLA classe II, doenças desmielinizantes.
Acute disseminated encephalomyelitis (ADEM) is a demyelinating disorder characterized by multifocal involvement of the central nervous system (CNS) white and gray matter and, in some cases, peripheral nervous system lesions1-5. ADEM can be defined as an acute or sub acute inflammatory or demyelinating event affecting multifocal areas of the CNS, with no detectable infectious agent or other cause; when this event follow a specific infection it is named post-infectious encephalomyelitis (PIEM) or, when associated to immunization is considered post-vaccination encephalomyelitis (PVEM)6-9. ADEM can progress with recurrent manifestation of multiple functional neurological systems, similar to multiple sclerosis (MS), and, in these cases, it is defined as multiphasic encephalomyelitis (MDEM)5,9. When the same functional neurological systems are involved, characterizing a stereotypic recurrent neurological deficit identical of the first demyelinating event, it is denominated recurrent encephalomyelitis (RDEM)5,9-12.Post-infectious forms of encephalomyelitis, also termed ADEM compose one of the several categories of inflammatory demyelinating disorders (IDD) of the CNS13,14. Recently, new criteria for pediatric population were proposed10. Pediatric monophasic ADEM includes obligatorily encephalopathy associated to multifocal areas of CNS demyelinating or inflammatory origin, after exclusion of any other cause10-12 and neuroimaging showing focal or multifocal lesions predominantly involving white matter, without evidence of previous lesions10. The pediatric RDEM is defined as a new event of ADEM, with recurrence of the same initial signs and symptoms, always associated to encephalopathy; magnetic resonance image (MRI) shows no new lesion and the original ones can have enlarged, during a interval time of three months, or more, of the remission, and at least one month after the last treatment with corticosteroids10-12. The pediatric MDEM diagnoses requires encephalopathy associated to new polysymptomatic neurological dysfunction, new MRI lesions, and an equal interval time of three months, or more, after the recovering of the first attack and at least one month after corticosteroids treatment ending20; the MRI needs to show new areas of involvement and complete or partial resolution of the first ADEM event11,12.
ADEM is thought to be an autoimmune disorder of the CNS targeting the myelin. Possibly, a T cell mediated autoimmune response to myelin basic protein, triggered by an infection or vaccination, underlies its pathogenesis15-18. Studies on peripheral immunocytes and on cerebrospinal fluid (CSF) revealed the presence of cytokine-mediated responses in ADEM19. As the cytokines profiles are mediated by the human leukocyte antigen (HLA), it can be speculated a possible association between ADEM and HLA alleles. ADEM followed poliomyelitis vaccine virus was reported in a Japanese child associated with the HLA-Cw3 and HLA-DR2, which are related to multiple sclerosis (MS) in Japan8. A linkage genetic study in children showed that ADEM was associated with HLA DRB1*01 and DRB1*017(03) in the Russian population, and these alleles are associated with MS in Russian patients20. In Brazil, MS has been associated with HLA DR2 in Caucasian21 patients and with HLA DQB1*0602 independently of DRB1*1501, in African-descendent patients of Rio de Janeiro City (RJC)21-23.
The aim of this study was to analyze the clinical features and the genetic association of HLA DRB1*1501, HLA DRB1*1503, HLA DQA1*0102, DQB1*0602 and HLA DPA1*0301 alleles in a group of Brazilian ADEM patients.
METHOD
Adult patients were classified as ADEM according to descriptions proposed by Brinar and Poser1, and pediatric patients were classified according to International Pediatric MS Study Group (IPMSSG)10. They were grouped as monophasic ADEM if no new neurological functional deficit or MRI lesion occurred until at least 3 months of the first event recovering, and at least one month after the treatment with corticosteroids. The patients included should be at least 8.5 years of follow up. The pediatric patients were classified as ADEM, MDEM or RDEM according to IPMSSG10, and adult ADEM,MDEM or RDEM patients according to Brinar and Poser studies1.
Clinical symptoms and disability were measured by the expanded disability scale score (EDSS)24 during the first demyelinating event and after recovered. The last evaluation in 2008 measured the interval time between the first and the actual EDSS. The brain MR imaging, cerebral spinal fluid (CSF) findings and the response to a standardized treatment during the acute phase of the disease and the follow up were assessed.
