ABSTRACT
Objective
To contribute our experience with surgical treatment of patients with mesial temporal lobe epilepsy (mTLE) undergoing anterior temporal lobectomy (ATL) or selective amygdalohippocampectomy (SelAH).
Method
This is a retrospective observational study. The sample included patients with medically refractory mTLE due to unilateral mesial temporal sclerosis who underwent either ATL or SelAH, at Hospital de Clinicas – UFPR, from 2005 to 2012. We report seizure outcomes, using Engel classification, cognitive outcomes, using measurements of verbal and visuospatial memories, as well as operative complications.
Result
Sixty-seven patients (33 ATL, 34 SelAH) were studied; median follow-up was 64 months. There was no statistically significant difference in seizure or neuropsychological outcomes, although verbal memory was more negatively affected in ATL operations on patients’ dominant hemispheres. Higher number of major complications was observed in the ATL group (p = 0.004).
Conclusion
Seizure and neuropsychological outcomes did not differ. ATL appeared to be associated with higher risk of complications.
temporal lobe epilepsy; amygdalo-hippocampal epilepsy; anterior temporal lobectomy; neuropsychological tests; seizures; postoperative complications
RESUMO
Objetivo
Contribuir com nossa experiência para o tratamento cirúrgico de pacientes com epilepsia do lobo temporal mesial submetidos a lobectomia temporal anterior (LTA) ou amigdalohipocampectomia seletiva (AHS).
Método
Estudo retrospectivo observacional. Foram incluídos pacientes com epilepsia refratária devido a esclerose mesial temporal unilateral, submetidos a LTA ou AHS no Hospital de Clínicas – UFPR, entre 2005-2012. Foram comparados os resultados cognitivos (análises de memórias verbal e visuoespacial), controle de crises (Engel) e complicações cirúrgicas.
Resultados
Sessenta e sete pacientes (33 LTA, 34 AHS) foram estudados; o período de acompanhamento médio foi de 64 meses. Não houve diferença no controle das crises ou resultado neuropsicológico, mas a memória verbal foi mais negativamente afetada nos pacientes submetidos à LTA no hemisfério dominante. Maior número de complicações graves ocorreu no grupo de LTA (p = 0.004).
Conclusão
Controle de crises e resultados neuropsicológicos não diferiram. LTA pareceu estar associada a um maior risco cirúrgico.
epilepsia do lobo temporal; epilepsia amígdalo-hipocampal; lobectomia temporal anterior; testes neuropsicológicos; crises convulsivas; complicações pós-operatórias
It is estimated that one third of patients with seizures have medically intractable epilepsy (MIE) – defined as failure of two antiepileptic medications given at appropriate doses11. Junna MR, Buechler R, Cohen-Gadol AA, Mandrekar J, Christianson T, Marsh WR et al. Prognostic importance of risk factors for temporal lobe epilepsy in patients undergoing surgical treatment. Mayo Clinic Proc. 2013;88(4):332-36. doi:10.1016/j.mayocp.2013.01.011,22. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission of Therapeutic Strategies. Epilepsia. 2010;51(6):1069-77.. Temporal lobe epilepsy (TLE) is the most common form of MIE. The most frequent pathologic substrate related to this condition is sclerosis and atrophy of the hippocampus – disease named mesial temporal sclerosis (MTS)33. Ramey WL, Martirosyan NL, Lieu CM, Hasham HA, Lemole GM Jr, Weinand ME. Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg. 2013;115(12):2411-8. doi:10.1016/j.clineuro.2013.09.035. Presently, there are multiple approaches to resection of TLE;, the most common being the standard anterior temporal lobectomy (ATL) and selective amygdalohippocampectomy (SelAH). In general, and regardless of the subtype of surgical approach, patients with MTS achieve postoperative seizure freedom in 59-89% of the times44. Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14(1):90-4. doi:10.1111/j.1468-1331.2006.01565.x. Studies have been focusing on the comparison of the two procedures, in terms of seizure and/or neurocognitive outcome, although a consensus is far from being reached.
In this context, this study contributes the Hospital de Clínicas – UFPR experience, as a reference hospital in Brazil, for the treatment of epilepsy. This is a retrospective observational study assessing seizure and neurocognitive outcomes, as well as postoperative complications, in patients diagnosed with MTS who were submitted to either ATL or SelAH at the Hospital de Clínicas from 2005 and 2012.
METHOD
Subjects
This is a retrospective observational study. Patients with medically refractory TLE due to unilateral MTS who underwent resective surgical therapy (either ATL or SelAH) at Hospital de Clínicas – UFPR from 2005 to 2012 were included. Data was collected by chart review. The study was approved by the local regulatory board. Informed consent was obtained from every individual involved in this research.
