Abstracts
Patent foramen ovale (PFO) closure is indicated in some cases to protect patients against embolic events. The aim of this study was to certify that the method of PFO closure to prevent microemboli (MES) is reliable, using contrast enhanced transcranial Doppler (cTCD) as a diagnostic and follow-up tool. METHODS: cTCD was performed before and after PFO closure in 20 patients. Results obtained a minimum of 12 months after the procedure were analyzed in this study. RESULTS: After the procedure, 14 patients (82%) showed no microemboli in cTCD at rest, but after provocative Valsalva maneuver (VM) microembolic phenomenon were still detected in 14 (70%): 7 (35%) <10 MES, 3 (15%) 10-20 MES and 4 (20%) had more than 20 MES ("curtain"). Only six of the total patients presented no MES in both resting and VM. CONCLUSION: These results showed a large percentage of patients with MES detection in a bubble study with transcranial Doppler more than one year after the procedure of PFO closure, showing right-to-left residual shunting. Despite the small number of patients, this study provides important data about this therapeutic decision.
foramen ovale; patent; ultrasonography; Doppler; transcranial; embolism; treatment
O fechamento do forame oval patente (FOP) é indicado em alguns casos para prevenir eventos embólicos. O objetivo deste estudo foi certificar que o fechamento do FOP previne contra microembolia usando o Doppler transcraniano contrastado (cTCD) como método diagnóstico e de controle. MÉTODOS: O cTCD foi realizado antes e depois do fechamento do FOP em 20 pacientes. Foram analisados somente os resultados obtidos após 12 meses do procedimento. RESULTADOS: Após o procedimento, 14 pacientes (82%) não apresentaram microembolia (MES) ao exame de repouso. Entretanto, após sensibilização com manobra de Valsalva (MV), detectou-se ainda passagem de MES em 14 (70%) dos pacientes: 7 (35%) <10 MES; 3 (15%) 10-20 MES e 4 (20%) com mais de 20 MES (padrão "cortina"). Somente seis pacientes não apresentaram sinais de MES em ambas as etapas do teste (repouso e MV). CONCLUSÃO: Grande porcentagem de pacientes apresentou MES após o procedimento para fechamento do FOP, o que é consistente com presença de shunt direito-esquerdo residual. Apesar do pequeno número de pacientes, este estudo apresenta dados que contribuem com esta importante decisão terapêutica.
forame oval patente; ultrassonografia Doppler transcraniana; embolia; tratamento
ARTICLE
Is the patent foramen ovale closure the best option?
O fechamento do foramen oval patente é a melhor opção?
Viviane Flumignan ZetolaI; Melissa Castello Branco e SilvaI; Marcos Christiano LangeI; Juliano Andre MuzzioI; Edison Matos NovakI; Admar MoraesII; Lineu Cesar WerneckI
IHospital de Clínicas, Neurology Division, Federal University of Paraná (UFPR), Curitiba PR, Brazil
IIHospital de Clínicas, Cardiology Division, UFPR, Curitiba PR, Brazil
Correspondence Correspondence: Viviane Flumignan Zetola Hospital de Clínicas, Divisão de Neurologia, Universidade Federal do Paraná Rua General Carneiro 181 80060-900 Curitiba PR - Brasil E-mail: viviane.zetola@gmail.com
ABSTRACT
Patent foramen ovale (PFO) closure is indicated in some cases to protect patients against embolic events. The aim of this study was to certify that the method of PFO closure to prevent microemboli (MES) is reliable, using contrast enhanced transcranial Doppler (cTCD) as a diagnostic and follow-up tool.
METHODS: cTCD was performed before and after PFO closure in 20 patients. Results obtained a minimum of 12 months after the procedure were analyzed in this study.
RESULTS: After the procedure, 14 patients (82%) showed no microemboli in cTCD at rest, but after provocative Valsalva maneuver (VM) microembolic phenomenon were still detected in 14 (70%): 7 (35%) <10 MES, 3 (15%) 1020 MES and 4 (20%) had more than 20 MES ("curtain"). Only six of the total patients presented no MES in both resting and VM.
CONCLUSION: These results showed a large percentage of patients with MES detection in a bubble study with transcranial Doppler more than one year after the procedure of PFO closure, showing right-to-left residual shunting. Despite the small number of patients, this study provides important data about this therapeutic decision.
Key words: foramen ovale, patent, ultrasonography, Doppler, transcranial, embolism, treatment.
