ABSTRACT
In this study, the effect of thymoquinone (TQ) on propylthiouracil (PTU)-induced memory impairment was investigated in juvenile rats. The rats were grouped into control, Hypo, Hypo-TQ5 and Hypo-TQ10. Propylthiouracil increased latency time in the Morris water maze test and decreased delay in entering the dark compartment in the passive avoidance test. Both 5 mg/kg and 10 mg/kg doses of TQ decreased latency time in the Morris water maze test and increased delay in entering the dark compartment in a passive avoidance test. The PTU also increased malondialdehyde and nitric oxide metabolites in the brain while reduced the thiol content and superoxide dismutase and catalase activities and serum T4 level. Both doses of TQ decreased malondialdehyde and nitric oxide metabolites in the brain while enhanced the thiol content and superoxide dismutase and catalase activities and serum T4 level. The results of the present study showed that TQ protected against PTU-induced memory impairments in rats.
hypothyroidism; propylthiouracil; memory
RESUMO
Neste estudo, foi investigado o efeito da timoquinona (TQ) contra deficiências de memória induzidas por propiltiouracilo (PTU) em ratos juvenis. Os ratos foram agrupados em grupos: controle, Hypo, Hypo-TQ5, e Hypo-TQ10. O PTU aumentou o tempo de latência no teste do labirinto aquático de Morris (MWM) e diminuiu o atraso para entrar no compartimento escuro no teste de evasão passiva (PA). Ambas as doses de TQ diminuíram o tempo de latência no teste de MWM e aumentaram o atraso para entrar no compartimento escuro no teste de PA. O PTU também aumentou os metabolitos de malondialdeído (MDA) e óxido nítrico (NO) no cérebro, enquanto reduziu o teor de tiol e as atividades de superóxido dismutasa (SOD) e catalasa (CAT) e o nível sérico de T4. Ambas as doses de TQ diminuíram os metabolitos de MDA e de NO no cérebro, aumentaram o conteúdo de tiol e as atividades de SOD e CAT e o nível de T4 no soro. Os resultados do presente estudo mostraram que a TQ protegeu contra deficiências de memória induzidas por PTU em ratos.
hipotireoidismo; propiltiouracila; memória
Thyroid hormones have been demonstrated to have important effects on the cellular basal metabolic rate and are considered a major regulator of energy metabolism, mitochondrial activity and oxygen consumption11. Martinez B, Hoyo P, Martin MA, Arenas J, Perez-Castillo A, Santos A. Thyroid hormone regulates oxidative phosphorylation in the cerebral cortex and striatum of neonatal rats. J Neurochem. 2001;78(5):1054-63. https://doi.org/10.1046/j.1471-4159.2001.00487.x
https://doi.org/10.1046/j.1471-4159.2001...
. It has been shown that reduction of thyroid hormones resulted in cognitive impairments, disrupted attention and depressed moods in adulthood22. Jackson IM. The thyroid axis and depression. Thyroid. 1998;8(10):951-6. https://doi.org/10.1089/thy.1998.8.951
https://doi.org/10.1089/thy.1998.8.951...
. In an experimental model using neonatal rats, hypothyroidism was reported to induce behavioral deficits and structural dysfunction33. Gong J, Liu W, Dong J, Wang Y, Xu H, Wei W et al. Developmental iodine deficiency and hypothyroidism impair neural development in rat hippocampus: involvement of doublecortin and NCAM-180. BMC Neurosci. 2010;11(1):50. https://doi.org/10.1186/1471-2202-11-50
https://doi.org/10.1186/1471-2202-11-50...
. In addition, scientific evidence has indicated that prenatal hypothyroidism disrupts learning and memory by decreasing dendritic spin density and attenuating synaptic function44. Gong J, Liu W, Dong J, Wang Y, Xu H, Wei W et al. Developmental iodine deficiency and hypothyroidism impair neural development in rat hippocampus: involvement of doublecortin and NCAM-180. BMC Neurosci. 2010;11(1):50.. Researchers have indicated that hypothyroidism is associated with a reduced number of neurons in the hippocampus of rats55. Alva-Sánchez C, Sánchez-Huerta K, Arroyo-Helguera O, Anguiano B, Aceves C, Pacheco-Rosado J. The maintenance of hippocampal pyramidal neuron populations is dependent on the modulation of specific cell cycle regulators by thyroid hormones. Brain Res. 2009;1271:27-35. https://doi.org/10.1016/j.brainres.2009.02.043
https://doi.org/10.1016/j.brainres.2009....
.
Brain oxidative damage has been found to be one of the main contributing factors in learning and memory impairment66. Anaeigoudari A, Soukhtanloo M, Shafei MN, Sadeghnia HR, Reisi P, Beheshti F et al. Neuronal nitric oxide synthase has a role in the detrimental effects of lipopolysaccharide on spatial memory and synaptic plasticity in rats. Pharmacol Rep. 2016;68(2):243-9. https://doi.org/10.1016/j.pharep.2015.09.004
https://doi.org/10.1016/j.pharep.2015.09...
. Changes in thyroid hormone levels can induce an oxidative stress condition in organs including the liver, heart, skeletal muscles and brain77. Beheshti F, Hosseini M, Shafei MN, Soukhtanloo M, Ghasemi S, Vafaee F et al. The effects of Nigella sativa extract on hypothyroidism-associated learning and memory impairment during neonatal and juvenile growth in rats. Nutr Neurosci. 2017;20(1):49-59. https://doi.org/10.1179/1476830514Y.0000000144
https://doi.org/10.1179/1476830514Y.0000...
,88. Baghcheghi Y, Salmani H, Beheshti F, Hosseini M. Contribution of Brain Tissue Oxidative Damage in Hypothyroidism-associated Learning and Memory Impairments. Adv Biomed Res. 2017;6(1):59. https://doi.org/10.4103/2277-9175.206699
https://doi.org/10.4103/2277-9175.206699...
. It has been documented that hypothyroidism can lead to a reduced level of glutathione and mitochondrial cytochrome c oxidase activity and overproduction of hydroxyl radicals99. Rahaman SO, Ghosh S, Mohanakumar KP, Das S, Sarkar PK. Hypothyroidism in the developing rat brain is associated with marked oxidative stress and aberrant intraneuronal accumulation of neurofilaments. Neurosci Res. 2001;40(3):273-9. https://doi.org/10.1016/S0168-0102(01)00237-1
https://doi.org/10.1016/S0168-0102(01)00...
