Abstracts
The evoked cerebral electric response when sequences of complex motor imagery (MI) task are executed several times is still unclear. This work aims at investigating the existence of habituation in the cortical response, more specifically in the alpha band peak of parietal and occipital areas (10-20 international system electroencephalogram, EEG, protocol). The EEG signals were acquired during sequences of MI of volleyball spike movement in kinesthetic and visual modalities and also at control condition. Thirty right-handed male subjects (18 to 40 years) were assigned to either an 'athlete' or a 'non-athlete' group, both containing 15 volunteers. Paired Wilcoxon tests (with α=0.05) indicates that sequential MI of complex tasks promotes cortical changes, mainly in the power vicinity of the alpha peak. This finding is more pronounced along the initial trials and also for the athletes during the modality of kinesthetic motor imagery.
motor imagery; alpha band; volleyball
A resposta elétrica cerebral evocada quando sequencias de imagética motora (MI) de tarefas complexas são executadas seguidamente no tempo permanecem desconhecidas. Este trabalho objetivou investigar a existência de habituação da resposta cortical, mais especificamente na banda do pico de alfa de áreas parietais e occipitais (sistema internacional 10-20, eletroencefalograma, protocolo de EEG). Os sinais de EEG foram adquiridos durante sequências de MI do movimento de ataque do voleibol nas modalidades cinestésica e visual, e também em condição de controle. Trinta voluntários adultos (entre 18 e 40 anos), destros, do gênero masculino foram agrupados como 'atletas' ou 'não-atletas', sendo cada grupo composto de 15 voluntários. Testes pareados de Wilcoxon (com α=0.05) indicaram que a MI sequencial de tarefas complexas promoveram alterações nas respostas corticais, mais especificamente na região ao redor do pico de alfa. Este achado foi mais pronunciado ao longo dos trechos iniciais e também nos atletas durante a modalidade cinestésica de imagética motora.
imagética motora; banda alfa; voleibol
ARTICLE
EEG changes during sequences of visual and kinesthetic motor imagery
Alterações no EEG durante sequencias de imagética motora visual e cinestésica
Marcus Vinicius StecklowI; Antonio Fernando Catelli InfantosiI; Maurício CagyII
IFederal University of Rio de Janeiro, Biomedical Engineering Program, Rio de Janeiro RJ, Brazil. Laboratório de Processamento de Imagens e Sinais Biológicos do Programa de Engenharia Biomédica (PEB) do Instituto Alberto Luiz Coimbra de Pós-graduação e Pesquisa em Engenharia (COPPE) da Universidade Federal do Rio de Janeiro, Rio de Janeiro RJ, Brazil
IIFluminense Federal University, Department of Epidemiology and Biostatistics, Niterói RJ, Brazil. Laboratório de Processamento de Imagens e Sinais Biológicos do Programa de Engenharia Biomédica (PEB) do Instituto Alberto Luiz Coimbra de Pós-graduação e Pesquisa em Engenharia (COPPE) da Universidade Federal do Rio de Janeiro, Rio de Janeiro RJ, Brazil
Correspondence Correspondence Marcus Vinicius Stecklow Av. Horácio Macedo 2030. Edifício do Centro de Tecnologia. Bloco H / sala 327 21941-914 Rio de Janeiro RJ - Brasil E-mail: marcus@peb.ufrj.br
ABSTRACT
The evoked cerebral electric response when sequences of complex motor imagery (MI) task are executed several times is still unclear. This work aims at investigating the existence of habituation in the cortical response, more specifically in the alpha band peak of parietal and occipital areas (10-20 international system electroencephalogram, EEG, protocol). The EEG signals were acquired during sequences of MI of volleyball spike movement in kinesthetic and visual modalities and also at control condition. Thirty right-handed male subjects (18 to 40 years) were assigned to either an 'athlete' or a 'non-athlete' group, both containing 15 volunteers. Paired Wilcoxon tests (with α=0.05) indicates that sequential MI of complex tasks promotes cortical changes, mainly in the power vicinity of the alpha peak. This finding is more pronounced along the initial trials and also for the athletes during the modality of kinesthetic motor imagery.
Key words: motor imagery, alpha band, volleyball.
