Abstracts
Using the pilocarpine model of epilepsy, we investigated the effects of alcohol consumption on the frequency of seizures in animals with epilepsy as well the underlying a possible association between alcohol intake and sudden unexpected death in epilepsy (SUDEP) occurrence. Rats were divided randomly into two groups: (A) rats with epilepsy and (B) rats with epilepsy that received a daily dose of ethanol solution (350 mg kg-1, i.p.) for 30 days. The basal frequency of seizures observed in the A and B groups during the first 30 days were 3.4±1.5 and 3.2±1.9 seizures per week per animal, respectively. In B group, it was observed a significant seizure increase (11.6±5.3) during the first 2 weeks of alcohol administration and quite interesting, one rat died suddenly after a generalized tonic-clonic seizure during this period. We concluded in our experimental study that exist a possible association between alcohol abuse and SUDEP occurrence.
epilepsy; alcohol; heart; seizure; SUDEP
Utilizando o modelo de epilepsia induzido pela pilocarpina, investigamos os efeitos do consumo de álcool sobre a frequência de crises epilépticas em animais com epilepsia, como também uma possível associação entre a ingestão de álcool e ocorrência de morte súbita e inesperada nas epilepsias (SUDEP). Os animais foram randomicamente divididos em dois grupos: (A) ratos com epilepsia e (B) ratos com epilepsia que receberam uma dose diária de etanol (350 mg kg-1, i.p.) por 30 dias consecutivos. A frequência basal de crises epilépticas observadas nos grupos A e B durante os primeiros 30 dias foram de 3,4±1,5 e 3,2±1,9 crises por semana/animal, respectivamente. No grupo B, ocorreu aumento significativo na frequência de crises (11,6±5,3) durante as duas primeiras semanas de administração do álcool e de forma interessante, um animal morreu subitamente após uma crise generalizada tônico-clonica durante esse período. Concluímos em nossa abordagem experimental que existe uma possível associação entre o consumo de álcool e a ocorrência de SUDEP.
epilepsia; álcool; coração; crise epiléptica; SUDEP
ARTICLE
Alcohol consumption and sudden unexpected death in epilepsy: experimental approach
Consumo de álcool e morte súbita em epilepsia: uma abordagem experimental
Carla A. ScorzaI; Roberta M. CysneirosII; Ricardo M. AridaIII; Vera C. TerraIV; Hélio R. MachadoIV; Antonio-Carlos G. de AlmeidaV; Esper A. CavalheiroI; Fulvio A. ScorzaI
IDisciplina de Neurologia Experimental, Universidade Federal de São Paulo/Escola Paulista de Medicina (UNIFESP/EPM), São Paulo SP, Brazil
IIPrograma de Pós-Graduação em Distúrbios do Desenvolvimento do Centro de Ciências Biológicas e da Saúde da Universidade Presbiteriana Mackenzie, São Paulo SP, Brazil
IIIDepartamento de Fisiologia, Universidade Federal de São Paulo/Escola Paulista de Medicina (UNIFESP/EPM), São Paulo SP, Brazil
IVDepartamento de Neurologia, Psiquiatria e Psicologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto SP, Brazil
VDepartamento de Engenharia Biomédica, Universidade Federal de São João Del Rei, São João Del Rei MG, Brazil
ABSTRACT
Using the pilocarpine model of epilepsy, we investigated the effects of alcohol consumption on the frequency of seizures in animals with epilepsy as well the underlying a possible association between alcohol intake and sudden unexpected death in epilepsy (SUDEP) occurrence. Rats were divided randomly into two groups: (A) rats with epilepsy and (B) rats with epilepsy that received a daily dose of ethanol solution (350 mg kg-1, i.p.) for 30 days. The basal frequency of seizures observed in the A and B groups during the first 30 days were 3.4±1.5 and 3.2±1.9 seizures per week per animal, respectively. In B group, it was observed a significant seizure increase (11.6±5.3) during the first 2 weeks of alcohol administration and quite interesting, one rat died suddenly after a generalized tonic-clonic seizure during this period. We concluded in our experimental study that exist a possible association between alcohol abuse and SUDEP occurrence.