. Hypothyroidism-induced oxidative damage in the hippocampus and amygdala has also been shown to have deleterious effects on learning and memory processes1010. Hosseini M, Dastghaib SS, Rafatpanah H, Hadjzadeh MA, Nahrevanian H, Farrokhi I. Nitric oxide contributes to learning and memory deficits observed in hypothyroid rats during neonatal and juvenile growth. Clinics (Sao Paulo). 2010;65(11):1175-81. https://doi.org/10.1590/S1807-59322010001100021
https://doi.org/10.1590/S1807-5932201000...
.
Thymoquinone (TQ), the main constituent of the volatile oil of Nigella sativa seeds, is well known to have antioxidant properties1111. Abdel-Zaher AO, Mostafa MG, Farghly HM, Hamdy MM, Omran GA, Al-Shaibani NK. Inhibition of brain oxidative stress and inducible nitric oxide synthase expression by thymoquinone attenuates the development of morphine tolerance and dependence in mice. Eur J Pharmacol. 2013;702(1-3):62-70. https://doi.org/10.1016/j.ejphar.2013.01.036
https://doi.org/10.1016/j.ejphar.2013.01...
. It has been suggested that TQ can prevent lipid peroxidation during cerebral ischemia-reperfusion injury in rat hippocampus1212. Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14(9):621-7. https://doi.org/10.1016/j.phymed.2006.12.005
https://doi.org/10.1016/j.phymed.2006.12...
. Thymoquinone has been documented to suppress inducible nitric oxide (NO) synthase expression in lipopolysaccharide-stimulated peritoneal macrophages of rats1313. El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N et al. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2(11):1603-11. https://doi.org/10.1016/S1567-5769(02)00139-X
https://doi.org/10.1016/S1567-5769(02)00...
. As well, TQ has been reported to produce anti-anxiety-like effects in mice by modulating the level of NO1414. Gilhotra N, Dhingra D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol Rep. 2011;63(3):660-9. https://doi.org/10.1016/S1734-1140(11)70577-1
https://doi.org/10.1016/S1734-1140(11)70...
. Considering these findings, the objective of this study was to evaluate the protective effect of TQ on learning and memory impairment and brain tissue oxidative damage in hypothyroid juvenile rats.
METHODS
Animals and drugs
Thirty-two male Wistar rats (21 days old, weighing 55–65 g) were purchased from the animal house of Mashhad University of Medical Sciences, Mashhad, Iran. The rats were kept in a standard condition (22 ± 2°C and 12-hour light/dark cycle) with free access to food and water. Work on the animals was carried out in accordance with procedures approved by the Committee on Animal Research of Mashhad University of Medical Sciences. The rats were divided into four groups: 1) Control; the animals received normal drinking water, 2) Hypo; the animals received drinking water supplemented with 0.05% propylthiouracil (PTU) for six weeks; 3) Hypo-TQ5 and 4) Hypo-TQ10; the animals of groups 3 and 4 received drinking water supplemented with 0.05% PTU and 5 mg/kg and 10 mg/kg of TQ respectively. After the behavioral tests, the animals were deeply anesthetized (urethane 1.4 g/kg) and blood samples were taken to confirm hypothyroidism using the radioimmunoassay method (Dai source, T4- RIA - CT). The brains were also removed and the hippocampus and cortical tissues separated to determine biochemical parameters.
Morris water maze test
The Morris water maze apparatus comprised a circular black pool (136 cm diameter, 60 cm high and 30 cm deep) divided into four quadrants; north, east, south and west. The pool was filled with water (24–26°C) and an escape platform (10 cm diameter and 28 cm high) was submerged 2 cm below the surface of the water in the center of the southwest quadrant. Outside the maze, visual cues were fixed around of the room, to help the animals navigate. The rats performed four trials daily, for five days. In each trial, the rat was released from one of four positions randomly. The animal was allowed to swim until it found and remained on the platform for 15 seconds. The time latency to reach the platform, the length of the swimming path and swimming speed were recorded by a video tracking system. On the probe day (sixth day), the platform was removed and the animals were allowed to swim for 60 seconds. The time spent and distance traveled in the target quadrant were recorded to compare between the groups1515. Anaeigoudari A, Soukhtanloo M, Reisi P, Beheshti F, Hosseini M. Inducible nitric oxide inhibitor aminoguanidine, ameliorates deleterious effects of lipopolysaccharide on memory and long term potentiation in rat. Life Sci. 2016;158:22-30. https://doi.org/10.1016/j.lfs.2016.06.019
https://doi.org/10.1016/j.lfs.2016.06.01...
.
Passive avoidance test
The passive avoidance test is based on negative reinforcement, used to study memory. The apparatus is made of two compartments (a light and a dark compartment with a grid floor), which are separated by a small gate. The rats were familiarized with the apparatus for two consecutive days for five minutes each day before the training session. In the training session, the animals were placed in the light compartment. When the animals entered into the dark compartment, the small gate was closed and they received an electric shock (2mA, two-second duration). In the retention phase, 3, 24 and 48 hours later, the animals were placed in the light compartment and the time latency for entering the dark compartment and the time spent in the light and dark compartments were recorded1616. Abareshi A, Hosseini M, Beheshti F, Norouzi F, Khazaei M, Sadeghnia HR et al. The effects of captopril on lipopolysaccharide induced learning and memory impairments and the brain cytokine levels and oxidative damage in rats. Life Sci. 2016;167:46-56. https://doi.org/10.1016/j.lfs.2016.10.026
https://doi.org/10.1016/j.lfs.2016.10.02...
.
Biochemical assessment
Measurement of malondialdehyde (MDA), total thiol and NO metabolites
Malondialdehyde levels and the total thiol content of hippocampal and cortical tissues were measured using a protocol that we have explained previously1717. Anaeigoudari A, Shafei MN, Soukhtanloo M, Sadeghnia HR, Reisi P, Beheshti F et al. Lipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazole [eng.]. Arq Neuropsiquiatr. 2015;73(9):784-90. https://doi.org/10.1590/0004-282X20150121
https://doi.org/10.1590/0004-282X2015012...
. Malondialdehyde, an index of lipid peroxidation, reacts with thiobarbituric acid and produces a red colored complex with a peak absorbance of 535 nm. Determination of the total thiol content was carried out using 2,2’-Dinitro-5,5’-dithiodibenzoic acid. This substance reacts with the sulfhydryl groups and produces a yellow colored complex with a peak absorbance of 412 nm. Nitric oxide metabolites (NO2/NO3) were determined using the Griess reagent method. In this method, 100 µl supernatant was added to the Griess reagent. Then the contents were transferred to a 96-well flat-bottomed micro-plate and absorbance was read at 520 nm using a micro-plate reader. The values were calculated from a standard calibration plot.