The genetic association study of the HLA DQB1*0602, DQA1*0102, DRB1*1501, DRB1*1503 and DPA1*0301 alleles compared the 15 ADEM/MDEM/RDEM patients with 84 healthy matched controls. It was considered for the present study only the genetic association analysis of monophasic ADEM patients.
Analyses of alleles by PCR methods
Genomic DNA was isolated from proteinase-K treated peripheral blood leukocytes by the salting-out method25. The analysed of sub regions of HLA DRB1, DQA1, DQB1 and DPA1 was performed by polymerase chain reaction (PCR) with sequence-specific primers. The genomic DNA was isolated from peripheral blood leukocytes by the salting-out method. Each 10 mL PCR reaction consisted of 100 ng genomic DNA, PCR buffer (50 mMKCl, 1.5 mM MgCl2, 10 mM Tris-HCl), 200 mM each of dATP, dCTP, dGTP and dTTP, 0.40 mM of the group-specific primers. PCR reaction mixture was obtained in total 25 uL of reaction including 10pM of each PCR primer specific HLA fragment amplification and 45 PCR cycles (95ºC 20 sec, 55-60ºC 20sec and 72ºC 30 sec) were run. The whole PCR reactions were directly electrophoresed in 2% ME agarose gels prestained with ethidium bromide (0.5 mg mL»1 gel). The gels were run at 10 V cm-1 for 15 min in 1X TBE buffer (1X TBE=80 mMTris base, 80 mM boric acid, 2 mM EDTA, pH 8.0), visualized under ultraviolet (UV) illumination and documented by photography.
The statistical analysis was performed by Fisher's exact test and significant p values (p, 0.05) were corrected for the number of comparisons made.
RESULTS
Among 226 patients with idiopathic inflammatory demyelinating disease (IIDD) of CNS follow-up in our University Hospital from 1992 to 2008, 25 (11.06%) were diagnosed as ADEM. Fifteen (8.4%) patients were included in this study. The follow up ranged from 8.9 years to 16 years. The mean age onset was 35.2 years (range 12 to 77 years-old), 55.3% were male. Eight patients (53.33%) were African-descendents.
Thirteen patients were adult and fulfill the ADEM/MDEM/REDEM characteristics1. Two patients presented less than 19 years old and fulfill criteria for pediatric ADEM10-12.
The two pediatric patients with ADEM presented encephalopathy and polysymptomatic neurological manifestations. In adult group of patients, encephalopathy occurred in three cases, one of them during a neuromyelitis optica (NMO) syndrome, and in two others during the first polysymptomatic demyelinating event.
ADEM was associated with previously infection in two patients (13.3%) and to hepatitis B vaccination in one (6.6%). ADEM occurred simultaneously to an infection in one (6.6%) but no microorganism was found in the virology and fungus/bacteriology culture of CSF. The CSF analysis, investigated during the acute phase of the initial event, was normal in 73.4% of the patients; three patients presented positive oligoclonal bands (20%) and one had an inflammatory CSF (6.6%) with severe pleocytosis and high protein level without a specific antigen. Two of the three MDEM patients showed positive oligoclonal bands (OGC).
Four patients (26.67%) presented large intracranial masses and underwent biopsy, as well as a patient with cervical lesion that was initially asumed as a tumor. Three patients (20%) presented multiphasic evolution and one case had a recurrent form of ADEM.
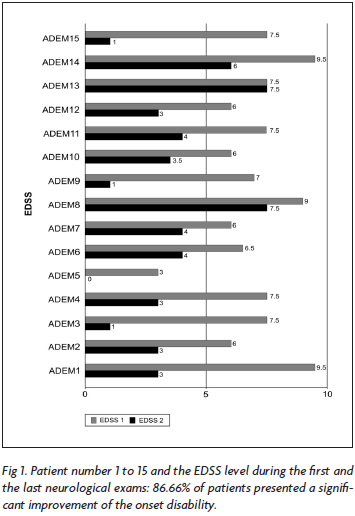
During the acute event, the EDSS ranged from 3.0 to 9.5 and the follow-up was associated with almost completely recovered or significant improvement in all 15 cases (Fig 1).
When we assess the progression of the 11 patients with ADEM and of the four patients with MDEM/RDEM, we clearly see the EDSS decrease after a monophasic event (Fig 2), and MDEM/RDEM patients progression were similar to the relapsing-remitting course of MS.