Key words: epilepsy, alcohol, heart, seizure, SUDEP.
RESUMO
Utilizando o modelo de epilepsia induzido pela pilocarpina, investigamos os efeitos do consumo de álcool sobre a frequência de crises epilépticas em animais com epilepsia, como também uma possível associação entre a ingestão de álcool e ocorrência de morte súbita e inesperada nas epilepsias (SUDEP). Os animais foram randomicamente divididos em dois grupos: (A) ratos com epilepsia e (B) ratos com epilepsia que receberam uma dose diária de etanol (350 mg kg-1, i.p.) por 30 dias consecutivos. A frequência basal de crises epilépticas observadas nos grupos A e B durante os primeiros 30 dias foram de 3,4±1,5 e 3,2±1,9 crises por semana/animal, respectivamente. No grupo B, ocorreu aumento significativo na frequência de crises (11,6±5,3) durante as duas primeiras semanas de administração do álcool e de forma interessante, um animal morreu subitamente após uma crise generalizada tônico-clonica durante esse período. Concluímos em nossa abordagem experimental que existe uma possível associação entre o consumo de álcool e a ocorrência de SUDEP.
Palavras-chave: epilepsia, álcool, coração, crise epiléptica, SUDEP.
Epilepsy is one of the most prevalent neurological conditions1 and people with epilepsy are more likely to die prematurely than those without epilepsy, and the most common epilepsy-related category of death is sudden unexpected death in epilepsy (SUDEP)1,2. The cause of SUDEP is still unknown; however, the most commonly suggested mechanisms are cardiac abnormalities during and between seizures2,3. Additionally, a number of associated factors for SUDEP have been reported but the results are not wholly consistent between studies. These include refractoriness of the epilepsy, presence of generalized tonic-clonic seizures, polytherapy with antiepileptic drugs, young age, duration of the seizure disorder ranging from 15 to 20 years, and early onset of epilepsy2-5. Unfortunately, all current studies about risk factors for SUDEP concentrated on evaluation of the above mentioned risk factors. Surprisingly, most previous studies have not been tested if cardiovascular risk factors like alcohol consumption play a pathogenic role in SUDEP.
As we known, alcohol and epilepsy are complexly interrelated and have been linked since Hippocrates6. Experimental and clinical studies have been shown that seizures may occur during alcohol intoxication7,8 and that patients with epilepsy who drink moderate or heavy amounts of alcohol could increase the risk of seizures6. Moreover, alcohol is a risk factor for ischemic cerebral infarction9 and increases the chances of head trauma10, both of which are known factors in inducing epilepsy. To our knowledge, there is no data describing with precision a possible relationship between alcohol intake and SUDEP events. As epilepsy and alcoholism are chronic diseases highly prevalent in the Brazilian population.
We evaluated in the present study the effects of alcohol consumption on the frequency of seizures in animals with epilepsy as well the underlying a possible association between alcohol intake and SUDEP occurrence.
METHOD
Adult male Wistar rats (n=20, 220-280 g) were housed under standard controlled conditions (7:00 a.m./7:00 p.m. light/dark cycle; 20-22ºC; 45-55% humidity) with food and water ad libitum. Rats were divided randomly into two groups: (A) rats with epilepsy (n=10), (B) rats with epilepsy that received a daily dose of 3.0 g kg-1 of a 30% ethanol solution via an oesophagic probe for 30 days (n=10). To do so, we used the pilocarpine model of epilepsy that provides a unique experimental condition for studying the human disorder11. In brief, 30 min after methylscopolamine injection (1 mg kg-1, s.c.), pilocarpine was administered (350 mg kg-1, i.p.) to rats. Seizure activity was monitored behaviorally and terminated with an i.p. injection of diazepam (10 mg/kg; Roche, Brazil) after 4 h of convulsive status epilepticus (SE). The animals were then allowed to evolve through the silent period to the chronic phase of the epilepsy model11. The frequency of SRSs was video-monitored (24 h per day) during all phases of the experiment. Basal frequency of seizures for each rat was determined by monitoring the seizures for 30 days. Following this period, the animals of group B received a daily dose of alcohol solution as described above. Alcohol intake was administrated at approximately the same time (between 10:00 and 12:00 h) during the whole procedure. Control rats (group A) received the same injections of methylscopolamine, pilocarpine and diazepam, but received saline solution instead of alcohol. To determine the number of seizures during this period three observers were recruited for all this behavioral analysis.