A total of 212 patients diagnosed with medically refractory MTS were submitted to resective surgery at our centre. Of these, sixty-seven met the inclusion criteria and were, included in this study (33 ATL; 34 SelAH). Median follow-up was 64 months. For the interest of statistical convenience, we analysed only the data up to the fifth year of follow-up. Demographic and clinical features of the patients can be seen at Table 1. Both groups were well matched as depicted in Table 1; therefore, these groups can be defined as being epidemiologically homogenous.
All patients had pre-surgical workup including video-EEG long-term monitoring, magnetic resonance imaging (MRI), and neuropsychological evaluation – if required, we performed additional tests such as invasive EEG, Wada test, SPECT and/or PET-CT –, as well as postoperative neuropsychological testing, and at least two years of follow-up after surgery. The exclusion criteria were as follows: (1) age less than 14 years, and (2) severe cognitive delay or mental retardation (these would not be able to be assessed by the same neuropsychological tests, what would preclude a reliable singular comparison).
Selection of patients to this study was not influenced by genre, ethnicity, social/economical/cultural status, or comorbidities – including psychiatric disorders (such as depression, psychosis, and anxiety), since these conditions are frequently concomitant in patients with TLE55. Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003;4 Suppl 4:26-30. doi:10.1016/j.yebeh.2003.10.003.
Surgical approach
As part of the teaching hospital Hospital de Clínicas – UFPR, our Epilepsy Surgical Program has two attending neurosurgeons that alternate weekly. One surgeon systematically uses the ATL approach, whereas the other systematically uses the SelAH approach. Patients were assigned to a surgeon, and consequently a surgical approach based on which week the operation was booked for. Therefore, a reliable comparison between the outcomes of the two surgical approaches is permitted because the patient distribution can be considered as random and the two groups can be considered as homogenous.
Operative techniques (ATL and SelAH)
Surgical treatment for TLE secondary to MTS aims to remove mesial temporal structures. Partial resection (approximately 3 cm in length) of the hippocampus, amygdala, and parahippocampal gyrus is performed, followed by total resection of the uncus.
In the ATL approach, the resection involves, in addition to the mesial structures, the superficial neocortical temporal gyri. These gyri should be removed even if they are not known to be epileptogenic, based on the observation that these structures (despite being healthy) would propagate seizure activity from temporal mesial sites66. Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52(1):89-94. doi:10.1002/ana.10260. Since more tissue is resected, some experts argue that the ATL technique confers higher chances of medium/long-term seizure control (Figures 1 and 2).
The SelAH technique, on the other hand, spares the neocortical gyri. Concerning the possible accesses, through which the resection of the hippocampus and adjacent structures is performed, they can be transsylvian, transinsular, subtemporal, or transcortical – via white matter on the middle temporal gyrus. The latter is the most used worldwide – and also at our service –, due to lower operative risks. The rationale behind choosing the SelAH remains on the fact that the main origin of epileptogenic activity lies on the mesial portion of the temporal lobe; therefore, additional resection of superficial cortex would not influence on seizure control77. Paglioli E, Palmini A, Portuguez M, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104(1):70-8. doi:10.3171/jns.2006.104.1.70. Moreover, this technique, for sparing the temporal neocortex, in theory results in less postoperative cognition deficits (including memory, language, and behaviour) (Figures 3, 4, and 5).
Complications
Recent systematic review on complications of epilepsy surgery proposed a slightly different classification of operative complications88. Hader WJ, Tellez-Zenteno J, Metcalfe A, et al. Complications of epilepsy surgery: A systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840-7. doi:10.1111/epi.12161. According to this study, minor complications would be the ones that resolved within three months after surgical intervention, whereas major complications are those that persisted beyond this period of time. Although this classification has been frequently used by other researchers99. Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43(2):170-4. doi:10.1046/j.1528-1157.2002.33800.x, we believe it would not be suitable for this study, given that it rates events exclusively by their duration.
According to our proposal, major complications are considered all those that prolong the Intensive Care Unit admission period after resection, lead to the need to redo urgent surgical procedure, as well as increase the risk of death (lethal potential). With this definition, all major vascular accidents, as well as severe infections, are considered major postoperative complications. Complications that do not fit the above-mentioned criteria are classified as minor. In terms of neurological deficits after procedure, we divided them into two groups, transitory and permanent, based on a 3-month cut off (transitory if the deficit resolves completely before 3 months postoperatively; permanent if it does not resolve by the 3rd month).
Neuropsychological testing
All patients were evaluated prior to and at least 6 months following surgery. Individuals with severe cognitive delay or mental retardation – due to the impossibility of them being assessed by standard neuropsychological tests –, as well as those who were not tested post-operatively, were excluded.
The neuropsychological protocol used by our center’s Epilepsy Service investigates visuospatial and verbal memories. The former was evaluated by the Rey-Osterrieth Complex Figure Test (ROCF). The parameter of most importance for this study, provided by the ROCF test, was our patients’ delayed recall ability. The verbal memory was evaluated by the Rey Auditory Verbal Learning Test (RAVL). From this test’s results, we could measure our patients’ long-term verbal memory.