The acute onset of the neurological symptoms was seen in eight patients (53.4%) and sub-acute in seven cases (46.6%). The polysymptomatic neurological signs and symptoms occurred in all patients, and were associated to encephalopathy in three adults MDEM patients and in two ADEM pediatric patients. The most frequent neurological syndrome was motor functional system involvement (86.6%), followed by the sensory symptoms (53.3%), consciousness and cognitive disturbance (33.3%), optical neuritis (26.6%), ataxia (20%), epileptic seizures (20%) and radiculopathy (6.6%).
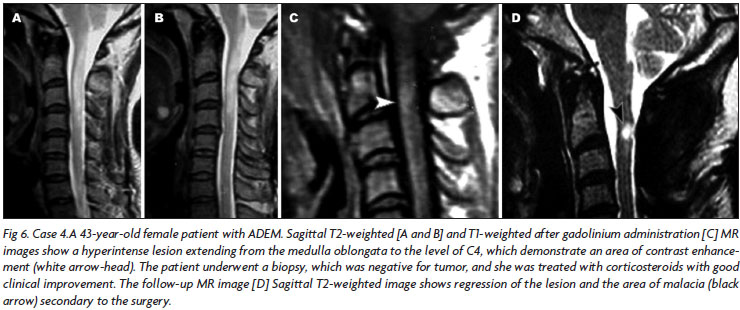
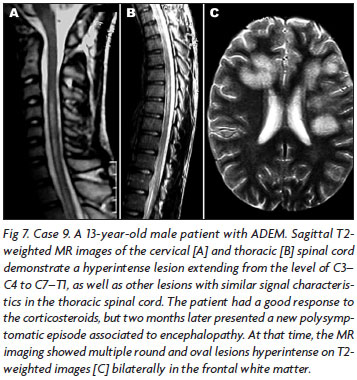
In most of the cases (n=8, 53.3%), brain MRI showed large, confluent or tumefactive lesions, usually with perilesional edema and mass effect (Figs 3, 4, 5), which were multiple in five and single in three cases. Three patients presented multiple small lesions (smaller than 5 cm), mainly involving sub cortical and peri ventricular regions, associated to gadolinium enhancement. One patient had a large longitudinal medullar lesion (Fig 6), which reached the bulbar region, and the other two had multifocal spinal cord lesions. In three cases, large lesions were seen in association to spinal cord lesions areas of signal abnormality (Fig 7).
Genetic association study result
The genetic association study of the HLA DRB1*1501, DRB1*1503, DQA1*0102, DQB1*0602, and DPA1*0301 was compared with 84 matched healthy controls (Table 1).
The results of the allelic association can be compared among the 11 monophasic ADEM patients according with the disease duration and EDSS. The HLA DQB1*0602 (90.9% versus 35.7%, p=0.0001) and HLA DRB1*1503 (45.4% versus 10.7%, p=0.01) were significantly associated with ADEM, as well as the DRB1*1501(36.3% versus 13.0% p=0.01) (Table 2).
DISCUSSION
The similar clinical characteristics of ADEM and MS constitute one of the major problems among the differential diagnosis of MS and other primary inflammatory demyelinating diseases of the CNS5-7,9,11,19,25,26. ADEM has been considered a disease with favorable long term diagnosis, which is not commonly seen in patients with MS9,11,13. Although ADEM is supposed to be a monophasic illness, the possibility of multiphasic or recurrent progression needs to be considered1,5,9-12. The differentiation between ADEM and a first attack of MS is difficult and has prognostic and therapeutic implications11,12,27. The search for biological markers to differential diagnosis between ADEM and MS has failed28. In pediatric patients, the diagnostic criteria for ADEM, MDEM and RDEM were recently proposed and have been applied10, but for adults it not clearly defined. The lack of ADEM diagnostic criteria in adults permitting a calculation of "conversion rate" for ADEM to MS is probably associated to a short observation period. Our study is longest than the previously longest follow-up of patients with ADEM3,4,29
In the present study we have evaluated 15 ADEM patients, eleven of them monophasic, three multiphasic (MDEM) and one with recurrent (RDEM) evolution. Two patients were pediatric. As previously reported in pediatric patients, ADEM patients from our series presented sub cortical white matter aggression, which progressed as isolated acute encephalitis, multifocal myelitis or both, and polysymptomatic neurologic symptoms10,11,13,30. The optic neuritis, transverse demyelinating myelitis, like Devic's syndrome14, hemiparesia, ataxia and brainstem syndromes were the neurological syndromes seen in our ADEM cases, resembling MS and NMO symptoms. One of our cases (patient 8) with encephalomyeloradiculoneuropathy illustrated the combination of ADEM with acute peripheral neuropathy, as has been showed by Poser1,9.