RESUMO
A resposta elétrica cerebral evocada quando sequencias de imagética motora (MI) de tarefas complexas são executadas seguidamente no tempo permanecem desconhecidas. Este trabalho objetivou investigar a existência de habituação da resposta cortical, mais especificamente na banda do pico de alfa de áreas parietais e occipitais (sistema internacional 10-20, eletroencefalograma, protocolo de EEG). Os sinais de EEG foram adquiridos durante sequências de MI do movimento de ataque do voleibol nas modalidades cinestésica e visual, e também em condição de controle. Trinta voluntários adultos (entre 18 e 40 anos), destros, do gênero masculino foram agrupados como 'atletas' ou 'não-atletas', sendo cada grupo composto de 15 voluntários. Testes pareados de Wilcoxon (com α=0.05) indicaram que a MI sequencial de tarefas complexas promoveram alterações nas respostas corticais, mais especificamente na região ao redor do pico de alfa. Este achado foi mais pronunciado ao longo dos trechos iniciais e também nos atletas durante a modalidade cinestésica de imagética motora.
Palavras-chave: imagética motora, banda alfa, voleibol.
Motor imagery (MI) has been considered a dynamic mental process associated with motor task simulation without real execution1 constituting a purely cognitive activity2. It has been shown that MI improves the muscular force3 and physical performance in many different tasks, such as surgical techniques4 and sport movements5. The MI usually is performed in kinesthetic modality (KMI), characterized by 'feeling' joint movements and muscles activations generating kinesthetic impressions, and in visual modality (VMI), when the subject forms himself a mental image, like still observing the such motor task.
It has been reported that KMI and VMI activate different neuron networks6, including the left parietal7 and visual cortex8. This cortical activation can be assessed by different techniques such as time response paradigms9, neuroimaging8 and electroencephalogram6,10-12 (EEG). Similar to external stimuli event-related potentials (ERP) visual13,14 and auditory15, the MI (so called internal stimuli) also originate ERPs12,16. In frequency domain, some studies16,17 have focused the cerebral changes observing the event-related desynchronization (ERD) and event-related synchronization (ERS). However, other studies only observe the behavior of power spectrum10,11,18.
Usually, the cerebral activity investigation with MI paradigms is based on the mean power in EEG frequency bands10,19, mainly in alpha band (8-13 Hz). In many works, the alpha band has been functionally divided in lower and upper sub-bands. However, these studies do not consider that such alpha activity may differ inter-individually according to inheritance20, age21, gender and cognitive state22,23. The variation is more pronounced at the frequency where the maximum power contribution occurs, so called the alpha band peak (ABP). Since the activity in alpha band is associated with mental effort during mental tasks, and also with the habituation of cerebral waves when stimuli are sequentially presented24, we hypothesize that an accommodation in cortical response may occur. Such accommodation would be reflected as changes in the frequency domain, more specifically in ABP of parietal and occipital areas, if a sequence of complex MI task is executed several times. Hence, this work aims at investigating the existence of habituation in alpha band power, focusing the frequencies around the alpha peak, during sequences of MI of the spike volleyball movement.
METHOD
The casuistry was composed of 15 elite athletes of indoor volleyball (GA) and 18 subjects without experience in this sport (GNA). All subjects of both groups were right handed. The volunteers answered a personal information questionnaire about athletic history and indicated no neurological disturbs nor the use of drugs that could affect the cognitive performance. All tests were carried out in the Laboratory of Cerebral Mapping and Motor Sensory Integration (Psychiatry Institute) and the Laboratory of Image and Signal Processing (Biomedical Engineering Program) both from Federal University of Rio de Janeiro. This study was submitted and approved by ethical committee, and all volunteers signed an informed consent.
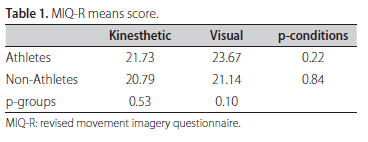
The Revised Movement Imagery Questionnaire25 (MIQ-R), concerning four movements and their respective motor imagery tasks in kinesthetic and visual modalities, was translated to Portuguese and then applied to ensure that all subjects could see or feel mental images with a minimum of clearness. As exclusion criterion, the sum of scores in each subscale below 15 yielded the exclusion of 3 subjects from GNA.