The enzymatic assays
Superoxide dismutase (SOD) activity was measured according the Madesh and Balasubramanian method. In this colorimetric assay, the SOD activity was determined at 570 nm. One unit of SOD was defined as the amount of enzyme that inhibits the rate of MTT reduction by 50%. The results were shown as units per gram tissue protein. Catalase activity was measured using a kit purchased from the Cayman Chemical Company.
Statistical analysis
All data are showed as mean ± SEM. The data of time, distance and speed during the five days of the Morris water maze were analyzed using repeated measures analysis of variance (ANOVA) followed by Tukey’s post hoc comparison test. The data from the probe trial in the Morris water maze, data from the passive avoidance test and biochemical data were compared by one-way ANOVA followed by Tukey’s post hoc comparison test. Differences were considered statistically significant when P < 0.05.
RESULTS
The results of the Morris Water Maze
In this study, the results showed that the escape latency and path traveled in the Hypo group were significantly greater than in the control group (p < 0.01 and p < 0.001). The animals in Hypo-TQ5 and Hypo-TQ10 groups had a significantly lower time latency and distance traveled to reach the platform in comparison with those of the Hypo group (p < 0.05 and p < 0.01). There were no significant differences in the time spent and the length of the swim path between the control, Hypo-TQ5 and Hypo-TQ10 groups (Figure 1A and 1B). In the probe trial, the animals of the Hypo group spent less time and traveled less distance in the target quadrant (Q1) than the control group (p < 0.001). The animals in the Hypo-TQ5 and Hypo-TQ10 groups spent more time and traveled a longer distance in Q1 than the Hypo group (p < 0.01 and p < 0.001). There was no significant difference in the time spent and the distance traveled in the Q1 between the control, Hypo-TQ5 and Hypo-TQ10 groups (Figure 2A and 2B). The results also revealed that there was no significant difference in the traveling speed between the groups (Figure 1C).
Comparison of the time latency (A) and traveled distance (B) to reach the platform and the swimming speed (C) between four groups in the Morris water maze test. Data are presented as mean ± SEM (n = 8 in each group). The time latency and the traveled distance of the Hypo group were significantly greater than those of the control group. The animals of the Hypo-TQ5 and Hypo-TQ10 groups took less time and distance to reach the platform than the Hypo group. There was no significant difference in the swimming speed between groups. **p < 0.01; ***p < 0.001 compared with the control group. +p < 0.05; ++p < 0.01 compared with the Hypo group.
The results of the time spent (A) and distance traveled (B) in target quadrant (Q1) on probe day, 24 hours after the last learning session. The platform was removed and the time spent and distance traveled in the target quadrant was compared between groups. Data are shown as mean ± SEM (n = 8 in each group). ***p < 0.001 compared with the control group; ++p < 0.01; +++p < 0.001 compared with the Hypo group.
The results of the passive avoidance test
The results of the passive avoidance test showed that the time latency to enter the dark compartment at 3, 24 and 48 hours after receiving a shock significantly decreased in the Hypo group in comparison to the control group (p < 0.05 and p < 0.01). The time latency to enter the dark compartment at 3, 24 and 48 hours after receiving a shock by the animals of the Hypo-TQ5 and Hypo-TQ10 was longer than the Hypo group (p < 0.05) (Figure 3A). In addition, there was no significant difference in the time latency to enter the dark compartment at 3, 24 and 48 hours after receiving a shock between the control, Hypo-TQ5 and Hypo-TQ10 groups.
Comparison of the time latency to enter the dark compartment (A) and the total time spent in the dark compartment (B) at 3, 24 and 48 hours after receiving a shock, in the experimental groups. Data are shown as mean ± SEM (n = 8 in each group). *p < 0.05; ** p < 0.01; *** p < 0.001 compared with the control group. +p < 0.05; ++p < 0.01; +++p < 0.001 compared with the Hypo group.
The total time spent in the dark compartment at 3, 24 and 48 hours after receiving a shock by the animals of the Hypo group was significantly longer than the control group (p < 0.001). The total time spent in the dark compartment at 3, 24 and 48 hours after receiving a shock in the Hypo-TQ5 and Hypo-TQ10 groups was significantly lower than the Hypo group (p < 0.01 and p < 0.001), however, when the total time spent in the dark component was compared between the control, Hypo-TQ5 and Hypo-TQ10, there was no significant difference between them (Figure 3B).
Biochemical results in hippocampal tissues
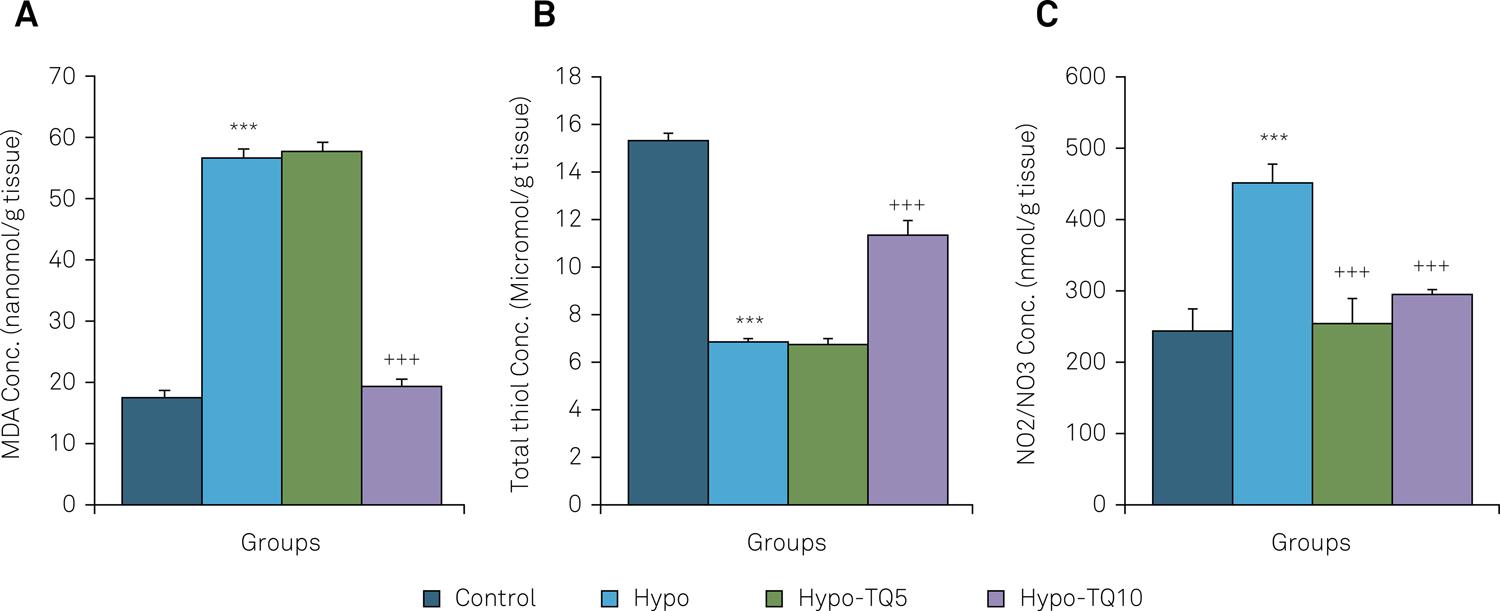
The MDA concentration in hippocampal tissues of the Hypo group was higher than the control group (p < 0.001). Administration of 10 mg/kg TQ decreased the MDA concentration in the hippocampal tissues of the Hypo-TQ10 group compared with the Hypo group (p < 0.001) (Figure 4A). There was no significant difference in the MDA concentration in hippocampal tissues between the Hypo and Hypo-TQ5 groups.