In addition, a twelve-years-old girl (patient 14) began the neurological manifestation with cognitive impairment and progression to bilateral optical neuritis, progressive motor deficit and pseudobulbar symptoms, resembling initially a sub acute pan encephalitis. The MRI showed lesions suggestive of Schilder's disease, and she recovered the tetraplegia and cognitive dysfunction almost completely after intravenous methylprednisolone (IVMP) treatment. Our findings are similar to the characteristic clinical features described by other authors who includes sudden onset of multifocal neurologic disturbances sometimes associated with a depressed level of consciousness and signs of an acute meningoencephalopathy, focal or generalized seizures and psychosis. Recently, encephalopathy, characterized by cognitive impairment, consciousness disturbance or psychiatric symptoms, is request in the diagnostic criteria of ADEM, MDEM or RDEM among pediatric patients10,12.
Our two pediatric patients presented all these symptoms and fulfill IPMSSG criteria10. One of them presented a consciousness impairment associated to transverse myelitis. The spinal cord MRI showed longitudinal transverse medullar extension lesion (LETML) (Fig 7), normal cranium MRI and inflammatory CSF. Treated with IVMP, improved after days, and two months latter presented a recurrence of the myelitis and consciousness impairment; at this moment, cranium MRI showed new large lesions (Fig 7). This patient illustrates step by step the new pediatric ADEM diagnoses10: he presented two polysymptomatic neurological manifestations, both of them associated to encephalopathy; we classified as monophasic ADEM because the "new manifestation" occurred before three months of the initial event, and is considered part of it. He was treated with IVMP and presented completely recovered.
As described by Hartung et al.17, our patients also reached several deficits within few days, followed by similar rapid remission. No one of our patients died, and the low lethality of ADEM was also found by other authors who call attention to the actual contrast with the high mortality rate in the past.
The eleven monophasic ADEM patients followed in our analysis did not present new clinical attack during the long follow-up ranged from almost 9 to 16 years, and the last MRI didn't show any new lesion. They can be considered classic cases of monophasic ADEM. In this group two patients presented NMO syndrome. One of them presented bilateral optical neuritis followed 48 hours later by acute transverse thoracic myelitis, with normal cranium MRI, spinal cord MRI showing LTMEL involving more than three segments, and normal CSF. This patient could fulfill Wingerchuket al.14 clinical criteria for NMO, but in this case the NMO syndrome was a parainfecctious demyelinating event, with all CSF culture negative. This patient presented lung tuberculosis, which was diagnosed and treated simultaneously with IVMP. He recovered completely and has been followed for the last 10 years with no new relapsing. The other NMO ADEM syndrome occurred in a patient who opened the symptoms with headache, visual disturbance, tetraplegia and consciousness impairment (Fig 6). This patient was submitted to cervical biopsy which showed an unspecific inflammatory process. The treatment with IVMP was followed by partial improvement and no new relapsing. The presence of encephalopathy is not seemed in diagnosis criteria of NMO.
Among the four MDEM patients, two were adult, and fulfill the new definition criteria for pediatric ADEM10-12 during the first event, characterized by encephalopathy, polysymptomatic neurological symptoms, and MRI multiple lesions. Nevertheless, the second subsequent relapsing in both of them was not associated to encephalopathy; they presented new neurological clinical manifestations and new MRI lesions. If we apply the pediatric criteria20, these two adult patients move, in this moment, to the diagnosis of clinical isolated syndrome (CIS)10; the reason is because they did not presented associated encephalopathy, and in this case their second demyelinating event is considered the first of a possible demyelinating condition that can, or not, be evaluated MS. These two adult patients continue evaluating with relapsing-remitting neurological symptoms after their second demyelinating event (classified as CIS) and after the third demyelinating event they should be moved to the diagnosis of MS (Figs 3 and 4) according the pediatric criteria10. Nevertheless, if we consider Brinar and Poser descriptions1, these two adult patients present MDEM. Our third MDEM patient presented polysymptomatic neurologic manifestation and multiple large lesions of white and gray matter involving cranium and cervical region, after less than four weeks of hepatitis B immunization. The evolution for relapsing-remitting neurological manifestations and new MRI lesions in the last years could move the ADEM diagnosis to MS, but accordingly to Brinar and Poser1, the temporal onset of a post-immunization event allow us to consider this case a post-infeccious ADEM, evaluating as MDEM.