Longitudinal neuropsychological evolution (in terms of verbal and visuospatial memories), prior and after surgery, was analysed using both (a) raw psychometric data and (b) Z-scores. The first method (a) considered cognitive improvement, worsening, or stability comparing neuropsychological tests raw measurements to the standard deviation of the control group (the population sample). The second method (b) classified improvement or worsening according to any change on the classification of memory based on the Z-score. This score was calculated based on raw neuropsychological clinical measurements, the mean of the control (population´s sample of Curitiba) for the cognitive test, and the standard deviation (SD) of the control. We then classified each patient, both at the pre and postoperative periods, as having normal memory (Z-score greater than -1.26) or deficits (mild, Z-score ranging from -1.6 to -1.26; moderate, from -2.2 to -1.6; orsevere, less than -2.2)1010. Strauss E, Sherman EMS, Spreen O. Compendium of neuropsychological tests: administration, norms and commentary. 3rd ed. New York: Oxford University Press; 2006..
RESULTS
Neuropsychological outcome
In pursuance of a broad overview, we compared memory performances of all patients before and after surgery (using the Z-score classification), regardless of the surgical technique. We found that in terms of verbal memory, 17.9% of patients improved, 23.9% worsened, and 58.2% had no change. In respect of visual memory, 16.7% improved, 10.6% worsened, and 72.7% had no change. In the following paragraphs, the results on more detailed analyses are shown.
Conforming to the first analytic method (a), there were no differences in the evolution of neuropsychological performance, in regards to both visuospatial memory (p = 0.182) and verbal memory (p = 0.386), for the two surgical approaches. Similarly, no differences were found in regards to the side operated on (Table 2).
We essentially found the same results when using the analytic method (b). Considering the time points prior and after surgery, there were no differences in the distribution of categories both in terms of visual memory (p = 0.117), and of verbal memory (p = 0.817). Of note, in the ATL group, when looking exclusively at the tests done prior to operation, less patients had visual memory classified as normal in relation to the SelAH group (p = 0.027). However, as shown above, considering the evolution before and after surgery, this observation did not result in differences for either group (Tables 3 and 4).
Further, analyses were done separately for the dominant and the nondominant subsets. Focusing on the ATL group, specifically the individuals operated on the dominant hemisphere, 60 percent of them had normal verbal memory preoperatively; this figure dropped to 20 percent after surgery. Still concerning the ATL on the dominant side, 20 percent of patients had severe verbal memory deficit prior to the ATL; after the procedure, 53.3% of patients were assessed as having severe verbal memory deficit. In regards to individuals who underwent ATL on the nondominant hemisphere, the verbal memory persisted the same without relevant change. Finally, in the whole ATL group, including dominant and nondominant, the visuospatial memory remained with no alteration throughout (Table 3).
When focusing on the SelAH group, on the other hand, there was no important difference in regards to evolution of cognitive status (including both verbal and visuospatial memories) – regardless of the side of operation (dominant versus nondominant) (Table 4).
After interpreting all these data and results, it was concluded that the only subgroup of patients that experienced a relevant cognitive deficit after surgery was the set of subjects who underwent ATL on the dominant hemisphere. Postoperatively, these patients showed an important decline in cognition, exclusively in verbal memory.
Seizure outcome
The evaluation of postoperative mid/long-term seizure control was done based on the Engel rating scale1111. Engel J Jr, Van Ness PC, Rasmussen T. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. New York: Raven; 1993. p. 609-21., which is the most commonly used outcome classification for epilepsy surgery patients1212. Schomer DL, Black PM. A 24-year-old woman with intractable seizures: review of surgery for epilepsy. JAMA. 2008;3;300(21):2527-38. doi:10.1001/jama.2008.709. This evaluation occurred, for every patient, at the 6th month after procedure, then annually up until the last clinical visit.
Table 5 shows, by percentage, the Engel rate at each evaluation for every patient in the study. Further, we categorized Engel rates I and II as ‘satisfactory’, and plotted the percentage of patients (from both groups, side to side) fitting the ‘satisfactory’ category, at the different clinical evaluations (Table 6). Regardless of the surgical approach, the number of patients who were rated Engel I or II was persistently higher than 70% throughout the 5-year period.
After a mean follow-up of 64 months, 82% of all patients had a satisfactory (Engel I or II) outcome, and 51.18% remained seizure-free (Engel Ia) throughout the first 5 years after procedure. In spite of the fact that seizure-freedom (Engel Ia) seemed to be reached more rapidly after SelAH than ATL during the first years after surgery, when we look at the entire follow-up of 5 years there are no statistically significant differences between the two groups.