Before the EEG acquisition, a 5-min video showing an expert athlete during execution of several trials of spike volleyball movement task in different points of view was displayed on a monitor for all subjects in order to demonstrate the correct execution of attack movement. Volunteers seated comfortably with closed eyes in a silent room, and Ag/AgCl electrodes were placed according the international 10-20 system26 with ear lobes reference.
EEG signals were acquired with BNT-36 electroencephalograph (EMSA - Rio de Janeiro), previously filtered (anti-aliasing of 100 Hz and high-pass of 0.1 Hz) and sampled at 240 Hz (using digital 60 Hz notch filter), in three different conditions: control condition (CON), kinesthetic motor imagery and visual motor imagery. CON was composed of unitary mental countdown started at number 1000 during 90 s and was registered preceding MI conditions and in the final of experiment. This counting task aimed at maintaining a standard mental engagement for all volunteers, which would be difficult to achieve if the subjects were requested "not to think in anything". KMI and VMI conditions had 30 target MI trials of volleyball attack randomly intermixed with 20 trials of handclapping in kinesthetic and visual motor imagery respectively. The start of each trial was triggered by a beep in one of two tonalities to indicate the task to be imagined (volleyball attack or handclap), preceded by a preparation beep presented 2 s before. This procedure was applied to avoid the subjects to anticipate the MI executions before the trigger sounds. After the end of KMI and VMI all subjects reported the imagery vividness of each condition block using the respective MIQ-R subscales.
In our study, the EEG signals from occipital (O1 and O2) and parietal regions (P3 and P4) were investigated. For each imagery trial, a 5 s epoch duration synchronized by the starting of the beep trigger in KMI (or VMI) condition was selected. For both experimental conditions, KMI and VMI, an algorithm27 based in first and second statistic moments was adapted and implemented to allow removing artifacts. By applying this procedure, 24 artifact-free epochs were selected for each of these conditions. In order to assess changes in alpha band peak (ABP) along the experiment, the 24 epochs set was sub-divided into three distinct sequences (S1, S2 and S3). The first eight consecutive epochs were included in S1, the next eight in S2 and the remaining in S3.
After subdividing each epoch into five segments of 1 s duration, the power spectral density (PSD) was then estimated using the Bartlett periodogram technique28 with rectangular window, performed in Matlab version 6.5 (The Mathworks Inc.), denoted by:
where  is the PSD of the epoch, M=5 the number of segments (no overlap) and
is the PSD of the epoch, M=5 the number of segments (no overlap) and  is the estimated spectrum of m-th segment, with spectral resolution of 1 Hz. The objective of using the rectangular window was minimizing near-frequency leakage of the spectrum obtained from original signal. Finally, the PSD of each sequence was calculated as the mean of the eight
is the estimated spectrum of m-th segment, with spectral resolution of 1 Hz. The objective of using the rectangular window was minimizing near-frequency leakage of the spectrum obtained from original signal. Finally, the PSD of each sequence was calculated as the mean of the eight  of the sequence epochs.
of the sequence epochs.
To account for the inter-individual alpha peak variability (frequency of maximal contribution of band power), the power was estimated within a narrow band of 2 Hz (ABP) centered in alpha peak using the trapezoidal method, which corresponds to calculating the area below the three points of discrete spectrum, laterally limited by frequencies ±1 Hz around the alpha peak. The MIQ-R scores and vividness during experiment were analyzed with independent-samples (inter-groups) and paired-samples (intra-groups) Student t-test (α=0.05). Since the EEG power can hardly be ascribed a Gaussian distribution, the Wilcoxon (paired, non-parametric) test (α=0.05) was used to evaluate the differences in ABP between S1, S2 and S3. Hence, for any of these tests, one has less than 5% probability of error when inferring to reject the null hypothesis of equality.
RESULTS
No statistical differences were found in MIQ-R mean scores between groups or MI modalities as indicated in t-test scores of Table 1. However, the MI of volleyball attack is much clearer in athletes imagining themselves than non-athletes according to the p-values indicated in the Table 2. The intra-group comparison indicated no significant differences in clearness between MI modalities for both groups.