The similarities in clinical, MRI and pathogenesis findings of ADEM and other inflammatory demyelinating diseases of CNS, as MS, NMO and Schilder disease, made some authors considered ADEM as part of the spectrum of IDD. The relationship between ADEM and MS remains a matter of controversy1, and our three MDEM adult cases illustrates these difficulties. We can't distinct the clinical manifestations of our MDEM patients as exclusive of ADEM. Nevertheless, epileptic seizures were high frequent than in MS patients (20% in our ADEM/MDEM series versus 5% reported by Poser in MS patients9). The presence of encephalopathy and temporal relationship with immunization or infection process suggest a distinct condition.
Considering the MRI findings, large and multifocal lesions in the brain and spinal cord can suggest the ADEM diagnosis, and those lesions were present in all patients of our series. In pediatric ADEM, the MRI large lesions are not included in diagnostic criteria10, and in adult patients it is still undefined. The MRI abnormalities are most frequently seen on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences as patchy, ill-defined areas of high-signal intensity. The lesions are frequently large, multiple, and asymmetric, involving the sub cortical and central white matter and cortical gray-white junction of cerebral hemispheres, cerebellum, brainstem, and spinal cord. The gray matter of the thalami and basal ganglia are commonly involved, typically in a symmetric pattern. Four patterns of cerebral involvement have been proposed to describe the MRI findings in patient with ADEM: (1) ADEM with small lesions; (2) ADEM with large, confluent, or tumefactive lesions, with extensive perilesional edema and mass effect; (3) ADEM with additional symmetric bithalamic involvement; and (4) acute hemorrhagic encephalomyelitis. The prevalence of gadolinium enhancement of the lesions is variable and depends on the stage of inflammation, being described in 30 to 100% of patients31,32. In our series, most of the cases presented the pattern associated with large, confluent or tumefactive lesions, which was seen in 53.3% of the patients. In addition, four cases showed the pattern of multiple small lesions. None of our patients presented the patterns with symmetric thalamic involvement or of the acute hemorrhagic encephalomyelitis.
The CSF analysis in our series showed pleocytosis in only one patient, different from literature that usually find it, but the rarity of intrathecal band found here is in according with other studies33,34. Besides these findings, CSF results are not distinctive enough to allow discrimination between ADEM and MS.
The interesting aspect of our series is the HLA allele association study. We found significantly association of HLA DQB1*0602, HLA DRB1*1501 and DRB1*1503 alleles with ADEM monophasic patients. The HLA DQB1*0602 allele confers genetic susceptibility to Brazilian MS patients from Rio de Janeiro City (RJC), independently of the genetic background21-23. The presence of a common allele conferring genetic susceptibility for both MS and ADEM in the same population was found by Idrissovaet al.20 among Russian MS and ADEM patients. In the present study, we found the HLA DQB1*0602 and HLA DRB1*1501 alleles sharing genetic susceptibility to MS and ADEM in patients from RJC. HLA DRB1*1501 confers MS susceptibility only to White RJC MS patients21, but the low number of ADEM patients didn't allow us to distinct African-descendents and White patients allelic association.
Concerning to HLA DRB1*1501, this allele has been continually pointed out as a protection factor to MS patients, associated to low morbidity when compared the MS outcome of patients who are DRB1*1501 negative22. The significantly association of ADEM patients with HLA DRB1*1501 allele can speculate about a protector role of these allele among the monophasic course and rapid improvement of those patients. Accordingly to this point of view, Idrissovaet al.20 also found association of HLA DRB1*01 allele with ADEM susceptibility in Russian patients. And like DRB1*1501, DRB1*01 has been described as an allele associated to low morbidity in MS patients.