Comparison of the MDA concentration (A), the total thiol content (B) and the level of nitric oxide metabolites (C) in hippocampal tissue of the four groups. Data are shown as mean ± SEM (n = 8 in each group). ***p < 0.001 compared with the control group; +++p < 0.001 compared with the Hypo group.
The total thiol content in the hippocampal tissues of the Hypo group was lower than the control group (p < 0.001). Administration of 10 mg/kg TQ enhanced the total thiol content in hippocampal tissues of the Hypo-TQ10 group compared to the Hypo group (p < 0.001) (Figure 4B). There was no significant difference in total thiol content between the Hypo and Hypo-TQ5 groups.
Nitric oxide metabolite concentration, NO2 or NO3, in the hippocampal tissues in the Hypo group was higher than the control group (p < 0.001). Administration of both doses of 5 mg/kg and 10 mg/kg TQ decreased the concentration of these metabolites in hippocampal tissue in the Hypo-TQ5 and Hypo-TQ10 groups in comparison with the Hypo group (p < 0.001) (Figure 4C).
According to the results of the current study, the SOD and CAT activities in the hippocampal tissues of the Hypo group were lower compared with the control group (p < 0.01 and p < 0.001). The SOD and CAT activity in the Hypo-TQ5 and Hypo-TQ10 groups was higher than the Hypo group (p < 0.001) (Figure 5A). In addition, we found no significant difference in the activities of SOD and CAT between the control, Hypo-TQ5 and Hypo-TQ10.
Comparison the activities of superoxide dismutase (SOD) (A) and catalase (CAT) (B) in hippocampal tissue between the four groups. Data are shown as mean ± SEM (n= 8 in each group). **p < 0.01; ***p < 0.001 compared with the control group; +++p < 0.001 compared with the Hypo group.
Biochemical results in cortical tissues
The MDA concentration in the cortical tissues of the Hypo group was higher than the control group (p < 0.001). In addition, injections of 5 mg/kg and 10 mg/kg TQ reduced the MDA level in cortical tissue in the Hypo-TQ5 and Hypo-TQ10 groups compared with the Hypo group (p < 0.05 and p < 0.01) (Figure 6A).
Comparison of the malondialdehyde (MDA) concentration (A), the total thiol content (B) and the level of nitric oxide metabolites (C) in cortical tissue of the four groups. Data are shown as mean ± SEM (n= 8 in each group). *** P< 0.001 compared with the control group. + P< 0.05 and +++ P< 0.001 compared with the Hypo group.
The total thiol content in the cortical tissues of the Hypo group was lower than the control group (p < 0.01). Administration of 10 mg/kg TQ enhanced the total thiol content in the cortical tissues of the Hypo-TQ10 group compared with the Hypo group (p < 0.001) (Figure 6B). There was no significant difference in total thiol content between the Hypo and Hypo-TQ5 groups.
Nitric oxide metabolite concentration, NO2 or NO3, in the cortical tissues in the Hypo group was higher than the control group (p < 0.001). Administration of TQ did not change the concentration of these metabolites in the cortical tissues in the Hypo-TQ5 and Hypo-TQ10 groups compared with the Hypo group (Figure 6C).
According to the results of current study, SOD and CAT activities in the cortical tissues of the Hypo group were lower in comparison with the control group (p < 0.01 and p < 0.001). The activity of SOD in the Hypo-TQ10 group was higher than the Hypo group (p < 0.001). Both doses including 5 mg/kg and 10 mg/kg TQ increased CAT activity in the cortical tissues of the Hypo-TQ5 and Hypo-TQ10 groups compared with the Hypo group (p < 0.001) (Figure 7A and 7B).
Comparison of the activities of SOD (A) and CAT (B) in cortical tissue between the four groups. Data are shown as mean ± SEM (n = 8 in each group). **p < 0.01; ***p < 0.001 compared with the control group; +++p < 0.001 compared with the Hypo group.