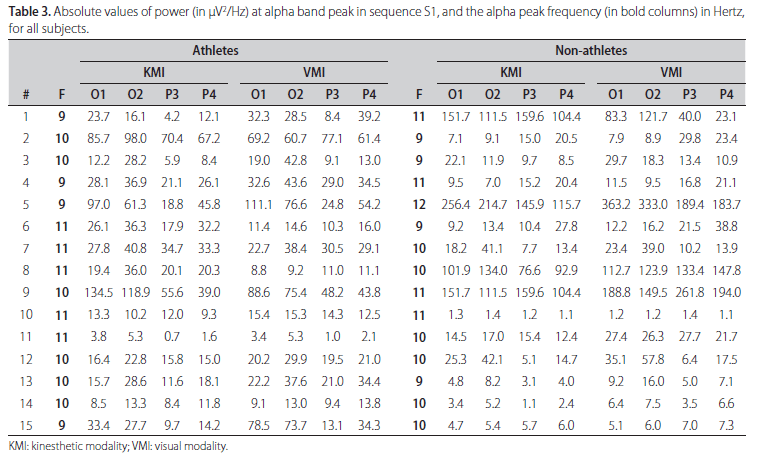
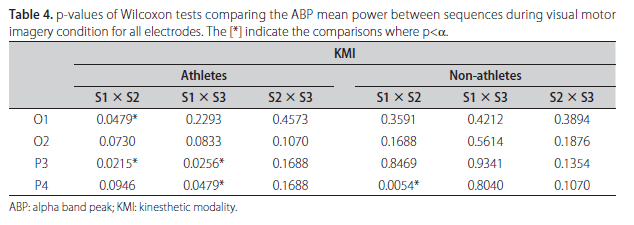
The absolute value of ABP power and its frequency for each subject during KMI and VMI, both for sequence S1, are shown in Table 3. From this table it is evident the inter-individual variability of such parameters between the groups of athletes and non-athletes, and also within each of the groups. For example, it is notable that although athletes #2 and #3 have the same alpha peak frequency (10 Hz), the power contribution at this frequency in derivation O1 during KMI are very different, 85.7 µV2/Hz for athlete #2 and only 12.2 µV2/Hz for athlete #3. Similar comment can be done about derivation O2 during VMI for non-athletes (#7 and #8) who have also alpha peak frequency at 10 Hz and power contribution at this frequency of 23.4 µV2/Hz for non-athlete #7 and 112.7 µV2/Hz for non-athlete #8. Wilcoxon test was applied to assess the null hypothesis (H0) of equal median power in ABP comparing the sequences in couples to both MI modalities. Statistical differences were observed between S1 and S2 in left occipital and parietal areas (O1 and P3), and only in parietal areas (P3 and P4) comparing S1 and S3 in KMI for athletes. Table 4 shows the results for all analysis with p-values obtained in Wilcoxon tests. The comparison between sequences for non-athletes reveals significant difference only between S1 and S2 in right parietal site. One example of ABP changes is illustrated on the Fig 1, where the left and right respective occipital [A,B] and parietal [C,D] sites in athlete #6 have mean power in S1 (continuous line) notoriously higher than S2 (hatched line) and S3 (dotted line) for both sites.
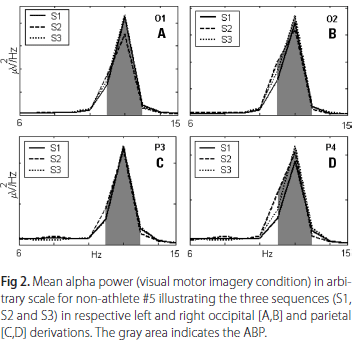
Observing Table 5, which summarizes the results of Wilcoxon tests between distinct moments in VMI, significant differences have been found in the athlete group for P4 between S1 and S2, and for O1 site between S1 and S3. In non-athlete group, no differences were found in any comparison. As observed in KMI, no differences were encountered between S2 and S3 for all sites in VMI for both groups. Fig 2 shows the left and right respective occipital [A,B] and parietal [C,D] derivations of non-athlete #5. Based on the morphology and the amplitude of the power spectrums, one cannot identify differences in mean alpha power between the three sequences (S1, S2 and S3).