Complications
Table 7 summarizes our descriptive classification (major or minor) of all surgical complications, and also distinguishes which of them resulted in neurological deficits (classified as being transitory or permanent).
Overall, 19 patients (15 ATL; 4 SelAH), or 28.35%, developed general complication(s) after surgery. There was not any death. In the SelAH group, there were two major and two minor complications; also in this set of patients, six developed postoperative neurological deficits, only one being permanent. In contrast, in the ATL group, there were 13 major and two minor complications; neurological deficits were nine, two being permanent. Comparing the major complications between the two techniques, rates are higher in the ATL group (p = 0.004). In regards to complications resulting in neurological deficits, there was no significant difference between the both approaches (p = 0.370). Besides complications, we believe that patient satisfaction is also important to consider. For that, we collected patients’ perception after procedure: 61 of the 67 patients (91%) were satisfied, and would undergo surgery again, if necessary.
DISCUSSION
The aim of this study was to compare the results of two techniques, ATL or SelAH, for patients diagnosed with medically refractory mesial TLE secondary to unilateral MTS, without any additional structural lesion. Quite a few studies have tried to identify the best surgical technique to treat patients with TLE, few of them, however, included patients with unilateral MTS as the sole underlying pathology1313. Tanriverdi T, Olivier A. Cognitive changes after unilateral cortico-amygdalohippocampectomy unilateral selective-amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17(2):91-9.,1414. Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after mesial temporal lobe epilepsy surgery: corticalamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2008;108(3):517-24. doi:10.3171/JNS/2008/108/3/0517,1515. Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013;104(1-2):94-104. doi:10.1016/j.eplepsyres.2012.09.007. With this goal, we carefully selected a series of patients diagnosed with unilateral MTS who were operated by one of our two attending neurosurgeons. Although there was no randomization per se, both groups were epidemiologically homogenous on most variables. Final data analysis was divided in three spheres: seizure outcome, cognitive outcome, and complications.
Concerning seizure outcomes, no statistically significant differences in seizure outcomes after ATL and SelAH were observed in our study. It has been described by most major series that postoperative seizure control does not differ significantly between the two approaches77. Paglioli E, Palmini A, Portuguez M, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104(1):70-8. doi:10.3171/jns.2006.104.1.70,1313. Tanriverdi T, Olivier A. Cognitive changes after unilateral cortico-amygdalohippocampectomy unilateral selective-amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17(2):91-9.,1414. Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after mesial temporal lobe epilepsy surgery: corticalamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2008;108(3):517-24. doi:10.3171/JNS/2008/108/3/0517,1515. Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013;104(1-2):94-104. doi:10.1016/j.eplepsyres.2012.09.007,1616. Tanriverdi T, Dudley RW, Hasan A, Al Jishi A, Al Hinai Q, Poulin N et al. Memory outcome after temporal lobe epilepsy surgery: corticoamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2010;113(6):1164-75. doi:10.3171/2009.10.JNS09677,1717. Yang XL, Lu QC, Xu JW, Wang GS, Liu Q. Predictors of outcome in the surgical treatment for epilepsy. Chin Med J (Eng). 2011;124(24):4166-71.,1818. Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446-50. doi:10.1002/ana.410400314,1919. Lee T, Mackenzie RA, Walker AJ, Matheson JM, Sachdev P. Effects of left temporal lobectomy and amygdalohippocampectomy on memory. J Clin Neurosci. 1997;4(3):314-9. doi:10.1016/S0967-5868(97)90098-9,2020. Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9(1):95-100. doi:10.1016/j.yebeh.2006.04.017,2121. Grivas A, Schramm J, Kral T, Lehe M, Helmstaedter C, Elger CE et al. Surgical treatment for refractory temporal lobe epilepsy in the elderly: seizure outcome and neuropsychological sequels compared with a younger cohort. Epilepsia. 2006;47(8):1364-72. doi:10.1111/j.1528-1167.2006.00608.x. Nonetheless, three studies have found better seizure-control in patients submitted to ATL44. Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14(1):90-4. doi:10.1111/j.1468-1331.2006.01565.x,2222. Mackenzie RA, Matheson J, Ellis M, Klamus J. Selective versus non-selective temporal lobe surgery for epilepsy. J Clin Neurosci. 1997;4(2):152-4. doi:10.1016/S0967-5868(97)90064-3,2323. Clusmann H, Kral T, Fackeldey E, Blümcke I, Heimstaedter C, Oertzen J et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry. 2004;75(11):1589-96. doi:10.1136/jnnp.2003.024208. Similarly, two recent systematic reviews concluded that ATL is associated with a reduced rate of seizure recurrence compared to SelAH2424. Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80(18):1669-76. doi:10.1212/WNL.0b013e3182904f82,2525. Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG. Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies: a systematic review. J Neurosurg. 2013;119(5):1089-97. doi:10.3171/2013.8.JNS121854.