RESUMO
O fechamento do forame oval patente (FOP) é indicado em alguns casos para prevenir eventos embólicos. O objetivo deste estudo foi certificar que o fechamento do FOP previne contra microembolia usando o Doppler transcraniano contrastado (cTCD) como método diagnóstico e de controle.
MÉTODOS: O cTCD foi realizado antes e depois do fechamento do FOP em 20 pacientes. Foram analisados somente os resultados obtidos após 12 meses do procedimento.
RESULTADOS: Após o procedimento, 14 pacientes (82%) não apresentaram microembolia (MES) ao exame de repouso. Entretanto, após sensibilização com manobra de Valsalva (MV), detectou-se ainda passagem de MES em 14 (70%) dos pacientes: 7 (35%) <10 MES; 3 (15%) 1020 MES e 4 (20%) com mais de 20 MES (padrão "cortina"). Somente seis pacientes não apresentaram sinais de MES em ambas as etapas do teste (repouso e MV).
CONCLUSÃO: Grande porcentagem de pacientes apresentou MES após o procedimento para fechamento do FOP, o que é consistente com presença de shunt direito-esquerdo residual. Apesar do pequeno número de pacientes, este estudo apresenta dados que contribuem com esta importante decisão terapêutica.
Palavras-Chave: forame oval patente, ultrassonografia Doppler transcraniana, embolia, tratamento.
Patent foramen ovale (PFO) is a congenital heart disease remnant of fetal circulation. The flap of the foramen ovale (septum primum) closes against the atrial septum (septum secundum) and normally fuses within the first two years of life. If the septum secundum covers the oval foramen, but does not seal to the septum primum, then a probable PFO exists that can be "opened" by the Valsalva or other maneuvers that increase right atrial pressure1,2. Prevalence of incomplete fusion is approximately 2527%1,3 and is associated with atrial septum aneurysm (ASA) in 5080% patients in a global population3. The opening between the left and right atria can introduce venous blood or venous thrombus to the arterial system by crossing into the left heart through a right-to-left shunt (RLS)1-5.
Studies have demonstrated that RLS emboli and PFO are significantly associated with cryptogenic ischemic stroke, transient ischemic attack (TIA) and migraine with aura in young adults (below 55 years of age)2-5.
Prevention against further embolic events by PFO closure is a controversial issue, however the procedure is indicated in some restricted cases6-13.
The aim of this study was to certify whether or not the PFO method of closure confirmed by transcranial Doppler prevented microemboli after a minimum of 12 months.
METHODS
We retrospectively analyzed 150 patients diagnosed with PFO from Hospital de Clínicas, Federal University of Paraná, and from a private clinic in Brazil. The study was conducted from October 2005 to October 2009. Only 20 patients were indicated for PFO closure, all of whom underwent a transesophageal echocardiography (ETE) exam to confirm the diagnosis. The indication for intervention procedure was decided by each patient's physician. Only the data of those patients who underwent PFO closure were analyzed in this study and only those for whom a standardized technique was performed by the same trained neurologists. We only considered the results obtained a minimum of 12 months after the procedure. These criteria were established to rule out cases of incomplete prosthesis epithelization in patients who underwent to endovascular closure.
Contrast-enhanced transcranial Doppler ultrasound (cTCD)
All cTCD studies were performed with the patient in a supine position in a controlled temperature environment (24 to 28ºC) by a trained neurologist (Doctors MCL, VFZ, and JAM). The equipment included a RIMED Smart Lite or a DWL Doppler Box, both with two 2-MHz transducers. Bilateral middle cerebral arteries (MCA) were insonated through the temporal window at a depth of 50 to 60 mm and fixed with a helmet, as described else-where. Contrast consisted of 10 mL air-mixed saline solution (9 mL of normal saline solution + 1 mL of air) injected as a bolus into a large right antecubital vein while resting (resting phase) and before the Valsalva maneuver (VM). The VM was performed five seconds after intravenous contrast injection and its effectiveness was monitored by a 25% decrease of MCA flow velocity. Both studies (resting phase and VM phase) were repeated three times, with each test lasting one minute. A RLS was considered positive when at least one air microbubble was detected on the spectral display of at least one of the monitored MCA12. Patients with a positive test were classified in four grades: negative (no microbubble), small RLS (≤10 MES (microemboli)), moderate RLS (1020 MES), and the latter subgroup was further labeled as a "curtain" or RLS if more than 20 MES appeared during MCA monitoring. Patients with negative or mild microbubble (MB) were classified as negative, and patients moderate or "curtain" were classified as positive2. A total of 20 patients were analyzed, with a mean age of 43 years (1167 years), 12 of whom were females (62,5%). Underlying etiologies for indication of the procedure were: stroke in 15 (75%) patients, transient ischemic attack in 3 (15%) and treatment refractory migraine with aura in two (10%) patients. All patients indicated for the procedure were classified as positive after VM at the time of diagnosis by cTCD.