RESULTS
Pilocarpine treatment sequentially induced the following behavioral changes: akinesia, facial automatisms, and limbic seizures consisting of forelimb clonus with rearing, salivation, and masticatory jaw movements and falling. This type of behavior built-up progressively into motor limbic seizures that recurred repeatedly and rapidly developed into status epilepticus. After SE, animals were comatose or unresponsive to their environment and akinetic. Behavior returned to normal over a 3- to 5-day period11,12. Spontaneous Recurrent Seizures (SRSs) in rats with epilepsy observed during the chronic period of the pilocarpine model of epilepsy were characterized by facial automatisms, forelimb clonus, rearing, loss of postural control and generalized clonic seizures lasting 40-60 s11.
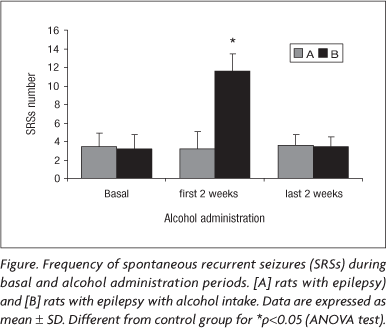
The basal frequency of seizures observed in the A and B groups during the first 30 days were 3.4±1.5 and 3.2±1.9 seizures per week per animal, respectively. In B group, it was observed a significant seizure increase to 11.6±5.3 during the first 2 weeks of alcohol administration (p<0.05). Moreover, during the last 2 weeks of alcohol administration, the number of SRSs returned to the previous basal level 3.4±1.2 (Figure). During the experimental procedure, animals from A group maintained the seizure frequency near the basal values related above (3.2±1.1 for the first 2 weeks and 3.6±1.3 for the last 2 weeks). Quite interesting, one rat (group B) died suddenly after a generalized tonic-clonic seizure during the second week of alcohol consumption.
DISCUSSION
To our knowledge, there are no experimental studies in literature describing a possible relationship between alcohol intake and SUDEP. The mainly data described here was the occurrence of SUDEP during alcohol consumption in rats with epilepsy. Furthermore, the present study also confirmed previous studies8 showing a significant increase of SRSs during alcohol administration. Exact knowledge regarding the association of alcohol abuse and SUDEP is lacking. Following this reasoning, a number of hypotheses could be put forward to explain our findings.
Firstly, our data demonstrated that the alcohol administration induces behavioral (increased frequency of seizures) changes during the chronic period of pilocarpine model of epilepsy and this fact could be direct related with the SUDEP event occurred in our study. As we know, SUDEP is responsible for 7.5% to 17% of all deaths in epilepsy and has an incidence among adults between 1:500 and 1:1,00013,14. Of the many risk factors suggested for SUDEP, higher frequency of seizures is the mainly consistent issue. Accordingly, among the rarely witnessed cases of SUDEP, the majority of patients proved to suffer a partial or generalized seizure immediately prior to death, suggesting a seizure-related cardiac or respiratory dysfunction15,16. In an elegant largest case-control study, Nilsson and colleagues17 demonstrated that seizure frequency is a strongest risk factor for SUDEP. In their study, fifty seven SUDEP cases were included, of whom 91% had undergone necropsy. The relative risk of SUDEP increased with number of seizures per year and the estimated relative risk was 10.16 (95% CI 2.94-35.18) in patients with more than 50 seizures per year, compared with those with up to two seizures per year. In this sense, as in our study a significant clustering of seizures was related during alcohol intake period, it seems to be clear a possible association between alcohol, seizure frequency and SUDEP.