In other words, a consensus on this matter has not yet been reached. There are a number of reasons that contribute to this lack of agreement. Few studies performed to date have aimed to compare seizure control after ATL and SelAH at one single center, due to the fact surgical programs generally choose one of these approaches to be used. Hence, comparisons can only be done with the collaboration of at least two centers. Further, ATL and SelAH groups usually have unequal number of patients. Also an issue, follow-up periods are frequently of just a couple of years44. Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14(1):90-4. doi:10.1111/j.1468-1331.2006.01565.x,1818. Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446-50. doi:10.1002/ana.410400314. Finally, most studies include individuals with TLE due to several different pathologies, such as tumors, malformations of cortical development, MTS, etc.; rarely do studies limit the recruitment to patients with MTS as the only epileptogenic source1818. Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446-50. doi:10.1002/ana.410400314. All these observations help create many potential biases, which ultimately weaken methodology strength1414. Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after mesial temporal lobe epilepsy surgery: corticalamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2008;108(3):517-24. doi:10.3171/JNS/2008/108/3/0517.
Along these lines, our study tried to minimize all these potential methodological pitfalls. All operations were performed at the same center, where ATL and SelAh were alternated every week. We carefully recruited similar number of patients for both groups. These individuals had similar clinical presentation, unilateral MTS on MRI without additional lesions, unilateral epileptiform discharges on EEG, and were homogenous on epidemiological variables. Further, our mean follow-up was more than 5 years, which is long compared to the majority of studies in this field.
Regarding neuropsychological outcomes, our study did not observe statistically significant differences between the two surgical approaches. However, we did note slight superiority on postoperative verbal memory in patients submitted to SelAH. Based on our analysis, the most important predictor of worse postoperative cognitive status was surgery on the dominant hemisphere, regardless of the technique.
As with seizure control, there is not yet a consensus in terms of cognitive outcomes after ATL and SelAH. Numerous studies concluded that there are no differences between the two approaches, including the systematic review conducted by Hu et al.1616. Tanriverdi T, Dudley RW, Hasan A, Al Jishi A, Al Hinai Q, Poulin N et al. Memory outcome after temporal lobe epilepsy surgery: corticoamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2010;113(6):1164-75. doi:10.3171/2009.10.JNS09677,2525. Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG. Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies: a systematic review. J Neurosurg. 2013;119(5):1089-97. doi:10.3171/2013.8.JNS121854,2626. Mansouri A, Fallah A, McAndrews MP, Cohn M, Mayor D, Andrade D et al. Neurocognitive and seizure outcomes of selective amygdalohippocampectomy versus anterior temporal lobectomy for mesial temporal lobe epilepsy. Epilepsy Res Treat. 2014;2014:ID 306382. doi:10.1155/2014/306382. Nevertheless, many other studies claim that SelAH confers lower cognitive morbidity, mostly involving language and verbal memory. In fact, many of these studies agree that ATL is particularly cognitively harmful when performed on the dominant hemisphere66. Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52(1):89-94. doi:10.1002/ana.10260,77. Paglioli E, Palmini A, Portuguez M, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104(1):70-8. doi:10.3171/jns.2006.104.1.70,2323. Clusmann H, Kral T, Fackeldey E, Blümcke I, Heimstaedter C, Oertzen J et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry. 2004;75(11):1589-96. doi:10.1136/jnnp.2003.024208,2727. Helmstaedter C, Grunwald T, Lehnertz K, Gleiβner U, Elger CE. Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: evidence from temporal lobe epilepsy. Brain Cogn. 1997;35(1):110-31. doi:10.1006/brcg.1997.0930,2828. Helmstaedter C, Richter S, Roske S, Oltmanns F, Schramm J, Lehmann TN. Differential effects of temporal pole resection with amygdalohippocampectomy versus selective amygdalohippocampectomy on material-specific memory in patients with mesial temporal lobe epilepsy. Epilepsia. 2008;49(1):88-97. doi:10.1111/j.1528-1167.2007.01386.x,2929. Kneebone AC, Lee GP, Wade LT, Loring DW. Rey Complex Figure: figural and spatial memory before and after temporal lobectomy for intractable epilepsy. J Int Neuropsychol Soc. 2007;13(4):664-71. doi:10.1017/S1355617707070828 – an observation that was also noted in our series.