We previously described that HLA DRB1*1503 is an allelic characteristic of African descent in both MS patients and controls, and this allele is not associated to the susceptibility or either protection to MS in Brazilian patients from the Rio de Janeiro City22. Among our ADEM patients, HLA DRB1*1503 confers genetic susceptibility. The concomitant presence of an allele attributed to MS low morbidity, as DRB1*1501, associated to HLA DRB1*1503, this last one associated to a genetic characteristic of African population (an original population who seems to be more resistant than others to MS) can suggest some kind of protection contributing to a monophasic pattern of ADEM demyelinating event. The low number of patients is a limit factor, but the power of our results is supported by the careful clinical and MRI analysis and the long follow-up. These results need to be confirmed in largest series of ADEM patients, and differential diagnosis between ADEM and MS in adults still remains35.
In conclusion, the prevalence of ADEM was 8.4% considering more than 8.5 years of follow-up in Neuroimmunological Unit of our University Hospital. The frequency of multiphasic and recurrent forms was smaller. The ADEM showed a similar prevalence in both genders, with age of presentation raging from 12 to 77 years, and similar frequency in White and African descendent. The acute and the polysymptomatic neurological presentation, consciousness impairment and epileptic seizures were common. The outcome was favorable, with EDSS showing significant decrease in most of the cases. The alleles HLA DQB1*0602, DRB1*1501 and DRB1*1503confers genetic susceptibility to ADEM in our series, and except to the DRB1*1503 association, these results are similar to our findings in MS patients from the Rio de Janeiro City.
Received 13 March 2009, received in final form 4 May 2009.
Accepted 11 May 2009.
Dra. Soniza Vieira Alves-Leon - Neurology Department / Hospital Universitário Clementino Fraga Filho/ UFRJ - Rua Professor Rodolpho Paulo Rocco 255 21941-913 Rio de Janeiro RJ - Brasil. E-mail: sonizavleon@globo.com
- 1 Brinar V, Poser CM. Disseminated encephalomyelitis in adults. Clin Neurol Neurosurg 2008;110:913-918.
- 2 Garg RK. Acute disseminated encephalomyelitis. Postgrad Med J 2003; 927:11-17.
- 3. Gupte G, Stonehouse M, Wassmer E, Coad NA, Whitehouse WP. Acute disseminated encephalomyelitis: a review of 18 cases in childhood. J Paediatr Child Health 2003;5:336-342.
- 4. Menge T, Hemmer B, Nessler S, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol 2005;62:1673-1680.
- 5. Poser CM. Multiple sclerosis and recurrent disseminated encephalomyelitis are different diseases. Arch Neurol 2008;65:674-675.
- 6. Alves-Leon SV, Lavrado FP, Fonseca M, Sohler MP, Alvarenga RP. Hepatitis B vacination in a case of CNS demyelination: multiphasic demyelination encephalomyelitis, postvaccinal demyelination or a new syndrome? Rev Neurol Argentina 2000;25:3.
- 7. Alves-Leon SV, Basto R, Dominguez R, et al. Neuromyelitisoptica (NMO) associated to tuberculosis infection: case report. Rev Neurológica 2002;12:1184.
- 8. Ozawa H, Noma S, Yoshida Y, Sekine H, Hashimoto T. Acute disseminated encephalomyelitis associated with poliomyelitis. Pediatric Neurol 2001;4:325.
- 9. Poser CM. Multiple sclerosis: diagnosis and treatment. Med Principles Pract 1993;3:1-16.
- 10. Krupp LB, Banwell B, Tenembaum S. for the International Pediatric MS Study Group. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurol 2007;68(Suppl2):S7-S12.
- 11. Tenembaum S, Chitnis T, Ness J, Hahn JS. International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology 2007; 68(Suppl 2):S23-S36.
- 12. Tenenbaum S. Disseminated encephalomyelitis in children. Clin Neurol Neurosurg 2008;110:928-938.
- 13. Wingerchuk DM. The clinical course of acute disseminated encephalomyelitis. Neurol Res 2006;28:341-347.
- 14. Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitisoptica. Neurology 2006;66:1485-1489.
- 15. Dale RC, Morovat A. Interleukin-6 and oligoclonal IgG synthesis in children with acute disseminated encephalomyelitis. Neuropediatrics 2003;3:141-145.
- 16. Durán I, Martinez-Carcere EM, Brieva L, et al. Similar pro-and anti-inflammatory cytokine production in the different clinical forms of multiple sclerosis. Mult Scler 2001;7:151-156.