DISCUSSION
The MIQ-R is composed of real execution and mental simulation in kinesthetic and visual MI of motor tasks such as bending and extending the knee in stand position and complex movement like jumping with both hands up, that is similar to some part of volleyball spike movement. Considering that MIQ-R have unfamiliar movements for both groups, there is no significant difference in MIQ-R scores between them. Nevertheless, the GA scores were slightly higher than those for GNA, which may be due to the high level of motor ability demanded from athletes executing volleyball movements during the real training sessions and matches when compared with inexperienced group. On the other hand, significant differences were found in clearness score of MI modalities of spike volleyball movement, statistically stronger in KMI, with athletes imagining more easily than non-athletes. This response is presumably because volleyball athletes execute this specific movement exhaustingly, which implies in a solid memory of the motor action.
For both groups, athletes and non-athletes, it is evident the inter-individual variability of the absolute value of ABP power and its frequency between the groups and also within each of them. From this, it is clear that one can not compare the results found for athletes and non-athletes without considering the inter-individual variability. About the time evolution of the experiment, one can point out that no significant differences have been found between sequences S2 and S3 neither in any derivation nor in any group. Although there were cases (derivations or groups, in both MI modalities) where no difference in ABP could be inferred throughout the whole experiment, whenever a difference has been achieved it occurred between S1 and S2 or between S1 and S3. This finding, although does not confirm, at least suggests that some level of habituation takes place along sequence S1, i.e. the beginning of the experiment. It could be interpreted as suggesting a quick phenomenon of habituation, followed by accommodation of ABP response to repetitive MI of stimulus (spike volleyball task). The work of Romero et al.12 also found the effect of habituation, but observing changes of ERP's amplitude in time domain, differently of this study that observe cortical changes with technique in frequency domain.
Comparing the results between groups, GA showed more cortical areas with habituation in ABP than GNA, especially during KMI. For GA, the ABP changes occurred mainly in sequences S2 and S3 compared to the initial one (S1). Since the alpha band power is usually associated with mental effort, this paradoxal response may suggest that athletes quickly learned how to execute the MI of spike volleyball. It is important to consider that athletes have large experience in real execution of spike volleyball movement, hence already having all the motor planning associated to it. On the other hand, the absence of changes in ABP for non-athletes may suggest that more time should be needed for this group to learn the execution of MI and planning the mental simulation of volleyball movement. The answer to this question demands future studies in order to confirm if there are changes in the cortical responses on the group control. Concerning the modality of MI, KMI promoted more physiological alterations than VMI, confirming that cortical activation does depend on the modality of MI executed6,8-11.
Finally, it worth to emphasize the difficulty of comparing our findings with those reported in the literature because we have analyzed the cerebral response to MI using a procedure based on the variation of ABP which could reflect the individual physiological pattern changes. Moreover, carrying out MI more times may help detecting statistical significant difference in ABP.
In conclusion, according to these findings, the motor imagery of sequences of complexes tasks, like the spike volleyball movement, in kinesthetic and visual modalities promotes cortical changes reflected in mean alpha power vicinity of alpha peak, pronouncedly during initial trials. This response is associated to the knowledge of real task execution and the modality of motor imagery executed. Studies using other statistical techniques and protocols, to analyze the behavior of alpha power during motor imagery, must be done to support these results.
Received 7 April 2009
Received in final form 21 December 2009
Accepted 29 December 2009
Support: CNPq and FAPERJ
- 1. Gentili R, Papaxanthis C, Pozzo T. Improvement and generalization of arm motor performance through motor imagery practice. Neuroscience 2006; 137:761-772.
- 2. Decety J, Ingvar DH. Brain structures participating in mental simulation of motor behavior: a neuropsychological interpretation. Acta Psychol 1990; 73:13-34.
- 3. Ranganathan VK, Sieminow V, Liu, JZ, Saghal V, Yue, GH. From mental power to muscle power: gaining strength by using the mind. Neuropsychologia 2004;42:944-956.
- 4. Hall JC. Imagery practice and the development of surgical skills. Am J Surg 2002;184:465-470.
- 5. Roure R, Collet C, Deschaumes-Molinaro C, Delhomme G, Dittmar A, Vernet-Maury E. Imagery quality estimated by autonomic response is correlated to sporting performance enhancement. Physiol Behav 1999;66:63-72.