In terms of operative complications, we found a high rate of general complications. This high rate resulted essentially from major complications, especially in the ATL group. In fact, there was a statistically significant difference in major complications between the ATL and SelAH groups. We could not find published studies that specifically compared operative complications between ATL and SelAH, therefore our subgroup data could not be compared to other centers’ results. However, in comparison to complications in epilepsy surgery in general (including different approaches to resective surgery, and invasive EEG implantation), our rate of total complications is categorically higher88. Hader WJ, Tellez-Zenteno J, Metcalfe A, et al. Complications of epilepsy surgery: A systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840-7. doi:10.1111/epi.12161. Notably, despite the relatively high overall complication rate, 91% of all operated patients were satisfied with having had epilepsy surgery and would undergo it again if necessary. This high satisfaction rate in our patients after epilepsy surgery is in agreement with other recent studies3030. Taft C, Sager Magnusson E, Ekstedt G, Malmgren K. Health-related quality of life, mood, and patient satisfaction after epilepsy surgery in Sweden: a prospective controlled observational study. Epilepsia. 2014;55(6):878-85. doi:10.1111/epi.12616. This finding reinforces the importance of surgical therapy in epilepsy – when well indicated.
We believe at least three reasons could address our incidence of operative complications. First, given that our center is a teaching-hospital, residents’ learning curve could have potentially affected the complication rate. Also, all patients needed to be followed for at least 2 years after surgery and have their charts thoroughly completed. As a result, these criteria could have selected a biased sample of patients who needed closer follow-up and care. Thirdly, there is a possibility that operative complications are under reported both in charts and in the literature.
We acknowledge that this study has limitations, basically because it is retrospective, and the number of enrolled patients is small. Another limitation lies in the fact that we incorporated data from the experience of two surgeons (one of them systematically performing ATL and the other SelAH); thus, the experience and skills of each surgeon should be taken into consideration when interpreting our study results. Nonetheless, although individual studies may not have enough power to detect differences that are statistically and clinically significant, these investigations are necessary and essential to produce reliable and important significant medical evidence when analysed on a broad perspective.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Ms. Yvonne DeWit for proofreading the manuscript.
References
-
1Junna MR, Buechler R, Cohen-Gadol AA, Mandrekar J, Christianson T, Marsh WR et al. Prognostic importance of risk factors for temporal lobe epilepsy in patients undergoing surgical treatment. Mayo Clinic Proc. 2013;88(4):332-36. doi:10.1016/j.mayocp.2013.01.011
-
2Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission of Therapeutic Strategies. Epilepsia. 2010;51(6):1069-77.
-
3Ramey WL, Martirosyan NL, Lieu CM, Hasham HA, Lemole GM Jr, Weinand ME. Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg. 2013;115(12):2411-8. doi:10.1016/j.clineuro.2013.09.035
-
4Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. Eur J Neurol. 2007;14(1):90-4. doi:10.1111/j.1468-1331.2006.01565.x
-
5Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003;4 Suppl 4:26-30. doi:10.1016/j.yebeh.2003.10.003
-
6Helmstaedter C, Reuber M, Elger CC. Interaction of cognitive aging and memory deficits related to epilepsy surgery. Ann Neurol. 2002;52(1):89-94. doi:10.1002/ana.10260
-
7Paglioli E, Palmini A, Portuguez M, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. J Neurosurg. 2006;104(1):70-8. doi:10.3171/jns.2006.104.1.70
-
8Hader WJ, Tellez-Zenteno J, Metcalfe A, et al. Complications of epilepsy surgery: A systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840-7. doi:10.1111/epi.12161
-
9Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: outcome, complications, and late mortality rate in 215 patients. Epilepsia. 2002;43(2):170-4. doi:10.1046/j.1528-1157.2002.33800.x
-
10Strauss E, Sherman EMS, Spreen O. Compendium of neuropsychological tests: administration, norms and commentary. 3rd ed. New York: Oxford University Press; 2006.
-
11Engel J Jr, Van Ness PC, Rasmussen T. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. New York: Raven; 1993. p. 609-21.
-
12Schomer DL, Black PM. A 24-year-old woman with intractable seizures: review of surgery for epilepsy. JAMA. 2008;3;300(21):2527-38. doi:10.1001/jama.2008.709
-
13Tanriverdi T, Olivier A. Cognitive changes after unilateral cortico-amygdalohippocampectomy unilateral selective-amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17(2):91-9.
-
14Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after mesial temporal lobe epilepsy surgery: corticalamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2008;108(3):517-24. doi:10.3171/JNS/2008/108/3/0517
-
15Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013;104(1-2):94-104. doi:10.1016/j.eplepsyres.2012.09.007
-
16Tanriverdi T, Dudley RW, Hasan A, Al Jishi A, Al Hinai Q, Poulin N et al. Memory outcome after temporal lobe epilepsy surgery: corticoamygdalohippocampectomy versus selective amygdalohippocampectomy. J Neurosurg. 2010;113(6):1164-75. doi:10.3171/2009.10.JNS09677
-
17Yang XL, Lu QC, Xu JW, Wang GS, Liu Q. Predictors of outcome in the surgical treatment for epilepsy. Chin Med J (Eng). 2011;124(24):4166-71.