- 17. Hartung HP, Achelos JJ, Zielasek J, et al. Circulating adhesion molecules and inflammatory mediators in demyelination: a review. Neurology 1995;45:22-32.
- 18. Ichiyama T, Shoji H, Kato M, et al. Cerebrospinal fluid levels of cytokines and soluble tumour necrosis factor receptor in acute disseminated encephalomyelitis. Eur J Pediatr 2002;3:133-137.
- 19. Kadhim H, De Prez C, Gazagnes MD, Sebire G. In situ cytokine immune responses in acute disseminated encephalomyelitis: insights into pathophysiologic mechanisms. Hum Pathol 2003;3:293-297.
- 20. Idrissova ZhR, Boldyreva MN, Dekonenko EP, et al. Acute disseminated encephalomyelitis in children: clinical features and HLA-DR linkage. Eur J Neurol 2003;5:537-546.
- 21. Papais-Alvarenga RM, Alves-Leon SV, Miranda Santos CM, et al. South Atlantic project: a Brazilian multiple sclerosis trial. In: Arriagada RC, Nogales-Gaete J (Eds). Esclerosis múltiple: una mirada Ibero Pan Americana. Santiago Chile: ArrynogEdiciones, 2002:35-45.
- 22. Alves-Leon SV, Papais-Alvarenga R, Magalhães M, Alvarenga M, Thuler LC, Fernandez y Fernandez O. Ethnicity-dependent association of HLA DRB1-DQA1-DQB1 alleles in Brazilian. Acta Neurol Scand 2007; 115:306-311.
- 23. Caballero A, Alves-Leon S, Papais-Alvarenga R, Fernandez O, Navarro G, Alonso A. DQB1*0602 confers genetic susceptibility to multiplesclerosis in Afro-Brazilians. Tissue Antigens 1999;5:524-526.
- 24. Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-1452.
- 25. Olerup O, Aldener A, Fogdell A. HLA- DQB1 and - DQA1 typing by PCR amplification with sequence-specifc primers (PCR-SSP) in 2 hours. Tissue Antigens 1993;41:119-134.
- 26. Alves-Leon, SV, Malfetano FR, Pimentel MLV, et al. Multiple sclerosis outcome and morbi-mortality of a Brazilian cohort patients. Arq Neuropsiquiatr 2008;66:671-677.
- 27. Tintore M, Rovira A, Martínez MJ, et al. Isolated demyelinating syndromes: comparison of different MRI criteria to predict conversion to clinically definite multiple sclerosis. Am J Neuroradiol 2000;21:702-706.
- 28. Chopra B, Abraham R, Abraham A. CSF beta-1 globulin--a potential marker in differentiating multiple sclerosis and acute disseminated encephalomyelitis: a preliminary study. Neurol India 2002;1:41-44.
- 29. Jin S, Hahn JS, Pohl D, Rensel M, Rao S. Diferential diagnosis and evaluation in pediatric multiple sclerosis. Neurology 2007;68(Suppl 2):S13-S22.
- 30. Lim KE, Hsu YY, Hsu WC, Chan CY. Multiple complete ring-shaped enhanced MRI lesions in acute disseminated encephalomyelitis. Clin Imaging 2003;4:281-284.
- 31. Holtmannspotter M, Inglese M, Rovaris M, Rocca MA, Codella M, Filippi M. A diffusion tensor MRI study of basal ganglia from patients with ADEM. J Neurol Sci 2003;1:27-30.
- 32. Hong PL, Ho HK, Cheng PW, Wong VC, Goh W, Chan FL. Childhood acute disseminated encephalomyelitis: the role of brain and spinal cord MRI. Pediatr Radiol 2002;1:59-66.
- 33. Hollinger P, Sturzenegger M, Mathis J, Schroth G, Hess CW. Acute disseminated encephalomyelitis in adults: a reappraisal of clinical, CSF, EEG, and MRI findings.J Neurol 2002;3:320-329.
- 34. Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B.Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology 2001;10:1313-1318.
- 35. Maranhão-Filho P. Multiple sclerosis or multiphasic disseminated encephalomyelitis ? A new question about an old problem: a case report. Arq Neuropsiquiatr 1996;3:505-509.
Publication Dates
-
Publication in this collection
21 Aug 2009 -
Date of issue
Sept 2009
History
-
Reviewed
04 May 2009 -
Received
13 Mar 2009 -
Accepted
11 May 2009