RESULTS
Upon PFO diagnosis by cTCD using the resting method, 3 patients (15%) showed no MES, 7 (35%) less than 10 MES, two (10%) 1020 MES and 8 (40%) showed more than 20 MES ("curtain). When VM was performed, 2 (10%) patients showed 1020 MES and 18 (90%) more than 20 MES ("curtain") (Table1).
Patients underwent the procedure using different device models: Amplatzer, Cardio-Seal, Premeri and Helex for PFO closure and 3 underwent surgery (15%). A minimum of 12 months (mean 24±12 months), after the PFO closure procedure cTCD was performed by the same trained neurologist who had carried out the diagnostic procedure.
The following results were found after PFO closure; when the resting method was employed: 14 (82%) patients had no MES, one (6%) showed more than 10 MES, two (12%) 1020 MES, and none showed a "curtain" effect. After the VM, six (30%) patients showed no MES, seven (35%) <10 MES, three (15%) 1020 MES and four (20%) showed a "curtain" effect. Thirteen patients (65%) were classified as negative and only seven (35%) as positive (Table 1).
The majority of patients, 17 (85%), including all 3 surgical subjects, showed no post procedure microemboli in cTCD at rest. Nevertheless, after provocative maneuver microembolic phenomenon was detected in 14 patients (70%): seven patients (35%) had less than 10 MES, 3 (15%) had 1020 MES, and the remaining four (20%) patients had more than 20 MES ("curtain").
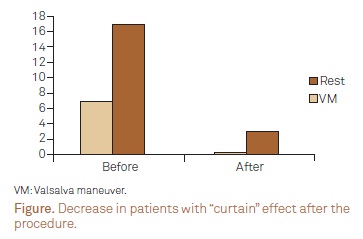
When cTCD diagnosis was performed, 8 (40%) patients showed more than 20 MES ("curtain") at rest and 18 (90%) after VM. Despite the high number of patients showing the "curtain" effect before the procedure, no patients showed more than 20 MES "curtain" at rest and only 4 patients (20%) after VM, following PFO closure. Thus a marked decrease in patients with "curtain" effect was seen (100% at rest and 78% after VM) (Figure).
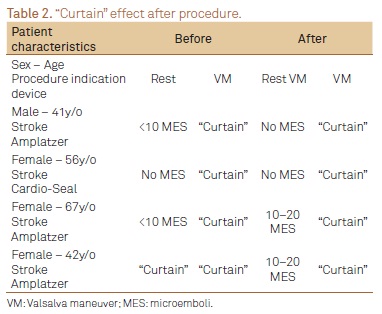
Among all patients, only 4 (20%) presented "curtain" effect after the procedure. The indication for the procedure in all cases was stroke. The subjects were predominantly women (75%) with a mean age of 51, 5 years (4167 years). Before PFO closure, one patient (25%) showed no MES, two (50%) 1020 MES and one (25%) presented the "curtain "effect. However, after PFO closure, 2 patients (50%) showed no MES while the other two had 1020 MES at rest. All patients presented the "curtain" effect after VM (Table 2).
Despite the obtained results, only six patients (30%) showed no MES after PFO closure both at rest and after VM, one of whom underwent surgery.
DISCUSSION
Since the first evidence suggesting that PFO was a probable cause for cryptogenic stroke, TIA, migraine with aura, dementia and obstructive apnea, different treatments for this congenital heart disease have been proposed by the medical community including: anticoagulation, antiplatelet agents, surgical procedure and transcatheter percutaneous closure13. However, to date, no consensus has been reached on the best treatment option and, although recommendations are available, there are no definitive guidelines for PFO treatment. The American Heart Association, American Stroke Association14,15, Cardiovascular Radiology and Intervention and The American Academy of Neurology in their guidelines14 make clear that any therapeutic options are level C evidence (recommendation based on expert opinion or serial cases). Clinical treatment, however, is class II evidence. In other words, there is a conflict or disagreement regarding the usefulness of therapeutic procedures. According to the guidelines, the available data is insufficient for recommendation of PFO closure for the first stroke episode; however, closure can be considered for a patient with stroke recurrence under optimal clinical treatment.