-
18Arruda F, Cendes F, Andermann F, Dubeau F, Villemure JG, Jones-Gotman M et al. Mesial atrophy and outcome after amygdalohippocampectomy or temporal lobe removal. Ann Neurol. 1996;40(3):446-50. doi:10.1002/ana.410400314
-
19Lee T, Mackenzie RA, Walker AJ, Matheson JM, Sachdev P. Effects of left temporal lobectomy and amygdalohippocampectomy on memory. J Clin Neurosci. 1997;4(3):314-9. doi:10.1016/S0967-5868(97)90098-9
-
20Morino M, Uda T, Naito K, Yoshimura M, Ishibashi K, Goto T et al. Comparison of neuropsychological outcomes after selective amygdalohippocampectomy versus anterior temporal lobectomy. Epilepsy Behav. 2006;9(1):95-100. doi:10.1016/j.yebeh.2006.04.017
-
21Grivas A, Schramm J, Kral T, Lehe M, Helmstaedter C, Elger CE et al. Surgical treatment for refractory temporal lobe epilepsy in the elderly: seizure outcome and neuropsychological sequels compared with a younger cohort. Epilepsia. 2006;47(8):1364-72. doi:10.1111/j.1528-1167.2006.00608.x
-
22Mackenzie RA, Matheson J, Ellis M, Klamus J. Selective versus non-selective temporal lobe surgery for epilepsy. J Clin Neurosci. 1997;4(2):152-4. doi:10.1016/S0967-5868(97)90064-3
-
23Clusmann H, Kral T, Fackeldey E, Blümcke I, Heimstaedter C, Oertzen J et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry. 2004;75(11):1589-96. doi:10.1136/jnnp.2003.024208
-
24Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80(18):1669-76. doi:10.1212/WNL.0b013e3182904f82
-
25Hu WH, Zhang C, Zhang K, Meng FG, Chen N, Zhang JG. Selective amygdalohippocampectomy versus anterior temporal lobectomy in the management of mesial temporal lobe epilepsy: a meta-analysis of comparative studies: a systematic review. J Neurosurg. 2013;119(5):1089-97. doi:10.3171/2013.8.JNS121854
-
26Mansouri A, Fallah A, McAndrews MP, Cohn M, Mayor D, Andrade D et al. Neurocognitive and seizure outcomes of selective amygdalohippocampectomy versus anterior temporal lobectomy for mesial temporal lobe epilepsy. Epilepsy Res Treat. 2014;2014:ID 306382. doi:10.1155/2014/306382
-
27Helmstaedter C, Grunwald T, Lehnertz K, Gleiβner U, Elger CE. Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: evidence from temporal lobe epilepsy. Brain Cogn. 1997;35(1):110-31. doi:10.1006/brcg.1997.0930
-
28Helmstaedter C, Richter S, Roske S, Oltmanns F, Schramm J, Lehmann TN. Differential effects of temporal pole resection with amygdalohippocampectomy versus selective amygdalohippocampectomy on material-specific memory in patients with mesial temporal lobe epilepsy. Epilepsia. 2008;49(1):88-97. doi:10.1111/j.1528-1167.2007.01386.x
-
29Kneebone AC, Lee GP, Wade LT, Loring DW. Rey Complex Figure: figural and spatial memory before and after temporal lobectomy for intractable epilepsy. J Int Neuropsychol Soc. 2007;13(4):664-71. doi:10.1017/S1355617707070828
-
30Taft C, Sager Magnusson E, Ekstedt G, Malmgren K. Health-related quality of life, mood, and patient satisfaction after epilepsy surgery in Sweden: a prospective controlled observational study. Epilepsia. 2014;55(6):878-85. doi:10.1111/epi.12616
Publication Dates
-
Publication in this collection
22 Dec 2015 -
Date of issue
Jan 2016
History
-
Received
25 May 2015 -
Reviewed
31 Aug 2015 -
Accepted
22 Sept 2015


 T1-weighted coronal MRI; resection is demarcated with gray-colored line. ATL: anterior temporal lobectomy; H: hippocampus; PH: parahippocampal gyrus; T1, T2 e T3: superior, middle, and inferior temporal gyri; SF: Sylvian fissure.
T1-weighted coronal MRI; resection is demarcated with gray-colored line. ATL: anterior temporal lobectomy; H: hippocampus; PH: parahippocampal gyrus; T1, T2 e T3: superior, middle, and inferior temporal gyri; SF: Sylvian fissure.
 T1-weighted coronal MRI; grey arrow targets the site of status-post right ATL. T2, T3, hippocampus, and parahippocampal gyrus were resected; amygdala was partially removed. ATL: anterior temporal lobectomy.
T1-weighted coronal MRI; grey arrow targets the site of status-post right ATL. T2, T3, hippocampus, and parahippocampal gyrus were resected; amygdala was partially removed. ATL: anterior temporal lobectomy.