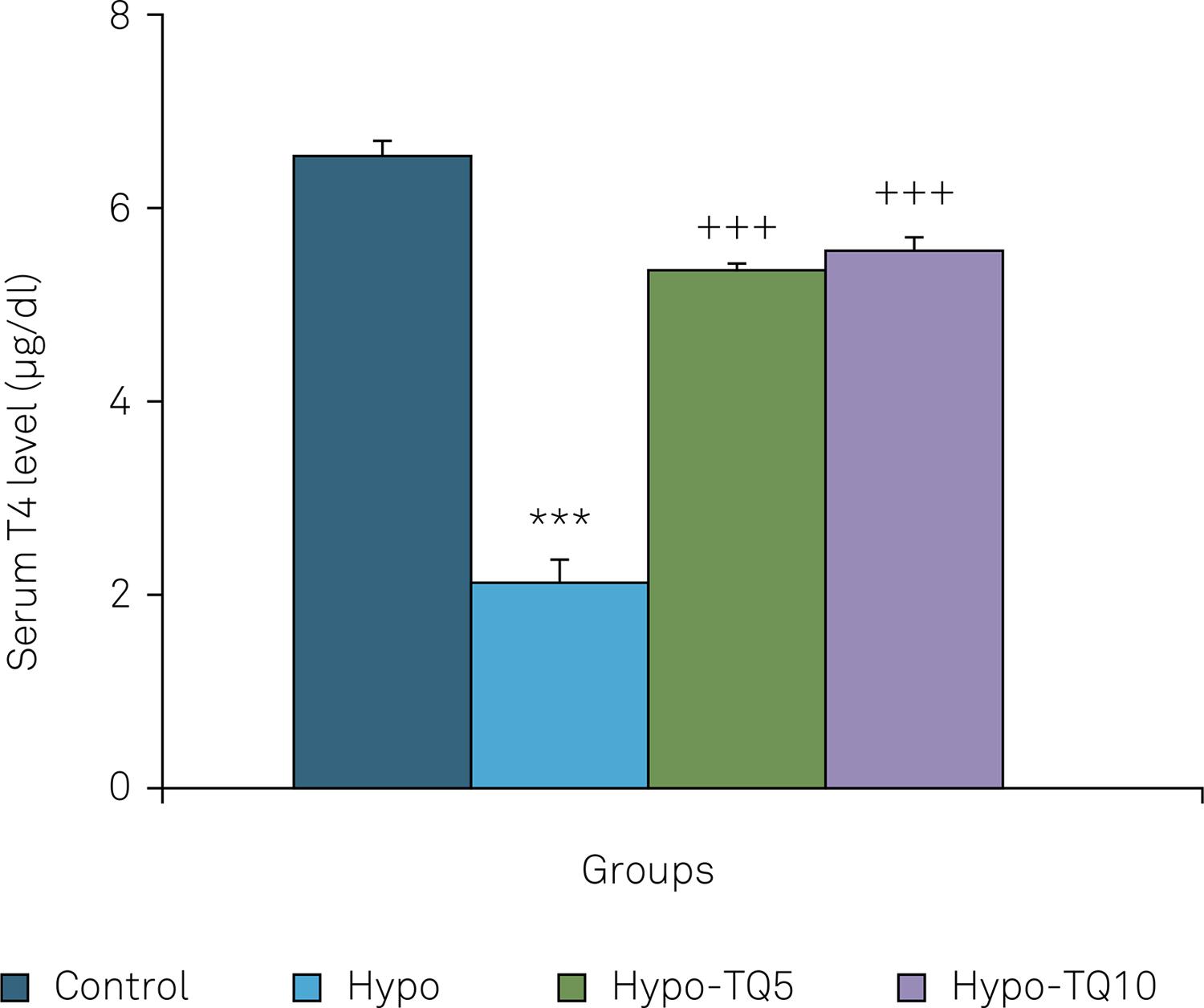
In addition, the serum T4 level in the Hypo group was lower than in the control group (p < 0.001). The serum level of T4 in the Hypo-TQ5 and Hypo-TQ10 groups was higher than the Hypo group (p < 0.001). There was no significant difference in the serum T4 level between the control, Hypo-TQ5 and Hypo-TQ10 groups (Figure 8).
Comparison of the serum level of T4 between the four groups. Data are shown as mean ± SEM (n = 8 in each group). ***p < 0.001 compared with the control group; +++p < 0.001 compared to the Hypo group.
DISCUSSION
Based on the results of the present study, hypothyroidism induced by PTU in juvenile rats resulted in learning and memory impairments. Previous studies have also shown that hypothyroidism disrupts learning and memory processes in neonatal and juvenile rats1818. Farrokhi E, Hosseini M, Beheshti F, Vafaee F, Hadjzadeh MA, Dastgheib SS. Brain tissues oxidative damage as a possible mechanism of deleterious effects of propylthiouracil- induced hypothyroidism on learning and memory in neonatal and juvenile growth in rats. Basic Clin Neurosci. 2014;5(4):285-94.. It has been documented that hypothyroidism attenuates hippocampal-related learning and memory, and long-term potentiation1919. Alzoubi KH, Aleisa AM, Gerges NZ, Alkadhi KA. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: behavioral and electrophysiological studies. J Neurosci Res. 2006;84(5):944-53. https://doi.org/10.1002/jnr.21014
https://doi.org/10.1002/jnr.21014...
. Reduced levels of thyroid hormones have also been reported to influence learning and memory through switching off myelinogenesis in the nervous system2020. Barradas PC, Ferraz AS, Ferreira AA, Daumas RP, Moura EG. 2‘3’Cyclic nucleotide 3’phosphodiesterase immunohistochemistry shows an impairment on myelin compaction in hypothyroid rats. Int J Dev Neurosci. 2000;18(8):887-92. https://doi.org/10.1016/S0736-5748(00)00028-9
https://doi.org/10.1016/S0736-5748(00)00...
. In addition, researchers have shown that thyroxine improved performances of rats in a radial arm maze task and ameliorated long-term potentiation impairments in the CA1 area of the hippocampus when it was administered to thyroidectomized rats2121. Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19(1):66-78. https://doi.org/10.1002/hipo.20476
https://doi.org/10.1002/hipo.20476...
. These scientific findings support the results of our study that PTU-induced hypothyroidism disrupted learning and memory in both the Morris water maze and passive avoidance tasks in rats.
It has been well documented that the balance between oxidants and antioxidants is essential for maintaining vital cellular and biochemical functions2222. Jones DP, Carlson JL, Mody VC Jr, Cai J, Lynn MJ, Sternberg P Jr. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28(4):625-35. https://doi.org/10.1016/S0891-5849(99)00275-0
https://doi.org/10.1016/S0891-5849(99)00...
. Changing the balance toward an enhancement of oxidants or reduction of antioxidants may result in cell damage, which can be defined as oxidative stress66. Anaeigoudari A, Soukhtanloo M, Shafei MN, Sadeghnia HR, Reisi P, Beheshti F et al. Neuronal nitric oxide synthase has a role in the detrimental effects of lipopolysaccharide on spatial memory and synaptic plasticity in rats. Pharmacol Rep. 2016;68(2):243-9. https://doi.org/10.1016/j.pharep.2015.09.004
https://doi.org/10.1016/j.pharep.2015.09...
. It has been demonstrated that excessive production of free radicals and brain oxidative damage result in learning and memory deficits, and compounds with antioxidant properties can improve these processes1717. Anaeigoudari A, Shafei MN, Soukhtanloo M, Sadeghnia HR, Reisi P, Beheshti F et al. Lipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazole [eng.]. Arq Neuropsiquiatr. 2015;73(9):784-90. https://doi.org/10.1590/0004-282X20150121
https://doi.org/10.1590/0004-282X2015012...