- 6. Neuper C, Shcherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: Differential effects of kinesthetic and visual-motor mode of imagery in single trial EEG. Cogn Brain Res 2005;25:668-677.
- 7. Rushworth MFS, Nixon PD, Renowden S, Wade DT, Passinghan RE. Left parietal cortex and motor att ention. Neuropsychologia 1997;35:1261-1273.
- 8. Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex 2004;14: 1246-1255.
- 9. Sirigu A, Duhamel JR. Motor and visual imagery as two complementary and neurally dissociable mental processes. J Cognitive Neurosci 2001;13:910-919.
- 10. Cremades JG. The effects of imagery perspective as a function of skill level on alpha activity. Int J Psychophysiol 2002;43:261-271.
- 11. Stecklow MV, Infantosi AFC, Cagy M. Alterações da banda alfa do eletrencefalograma durante imagética motora visual e cinestésica Arq Neuropsiquiatr 2007;65:1084-1088.
- 12. Romero DH, Lacourse MG, Lawrence KE, Schandler S, Cohen MJ. Event-related potentials as a function of movement parameter variations during motor imagery and isometric action. Behav Brain Res 2000;117:83-96.
- 13. Lew GS, Polich J. P300, habituation, and response mode. Physiol Behav 1993;53: 111-117.
- 14. Infantosi AFC, Miranda de Sá, AMFL. A statistical test for evaluating the event-related synchronization/desynchronization and its potential use in Brain-Computer-Interfaces. IFMBE Proceedings 2007;18:1122-1126.
- 15. Pan JP, Takeshita T, Morimoto K. P300 habituation from auditory single-stimulus and oddball paradigms. Int J Psychophysiol 2000;37:149-153.
- 16. Pfurtscheller G, Brunner C, Schlögl A, Lopes da Silva FH. Mu rhythm (de)synchronization and EEG single-trial classification of different motor imagery tasks. NeuroImage 2006;31:153-159.
- 17. Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842-1857.
- 18. Marks DF, Isaac AR. Topographical distribution of EEG activity accompanying visual and motor imagery in vivid and non-vivid imagers. (electroencephalographic activity) (Imagery and Motor Processes). Br J Psychol 1995; 86:271-282.
- 19. Inoue S, Akiyama Y, Izumi Y, Nishijima S. The development of BCI using alpha waves for controlling the robot arm. IEICE Trans Commun 2008;91:2125-2132.
- 20. Smit CM, Wright MJ, Hansell NK, Geffen GM, Martin NG. Genetic variation of individual alpha frequency (IAF) and alpha power in a large adolescent twin sample. Int J Psychophysiol 2006;61:235-243.
- 21. Böttger D, Herrmann CS, Cramon DY. Amplitude differences of evoked alpha and gamma oscillations in two different age groups. Int J Psychophysiol 2002;45:245-251.
- 22. Clark CR, Veltmeyer MD, Hamilton RJ, et al. Spontaneous alpha peak frequency predicts working memory performance across the age span. Int J Psychophysiol 2004;53:1-9.
- 23. Posthuma D, Neale MC, Boomsma DI, Geus EJC. Are smarter brains running faster? Heritability of alpha peak frequency, IQ, and their interrelation. Behav Genet 2001;31:567-579.
- 24. Ravden D, Polich J. On P300 measurement stability: habituation, intra-trial block variation, and ultradian rhythms. Biol Psychol 1999;51:59-76.
- 25. Hall CR, Martin KA. Measuring movement imagery abilities: a revision of movement imagery questionnaire. J Ment Imagery 1997;21:143-154.
- 26. Jasper HH. The ten twenty electrode system of International Federation. Electroencephalography and Clinical Neurophysiology, 1958;10:917-975.
- 27. Tierra-Criollo CJ, Infantosi AFC. Low-frequency oscillations in human tibial somatosensory evoked potentials. Arq Neuropsiquiatr 2006;64:402-406.
- 28. Shiavi R. Introduction to applied statistical signal analysis, 2nd Ed. Nashville, Tennessee: Academic Press, 1999.
Correspondence
Publication Dates
-
Publication in this collection
11 Aug 2010 -
Date of issue
Aug 2010
History
-
Reviewed
21 Dec 2009 -
Received
07 Apr 2009 -
Accepted
29 Dec 2009