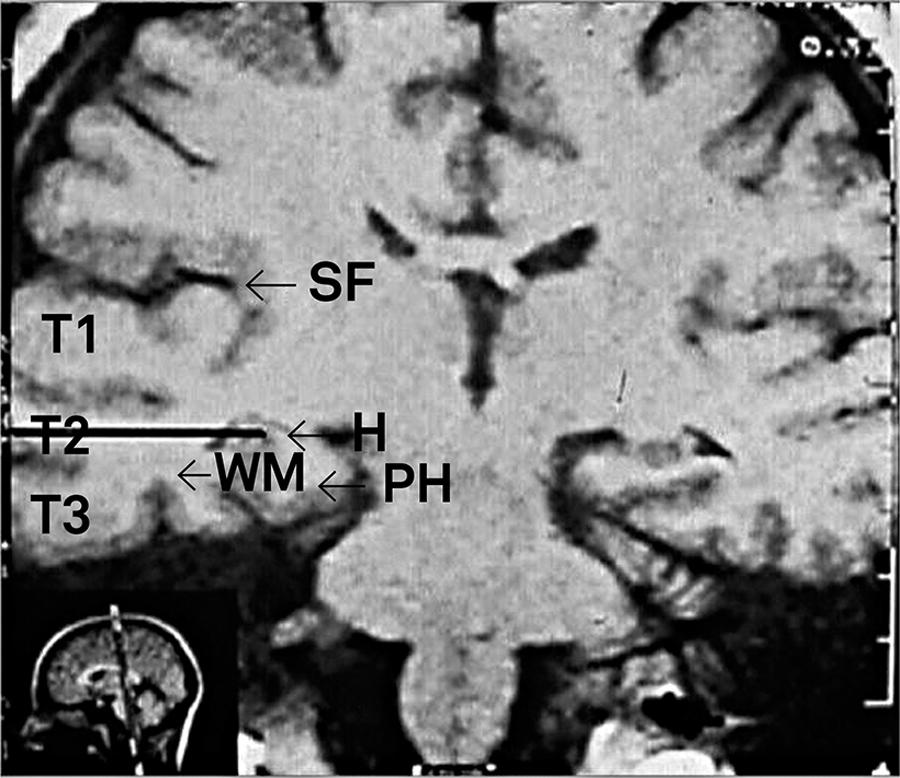 T1-weighted coronal MRI. The black line corresponds to the corridor of dissection (with a 3 cm incision) through T2 to the mesial structures. SelAH: selective amygdalohippocampectomy; WM: white matter; H: hippocampus; PH: parahippocampal gyrus. T1, T2 e T3: superior, middle, and inferior temporal gyri; SF: Sylvian fissure.
T1-weighted coronal MRI. The black line corresponds to the corridor of dissection (with a 3 cm incision) through T2 to the mesial structures. SelAH: selective amygdalohippocampectomy; WM: white matter; H: hippocampus; PH: parahippocampal gyrus. T1, T2 e T3: superior, middle, and inferior temporal gyri; SF: Sylvian fissure.
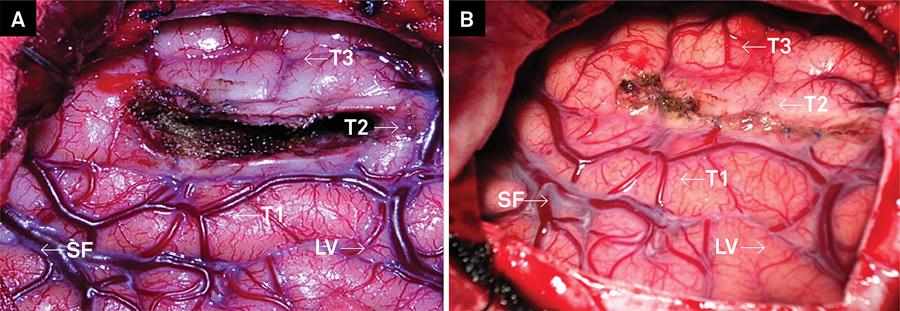 (A) Final surgical cavity, after resection and hemostasis. (B) View after suture of the cortical incision. SelAH: selective amygdalohippocampectomy; SF: Sylvian fissure; LV: Labbé vein.
(A) Final surgical cavity, after resection and hemostasis. (B) View after suture of the cortical incision. SelAH: selective amygdalohippocampectomy; SF: Sylvian fissure; LV: Labbé vein.
 T1-weighted coronal MRI. MRI coronal T1 sequence. Black arrow targets the site of status-post right SelAH, mesial structures were appropriately resected; SelAH: selective amygdalohippocampectomy; H: hippocampus; PH: parahippocampal gyrus.
T1-weighted coronal MRI. MRI coronal T1 sequence. Black arrow targets the site of status-post right SelAH, mesial structures were appropriately resected; SelAH: selective amygdalohippocampectomy; H: hippocampus; PH: parahippocampal gyrus.