. Previously, we demonstrated the role of brain tissue oxidative damage in learning and memory deficits in rats1515. Anaeigoudari A, Soukhtanloo M, Reisi P, Beheshti F, Hosseini M. Inducible nitric oxide inhibitor aminoguanidine, ameliorates deleterious effects of lipopolysaccharide on memory and long term potentiation in rat. Life Sci. 2016;158:22-30. https://doi.org/10.1016/j.lfs.2016.06.019
https://doi.org/10.1016/j.lfs.2016.06.01...
. Oxidative stress followed by PTU administration has also been suggested to have negative effects on learning and memory77. Beheshti F, Hosseini M, Shafei MN, Soukhtanloo M, Ghasemi S, Vafaee F et al. The effects of Nigella sativa extract on hypothyroidism-associated learning and memory impairment during neonatal and juvenile growth in rats. Nutr Neurosci. 2017;20(1):49-59. https://doi.org/10.1179/1476830514Y.0000000144
https://doi.org/10.1179/1476830514Y.0000...
. Based on the results of previous studies, PTU-induced memory deficits in neonatal and juvenile rats were also accompanied with an overproduction of NO metabolites in the hippocampus2323. Hosseini M, Headari R, Oryan S, Hadjzadeh MA, Saffarzadeh F, Khazaei M. The effect of chronic administration of L-arginine on the learning and memory of estradiol-treated ovariectomized rats tested in the morris water maze. Clinics (Sao Paulo). 2010;65(8):803-7. https://doi.org/10.1590/S1807-59322010000800011
https://doi.org/10.1590/S1807-5932201000...
. In the current study, PTU-related learning and memory impairments were also associated with oxidative stress in the brain tissue of rats. Enhanced levels of MDA and NO metabolites and reduced content of total thiol groups and activity of SOD and CAT in both hippocampal and cortical tissues in the Hypo group, relative to the control group, support this claim. Considering these findings, it seems that hypothyroidism-induced oxidative stress followed by PTU administration resulted in learning and memory impairments in juvenile rats in the present study.
The beneficial effects of Nigella sativa, and compounds derived from it, on learning and memory in animal and human studies2424. Bin Sayeed MS, Asaduzzaman M, Morshed H, Hossain MM, Kadir MF, Rahman MR. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol. 2013;148(3):780-6. https://doi.org/10.1016/j.jep.2013.05.004
https://doi.org/10.1016/j.jep.2013.05.00...
have been documented. In a study using an eight-arm radial arm maze, Nigella sativa oil was reported to improve learning and memory in rats2525. Sahak MKA, Mohamed AM, Hashim NH, Hasan Adli DS. Nigella sativa oil enhances the spatial working memory performance of rats on a radial arm maze. Evid Based Complement Alternat Med. 2013;2013:ID180598. https://doi.org/10.1155/2013/180598
https://doi.org/10.1155/2013/180598...
. It has also been found that Nigella sativa may improve spatial learning and memory impairments induced by global cerebrovascular hypoperfusion in rats2626. Azzubaidi MS, Saxena AK, Talib NA, Ahmed QU, Dogarai BB. Protective effect of treatment with black cumin oil on spatial cognitive functions of rats that suffered global cerebrovascular hypoperfusion. Acta Neurobiol Exp (Wars). 2012;72(2):154-65.. Additionally, Nigella sativa has been shown to have protective effects against learning and memory impairment caused by diabetes2727. Roghani M. The effect of Nigella sativa on learning and memory in male diabetic rats. Basic Clin Neurosci. 2009;1(1):32-4.. In the present study, pretreatment with TQ, an important component of Nigella sativa, also restored learning and memory deficits caused by PTU in rats in both the Morris water maze and passive avoidance tests.
The underlying mechanisms of the beneficial effects of TQ on learning and memory have not been understood precisely. Thymoquinone has been reported to possess antihypertensive, anti-inflammatory and antioxidant properties2828. Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299-305. https://doi.org/10.1002/ptr.1309
https://doi.org/10.1002/ptr.1309...
. The antioxidant effects of TQ have been shown in vivo and in vitro2929. Beheshti F, Karimi S, Vafaee F, Shafei MN, Sadeghnia HR, Hadjzadeh MA et al. The effects of vitamin C on hypothyroidism-associated learning and memory impairment in juvenile rats. Metab Brain Dis. 2017;32(3):703-15. https://doi.org/10.1007/s11011-017-9954-y
https://doi.org/10.1007/s11011-017-9954-...
. In recent studies, TQ has been suggested as a potent mitochondria-targeted antioxidant against neurodegenerative diseases. For example, it has been reported that TQ improves the rotenone-induced Parkinson’s disease symptoms via its antioxidant effect3030. Ebrahimi SS, Oryan S, Izadpanah E, Hassanzadeh K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol Lett. 2017;276:108-14. https://doi.org/10.1016/j.toxlet.2017.05.018
https://doi.org/10.1016/j.toxlet.2017.05...
. In addition, scientific findings show that TQ exerts a protective effect against pilocarpine-induced brain injury and it ameliorates brain damage through an antioxidant pathway in a status epilepticus rat model3131. Shao YY, Li B, Huang YM, Luo Q, Xie YM, Chen YH. Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model. Transl Neurosci. 2017;8(1):9-14. https://doi.org/10.1515/tnsci-2017-0003
https://doi.org/10.1515/tnsci-2017-0003...
. It has also been shown that TQ reduces lipid peroxidation in the hippocampal tissue of rats in a global cerebral ischemia-reperfusion injury model1212. Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14(9):621-7. https://doi.org/10.1016/j.phymed.2006.12.005
https://doi.org/10.1016/j.phymed.2006.12...
. In the present study, administration of TQ decreased the oxidative stress status, which was shown by decreased levels of MDA and NO metabolites and increased content of total thiol, and the enhanced activity of SOD and CAT in the brain tissue of hypothyroid rats. Considering these findings, the antioxidant effects of TQ may have contributed to improve the detrimental effects of PTU on learning and memory in our study.
The administration of PTU during juvenile and growth periods has been reported to have a noticeable effect on thyroid gland growth and reduction of serum T4 concentration in rats2929. Beheshti F, Karimi S, Vafaee F, Shafei MN, Sadeghnia HR, Hadjzadeh MA et al. The effects of vitamin C on hypothyroidism-associated learning and memory impairment in juvenile rats. Metab Brain Dis. 2017;32(3):703-15. https://doi.org/10.1007/s11011-017-9954-y
https://doi.org/10.1007/s11011-017-9954-...