A second argument in favor of SUDEP in the case reported here includes a possible cardiac arrhythmia precipitated by increased seizure frequency mediated by alcohol consumption. In this sense, it has been demonstrated an increase of mean heart rate and arrhythmias after heavy drinking, suggesting an exaggerated sympathetic reaction in these events18,19. Similarly, cardiac arrhythmia precipitated by seizure discharge acting via the autonomic nervous system has been postulated as a cause of SUDEP20. This may include stress related release of catecholamines from the adrenal medulla predisposing to cardiac arrhythmias changes in the autonomic control of the heart20-24.
Third, the increased frequency of seizures mediated by alcohol administration described in our study could also be related with synaptic integration and plasticity of new neurons in the hippocampal formation. In 2002, Pawlak and colleagues25 demonstrated that 14 days of ethanol administration caused 2-fold increase in the number of proliferating cells in subgranular zone of dentate gyrus, suggesting that long-term ethanol intoxication causes damage to hippocampal subfields, but not to DG which can be counterbalanced by ongoing neurogenesis. Concerning epilepsy, an increased neurogenesis is also reported in several experimental models and in human adult epileptic tissue obtained after hippocampectomy26. Quite interesting, as news hilar-ectopic dentate granule cells are migrate aberrantly, abnormally integrated and hyperexcitable, contributing with this to seizure development or progression of recurrent seizures27 and seizure severity and frequency are the most important risk factors for SUDEP4, it is plausible to believe that this "reverberant endogenous mechanism" could influence negatively the cardiovascular system of the patient with epilepsy leading to cardiac abnormalities and hence SUDEP.
Last, from twenty animals with epilepsy evaluated in our study, just one developed SUDEP and probably associated with alcohol intake. In these lines, we believe and are totally in agreement with Nashef and colleagues28 hypothesis that exist a genetic susceptibility to epilepsy and sudden cardiac death. In their elegant paper, the authors concluded that although, at present, bridging evidence between cardiac inherited gene determinants and SUDEP is lacking, the possibility of a coexisting "mild" susceptibility to sudden cardiac death, be it independent of or related to the epilepsy, which becomes symptomatic in the presence of uncontrolled seizures28.
In sum, although recent epidemiological studies have been helpful in identifying the patient at risk and have thus provided clues as to the mechanisms behind SUDEP, there is no single risk factor common to all cases3, which suggests that alcohol abuse may have an interesting role in this scenario. Finally, further experimental and clinical studies are needed to gain a better understanding of the role of alcohol consumption as a potential risk factor to SUDEP, but in the mean time caution with occurrence of SUDEP continuous to be prudent and necessary.
Received 4 February 2009, received in final form 7 July 2009. Accepted 3 August 2009.
The authors thank FAPESP, CInAPCe-FAPESP and CNPq for supporting this study.
Dr. Fulvio Alexandre Scorza - Disciplina de Neurologia Experimental - Rua Botucatu 862 - 04023-900 São Paulo SP - Brasil. E-mail: scorza.nexp@epm.br
- 1. Duncan JS, Sander JW, Sisodiya SM, et al. Adult epilepsy. Lancet 2006; 367:1087-1100.
- 2. Stollberger C, Finsterer J. Cardiorespiratory findings in sudden unexplained/unexpected death in epilepsy (SUDEP). Epilepsy Res 2004; 59:51-60.
- 3. Tomson T, Walczak T, Sillanpaa M, et al. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia 2005;46:54-61.
- 4. Ryvlin P, Montavont A, Kahane P. Sudden unexpected death in epilepsy: from mechanisms to prevention. Curr Opin Neurol 2006;19:194-199.