In recent decades, promising results of transcatheter percutaneous PFO closure have been reported, including a decrease in stroke recurrence, as well as low morbidity and mortality of the procedure. Consequently, this has led to an increase in the number of procedures performed worldwide and the emergence of different devices2,3,5,6,8,9,16. Despite these promising results, some cautious recommendations for PFO closure criteria have been recently established. The results include: a history consistent with paradoxical embolism, recurrent stroke events despite medical therapy, the combination of PFO and atrial septal aneurysm, large PFO size or an underlying hypercoagulable state13.
In the present study, the several different models of device for PFO closure included: Amplatzer, Cardio-Seal, Premeri and Helex, and, in addition, three patients underwent surgery. The indication for surgery or the use of a particular device was made by the performing surgeon. In this particular study, the goal was not to compare the data between different devices or with surgery. The efficacy of the procedure was assessed after transcatheter percutaneous PFO closure using the following methods: transthoracic echocardiography (TTC), transesophageal echocardiography (TEE) and transcranial Doppler (cTCD). In this study, cTCD was elected for the diagnosis of right-to-left shunt2,7,8 as it is a noninvasive, low cost, well tolerated diagnostic method available in our clinical practice2,16 and is a suitable microemboli diagnostic tool. The improved sensitivity of cTCD for detecting residual RLS2,7 is one possible reason for the high permanence of RLS after PFO closure. TEE was also used as a follow-up tool after PFO closure. However, enhanced contrast was not used in the follow-up exam. This fact coupled with the difficulty in achieving the VM can lead to misdiagnoses of RLS in post procedure exams thus decreasing sensitivity of the method. In the present study, we did not analyze nor compare the results of TEE against those of cTCD after PFO closure. A recent study documented effective procedural success in 86% of patients using TEE during the follow-up17, but the two methods were not compared.
Our study demonstrated that a large proportion of patients (more than 80%) showed no RLS when using the resting method after PFO closure. By contrast, after VM, 14 (70%) showed RLS after the procedure. Despite differences in the methodology, Harms et al.7, in 236 patients, and Braun et al.6 demonstrated RLS in almost 44% of patients after the procedure. Notably in our data, only six patients (30%) showed no MES at rest and after VM, and surgery was performed in one subject. In the study by Braun et al.6, only 56% of patients presented no MB after the procedure. The high number of patients showing RLS after VM may suggest that, in this particular study, the results for the procedure were not satisfactory.
It is also important to emphasize that before PFO closure 18 patients (90%) showed the "curtain" effect after VM on cTCD, but after the procedure the number decreased to only 4 patients (20%). It is also important to emphasize that before PFO closure, 18 patients (90%) showed the "curtain" effect after VM on cTCD, but after the procedure the number of patients decreased to only 4 (20%). Our data demonstrated a marked decline of about 78% in the remaining patients showing the "curtain" effect in cTCD after PFO closure after VM. These findings raise the question as to whether or not a decrease of total MES is a protective factor in further prevention of symptomatic microemboli events.
Despite the substantial reduction in patients showing the "curtain" effect after PFO closure, four (20%) patients remained in the "curtain" classification after the procedure. This result may be explained by different times necessary for proper healing after the procedure, technical failure during PFO closure or by the device itself. Regardless of the outcome of this hypothesis, these patients should be further evaluated in order to draw more definitive conclusions.
Some anatomical factors are associated with failure of PFO closure. One example is ASA that tended to be over represented in patients with closure failure8. In addition, some atrium septum defect (ASD), which may not be corrected by most of the devices, can only be detected at the time of the procedure16. Small ASD, fenestrations and ASA present another challenge in PFO closure, since different morphology and anatomy makes it more difficult to create a perfect device that closes both ASD/ASA and PFO without remaining RLS16. Although some of the patients in this study had ASA or ASD, this was not analyzed in our results.
Microemboli after transcatheter percutaneous closure can occur due to an extra cardiac shunt such as a pulmonary arteriovenous fistula18,19. The timing from contrast injection to identification of the first bubble on the medium cerebral artery can be used to differentiate between a cardiac and an extra cardiac shunt. If the first bubble is identified after 11 seconds, RLS is considered cardiac whereas, if it is identified in more than 14 seconds, it can be considered extracardiac2. In our study, extracardiac RLS was not investigated and no patients were included with high risk of pulmonary arteriovenous fistula, such as subjects with renal and liver failure or high altitude citizens.