,3232. Baghcheghi Y, Beheshti F, Salmani H, Soukhtanloo M, Hosseini M. Protective effect of PPARγ agonists on cerebellar tissues oxidative damage in hypothyroid rats. Neurology research international. 2016;2016:ID1952561. httpS://doi.org/10.1155/2016/1952561
httpS://doi.org/10.1155/2016/1952561...
. In line with these findings, in the current study, the administration of PTU significantly decreased the serum T4 level in the Hypo group compared to the control group. In addition, it has been proposed that antioxidant compounds can apply protective effects on the thyroid gland. For example, vitamin C, as a potent antioxidant, has been shown to protect the thyroid gland against toxic compounds such as PTU2929. Beheshti F, Karimi S, Vafaee F, Shafei MN, Sadeghnia HR, Hadjzadeh MA et al. The effects of vitamin C on hypothyroidism-associated learning and memory impairment in juvenile rats. Metab Brain Dis. 2017;32(3):703-15. https://doi.org/10.1007/s11011-017-9954-y
https://doi.org/10.1007/s11011-017-9954-...
. It has also been reported that antioxidant agents, including vitamin C, can improve the abnormalities in serum T4, T3 and thyroid stimulating hormone concentration in hypothyroid patients3333. Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab. 2014;99(6):E1031-4. https://doi.org/10.1210/jc.2013-4360
https://doi.org/10.1210/jc.2013-4360...
. In the present study, administration of TQ restored the PTU-induced abnormality in the thyroid gland, which was associated with the improvement in learning and memory in both the Morris water maze and passive avoidance tests in rats. In support of this data, the administration of TQ enhanced the serum level of T4 hormone in the Hypo-TQ5 and Hypo-TQ10 groups relative to the Hypo group. The antioxidant effects of TQ mentioned above, and the balancing effect of this compound via improvement of the oxidative status on thyroid hormones, may be one of the possible mechanisms that indirectly contribute to the improvement of learning and memory impairment caused by PTU in the present study. However, the exact mechanism needs to be investigated further in the future.
In summary, the results of the current study showed that treatment with TQ can reverse the negative effects of PTU-induced hypothyroidism on the learning and memory of juvenile rats. The antioxidant properties of TQ against brain oxidative damage may be involved in the improvement of learning and memory impairment caused by PTU.
References
-
1Martinez B, Hoyo P, Martin MA, Arenas J, Perez-Castillo A, Santos A. Thyroid hormone regulates oxidative phosphorylation in the cerebral cortex and striatum of neonatal rats. J Neurochem. 2001;78(5):1054-63. https://doi.org/10.1046/j.1471-4159.2001.00487.x
» https://doi.org/10.1046/j.1471-4159.2001.00487.x -
2Jackson IM. The thyroid axis and depression. Thyroid. 1998;8(10):951-6. https://doi.org/10.1089/thy.1998.8.951
» https://doi.org/10.1089/thy.1998.8.951 -
3Gong J, Liu W, Dong J, Wang Y, Xu H, Wei W et al. Developmental iodine deficiency and hypothyroidism impair neural development in rat hippocampus: involvement of doublecortin and NCAM-180. BMC Neurosci. 2010;11(1):50. https://doi.org/10.1186/1471-2202-11-50
» https://doi.org/10.1186/1471-2202-11-50 -
4Gong J, Liu W, Dong J, Wang Y, Xu H, Wei W et al. Developmental iodine deficiency and hypothyroidism impair neural development in rat hippocampus: involvement of doublecortin and NCAM-180. BMC Neurosci. 2010;11(1):50.
-
5Alva-Sánchez C, Sánchez-Huerta K, Arroyo-Helguera O, Anguiano B, Aceves C, Pacheco-Rosado J. The maintenance of hippocampal pyramidal neuron populations is dependent on the modulation of specific cell cycle regulators by thyroid hormones. Brain Res. 2009;1271:27-35. https://doi.org/10.1016/j.brainres.2009.02.043
» https://doi.org/10.1016/j.brainres.2009.02.043 -
6Anaeigoudari A, Soukhtanloo M, Shafei MN, Sadeghnia HR, Reisi P, Beheshti F et al. Neuronal nitric oxide synthase has a role in the detrimental effects of lipopolysaccharide on spatial memory and synaptic plasticity in rats. Pharmacol Rep. 2016;68(2):243-9. https://doi.org/10.1016/j.pharep.2015.09.004
» https://doi.org/10.1016/j.pharep.2015.09.004 -
7Beheshti F, Hosseini M, Shafei MN, Soukhtanloo M, Ghasemi S, Vafaee F et al. The effects of Nigella sativa extract on hypothyroidism-associated learning and memory impairment during neonatal and juvenile growth in rats. Nutr Neurosci. 2017;20(1):49-59. https://doi.org/10.1179/1476830514Y.0000000144
» https://doi.org/10.1179/1476830514Y.0000000144 -
8Baghcheghi Y, Salmani H, Beheshti F, Hosseini M. Contribution of Brain Tissue Oxidative Damage in Hypothyroidism-associated Learning and Memory Impairments. Adv Biomed Res. 2017;6(1):59. https://doi.org/10.4103/2277-9175.206699
» https://doi.org/10.4103/2277-9175.206699 -
9Rahaman SO, Ghosh S, Mohanakumar KP, Das S, Sarkar PK. Hypothyroidism in the developing rat brain is associated with marked oxidative stress and aberrant intraneuronal accumulation of neurofilaments. Neurosci Res. 2001;40(3):273-9. https://doi.org/10.1016/S0168-0102(01)00237-1
» https://doi.org/10.1016/S0168-0102(01)00237-1 -
10Hosseini M, Dastghaib SS, Rafatpanah H, Hadjzadeh MA, Nahrevanian H, Farrokhi I. Nitric oxide contributes to learning and memory deficits observed in hypothyroid rats during neonatal and juvenile growth. Clinics (Sao Paulo). 2010;65(11):1175-81. https://doi.org/10.1590/S1807-59322010001100021
» https://doi.org/10.1590/S1807-59322010001100021 -
11Abdel-Zaher AO, Mostafa MG, Farghly HM, Hamdy MM, Omran GA, Al-Shaibani NK. Inhibition of brain oxidative stress and inducible nitric oxide synthase expression by thymoquinone attenuates the development of morphine tolerance and dependence in mice. Eur J Pharmacol. 2013;702(1-3):62-70. https://doi.org/10.1016/j.ejphar.2013.01.036