- 5. Scorza FA, Colugnati DB, Pansani AP, Sonoda EY, Arida RM, Cavalheiro EA. Preventing tomorrow's sudden cardiac death in epilepsy today: what should physicians know about this? Clinics 2008;63:389-394.
- 6. Hauser WA, Ng SKC, Brust JCM. Alcohol, seizures and epilepsy. Epilepsia 1988;29(Suppl 2):S66-S78.
- 7. Freedland ES, McMicken DB. Alcohol-related seizures. Part 1. Pathophysiology, differential diagnosis, and evolution. J Emerg Med 1993;11:463-473.
- 8. Scorza FA, Arida RM, Cysneiros RM, Priel MR, de Albuquerque M, Cavalheiro EA. The effects of alcohol intake and withdrawal on the seizures frequency and hippocampal morphology in rats with epilepsy. Neurosci Res 2003;47:323-328.
- 9. Hilbom ME, Kaste M. Ethanol intoxication: a risk factor for isquemic brain infarctation. Stroke 1983;14:694-699.
- 10. Corrigan GE. Alcoholic head trauma triad. J Stud Alcohol 1977;36:541-549.
- 11. Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci 1995; 16:33-37.
- 12. Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia 1991;32:778-782.
- 13. Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol 2000;428:240-253.
- 14. Pedley TA, Hauser WA. Sudden death in epilepsy: a wake-up call for management. Lancet 2002;359:1790-1791.
- 15. Leestma JE, Annegers JF, Brodie MJ, et al. Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia 1997;38:47-55.
- 16. Jehi L, Najm IM. Sudden unexpected death in epilepsy: impact, mechanisms, and prevention. Cleve Clin J Med. 2008;Suppl 2:S66-S70.
- 17. Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet 1999;353:888-893.
- 18. Mäki T, Toivonen L, Koskinen P, Näveri H, Härkönen M, Leinonen H. Effect of ethanol drinking, hangover, and exercise on adrenergic activity and heart rate variability in patients with a history of alcohol-induced atrial fibrillation. Am J Cardiol 1998;82:317-322.
- 19. Denison H, Jern S, Jagenburg R, Wendestam C, Wallerstedt S. Influence of increased adrenergic activity and magnesium depletion on cardiac rhythm in alcohol withdrawal. Br Heart J 1994;72:554-560.
- 20. Lathers CM, Schraeder PL, Bungo MW. The mystery of sudden death: mechanisms for risks. Epilepsy Behav 2008;12:3-24.
- 21. Opeskin K, Harvey AS, Cordner SM, Berkovic SF. Sudden unexpected death in epilepsy in Victoria. J Clin Neurosci 2000;7:34-37.
- 22. Lathers CM, Kelliher GJ, Roberts J, Beasley AB. Nonuniform cardiac sympathetic nerve discharge: mechanism for coronary occlusion and digitalis-induced arrhythmia. Circulation 1978;57:1058-1065.
- 23. Mameli O, Mameli P, Tolu E, et al. Analysis of central cardioarrhythmogenic triggers in experimental epilepsy. Epilepsy Res 1990;7:210-218.
- 24. Schraeder PL, Lathers CM. Paroxysmal autonomic dysfunction, epileptogenic activity and sudden death. Epilepsy Res 1989;3:55-62.
- 25. Pawlak R, Skrzypiec A, Sulkowski S, Buczko W. Ethanol-induced neurotoxicity is counterbalanced by increased cell proliferation in mouse dentate gyrus. Neurosci Lett 2002;327:83-86.
- 26. Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist 2003;9:261-272.
- 27. Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia 2008; 49(Suppl 5):S19-S25.
- 28. Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia 2007;48:859-871.
Publication Dates
-
Publication in this collection
16 Dec 2009 -
Date of issue
Dec 2009
History
-
Received
04 Feb 2009 -
Reviewed
07 July 2009 -
Accepted
03 Aug 2009