The ideal time at which to consider epithelization healing and true PFO closure after the procedure remains controversial, but studies have shown that a progressive decrease in RLS is reported after several months3,6,16.. However, it is necessary to validate a precise time for epithelization healing for better assessment of the procedure. In our study, we did not validate one year period for prosthesis epithelization. These patients are being followed-up to verify whether epithelization is an ongoing process that leads to full epithelization healing, thus contributing to our data.
Based on our research, it can be concluded that PFO closure continues to be a highly controversial subject both in terms of the indication for best treatment and of assessing the true effectiveness of the treatment. Some PFO treatment clinical trials are currently underway, and it is hoped that the outcomes of these trials can yield further evidence to elucidate the best PFO treatment. Due to the lack of studies with large numbers of patients, controversial issues and poor evidence on best treatment for PFO, it is essential to analyze each patient on an individual basis in order to make the best possible therapeutic decision.
Received 14 February 2012
Received in final form 06 September 2012
Accepted 13 September 2012
Conflict of interest: There is no conflict of interest to declare.
- 1. Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol 2001;38:613-623.
- 2. Lange MC, Zétola VF, Souza AM, et al. Transcranial doppler for patent foramen ovale screening: is there a good correlation with transesophagal echocardiography? Arq Neuropsiquiatr 2008;66:785-789.
- 3. Wu LA, Malouf JF, Dearani JA, et al. Patent foramen ovale in cryptogenic stroke: current understanding and management options. Arch Internal Med 2004;164:950-956.
- 4. Liboni W, Molinari F, Allais GB, et al. Patent foramen ovale detected by near-infrared spectroscopy in patients suffering from migraine with aura. Neurol Sci 2008;29:S182-S185.
- 5. Homma S, Sacco RL, Tulio MRD, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625-2631.
- 6. Braun M, Gliech V, Boscheri A, et al. Transcatheter closure of patent foramen ovale (PFO) in patients with paradoxical embolism.Periprocedural safety and mid-term follow-up results of three different device occluder systems. Eur Heart J 2004;25:424-430.
- 7. Harms V, Reisman M, Fuller CJ, et al. Outcomes after transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Am J Cardiol 2007;99:1312-1315.
- 8. Anzola GP, Morandi E, Cassilli F, Onorato E. Does transcatheter closure of patent foramen ovale really "shut the door"? A prospective study with transcranial Doppler. Stroke 2004;35:2140-2144.
- 9. Bridges ND, Hellenbrand W, Latson L, Filiano J, Newburger JW, Lock JE. Transcatheter closure of patent foramen ovale after presumed paradoxical embolism. Circulation 1992;86:1902-1908.
- 10. Windecker S, Meier B. Is closure recommended for patent foramen ovale and cryptogenic stroke? Patent foramen ovale and cryptogenic stroke: to close or not to close? Closure: what else! Circulation 2008;118:1989-1998.
- 11. Tong DC, Becker KJ. Patent foramen ovale and recurrent stroke: closure is the best option: no. Stroke 2004;35:804-805.
- 12. Donnan GA, Davis SM. Patent foramen ovale and stroke: closure by further randomized trial is required! Stroke 2004;35:806.
- 13. Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology 2009;73:89-97.
- 14. Sacco RL, Adams R, Alberts MJ, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006;113:409-449.
- 15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stoke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227-276.
- 16. Serena J, Segura T, Pérez-Ayuso MJ, Bassaganyas J, Molins A, Dávalos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke 1998;29:1322-1328.
- 17. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991-999.
- 18. Jerusum JT, Fuller CJ, Renz J, Krabill KA, Spencer MP, Reisman M. Diagnosis of secondary right-to-left shunt with balloon occlusion of patent foramen ovale and power M-mode transcranial Doppler. JACC Cardiovasc Interv 2009;2:561-567.
- 19. Aguirregomozcorta M, Ustrell X, Ramió-Torrentà LL, Serena J. [Diagnosis of isolated pulmonary arterio-venous fistula using contrast transcranial Doppler]. Neurologia 2006;21:40-43.
Correspondence:
Publication Dates
-
Publication in this collection
03 Jan 2013 -
Date of issue
Dec 2012
History
-
Received
14 Feb 2012 -
Accepted
13 Sept 2012 -
Reviewed
06 Sept 2012