» https://doi.org/10.1016/j.ejphar.2013.01.036 -
12Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14(9):621-7. https://doi.org/10.1016/j.phymed.2006.12.005
» https://doi.org/10.1016/j.phymed.2006.12.005 -
13El-Mahmoudy A, Matsuyama H, Borgan MA, Shimizu Y, El-Sayed MG, Minamoto N et al. Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophages. Int Immunopharmacol. 2002;2(11):1603-11. https://doi.org/10.1016/S1567-5769(02)00139-X
» https://doi.org/10.1016/S1567-5769(02)00139-X -
14Gilhotra N, Dhingra D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol Rep. 2011;63(3):660-9. https://doi.org/10.1016/S1734-1140(11)70577-1
» https://doi.org/10.1016/S1734-1140(11)70577-1 -
15Anaeigoudari A, Soukhtanloo M, Reisi P, Beheshti F, Hosseini M. Inducible nitric oxide inhibitor aminoguanidine, ameliorates deleterious effects of lipopolysaccharide on memory and long term potentiation in rat. Life Sci. 2016;158:22-30. https://doi.org/10.1016/j.lfs.2016.06.019
» https://doi.org/10.1016/j.lfs.2016.06.019 -
16Abareshi A, Hosseini M, Beheshti F, Norouzi F, Khazaei M, Sadeghnia HR et al. The effects of captopril on lipopolysaccharide induced learning and memory impairments and the brain cytokine levels and oxidative damage in rats. Life Sci. 2016;167:46-56. https://doi.org/10.1016/j.lfs.2016.10.026
» https://doi.org/10.1016/j.lfs.2016.10.026 -
17Anaeigoudari A, Shafei MN, Soukhtanloo M, Sadeghnia HR, Reisi P, Beheshti F et al. Lipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazole [eng.]. Arq Neuropsiquiatr. 2015;73(9):784-90. https://doi.org/10.1590/0004-282X20150121
» https://doi.org/10.1590/0004-282X20150121 -
18Farrokhi E, Hosseini M, Beheshti F, Vafaee F, Hadjzadeh MA, Dastgheib SS. Brain tissues oxidative damage as a possible mechanism of deleterious effects of propylthiouracil- induced hypothyroidism on learning and memory in neonatal and juvenile growth in rats. Basic Clin Neurosci. 2014;5(4):285-94.
-
19Alzoubi KH, Aleisa AM, Gerges NZ, Alkadhi KA. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: behavioral and electrophysiological studies. J Neurosci Res. 2006;84(5):944-53. https://doi.org/10.1002/jnr.21014
» https://doi.org/10.1002/jnr.21014 -
20Barradas PC, Ferraz AS, Ferreira AA, Daumas RP, Moura EG. 2‘3’Cyclic nucleotide 3’phosphodiesterase immunohistochemistry shows an impairment on myelin compaction in hypothyroid rats. Int J Dev Neurosci. 2000;18(8):887-92. https://doi.org/10.1016/S0736-5748(00)00028-9
» https://doi.org/10.1016/S0736-5748(00)00028-9 -
21Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19(1):66-78. https://doi.org/10.1002/hipo.20476
» https://doi.org/10.1002/hipo.20476 -
22Jones DP, Carlson JL, Mody VC Jr, Cai J, Lynn MJ, Sternberg P Jr. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28(4):625-35. https://doi.org/10.1016/S0891-5849(99)00275-0
» https://doi.org/10.1016/S0891-5849(99)00275-0 -
23Hosseini M, Headari R, Oryan S, Hadjzadeh MA, Saffarzadeh F, Khazaei M. The effect of chronic administration of L-arginine on the learning and memory of estradiol-treated ovariectomized rats tested in the morris water maze. Clinics (Sao Paulo). 2010;65(8):803-7. https://doi.org/10.1590/S1807-59322010000800011
» https://doi.org/10.1590/S1807-59322010000800011 -
24Bin Sayeed MS, Asaduzzaman M, Morshed H, Hossain MM, Kadir MF, Rahman MR. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol. 2013;148(3):780-6. https://doi.org/10.1016/j.jep.2013.05.004
» https://doi.org/10.1016/j.jep.2013.05.004 -
25Sahak MKA, Mohamed AM, Hashim NH, Hasan Adli DS. Nigella sativa oil enhances the spatial working memory performance of rats on a radial arm maze. Evid Based Complement Alternat Med. 2013;2013:ID180598. https://doi.org/10.1155/2013/180598
» https://doi.org/10.1155/2013/180598 -
26Azzubaidi MS, Saxena AK, Talib NA, Ahmed QU, Dogarai BB. Protective effect of treatment with black cumin oil on spatial cognitive functions of rats that suffered global cerebrovascular hypoperfusion. Acta Neurobiol Exp (Wars). 2012;72(2):154-65.
-
27Roghani M. The effect of Nigella sativa on learning and memory in male diabetic rats. Basic Clin Neurosci. 2009;1(1):32-4.
-
28Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299-305. https://doi.org/10.1002/ptr.1309
» https://doi.org/10.1002/ptr.1309 -
29Beheshti F, Karimi S, Vafaee F, Shafei MN, Sadeghnia HR, Hadjzadeh MA et al. The effects of vitamin C on hypothyroidism-associated learning and memory impairment in juvenile rats. Metab Brain Dis. 2017;32(3):703-15. https://doi.org/10.1007/s11011-017-9954-y
» https://doi.org/10.1007/s11011-017-9954-y -
30Ebrahimi SS, Oryan S, Izadpanah E, Hassanzadeh K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol Lett. 2017;276:108-14. https://doi.org/10.1016/j.toxlet.2017.05.018
» https://doi.org/10.1016/j.toxlet.2017.05.018 -
31Shao YY, Li B, Huang YM, Luo Q, Xie YM, Chen YH. Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model. Transl Neurosci. 2017;8(1):9-14. https://doi.org/10.1515/tnsci-2017-0003
» https://doi.org/10.1515/tnsci-2017-0003 -
32Baghcheghi Y, Beheshti F, Salmani H, Soukhtanloo M, Hosseini M. Protective effect of PPARγ agonists on cerebellar tissues oxidative damage in hypothyroid rats. Neurology research international. 2016;2016:ID1952561. httpS://doi.org/10.1155/2016/1952561
» httpS://doi.org/10.1155/2016/1952561 -
33Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metab. 2014;99(6):E1031-4. https://doi.org/10.1210/jc.2013-4360
» https://doi.org/10.1210/jc.2013-4360
-
Support: Jiroft University of Medical Sciences.
Publication Dates
-
Publication in this collection
Jan 2018
History
-
Received
06 June 2017 -
Reviewed
11 Aug 2017 -
Accepted
29 Sept 2017