ABSTRACT
Multiple sclerosis (MS) is a complex autoimmune and neurodegenerative disease of the central nervous system. Since MS affects mostly fertile women, pregnancy issues often arise in daily practice. The present study assessed the use of postpartum intravenous immunoglobulin (IVIG) in MS.
Methods
The authors individually searched for records using PubMed, Medline, EMBASE, Cochrane, SciELO, LILACS, and Google Scholar, using the terms “multiple sclerosis” OR “MS” AND “pregnancy” OR “gestation” OR “partum” OR “post-partum” OR “puerperium” AND “immunoglobulin”.
Results
The initial search returned 321 papers. There were 11 eligible articles selected for the review. In total, 380 patients had received post-natal IVIG to reduce the number of postpartum relapses. The unadjusted number needed to treat was 6.3 for the quantitative and 5.8 for the qualitative analyses.
Conclusion
The therapeutic effect of IVIG for prevention of postnatal relapses in MS could not clearly be established in this meta-analysis.
multiple sclerosis; pregnancy; immunoglobulins; Neurology; systematic review; meta-analysis
RESUMO
Esclerose múltipla (EM) é uma complexa doença autoimune e neurodegenerativa do sistema nervoso central. Uma vez que EM afeta principalmente mulheres em idade fértil, assuntos relacionados à gravidez frequentemente surgem na prática diária. O presente estudo avaliou o uso pós-parto de imunoglobulina (IVIG) na EM.
Métodos
Os autores individualmente pesquisaram as bases de dados PubMed, Medline, EMBASE, Cochrane, SciELO, LILACS, e Google Scholar usando os termos “multiple sclerosis” OR “MS” AND “pregnancy” OR “gestation” OR “partum” OR “post-partum” OR “puerperium” AND “immunoglobulin”.
Resultados
A pesquisa inicial retornou 321 artigos. Havia 11 artigos elegíveis para a revisão. No total, havia relato de 380 pacientes que receberam IVIG após a gravidez visando reduzir o número de surtos. O número necessário para tratar não ajustado foi 6,3 para análise quantitativa e 5,8 para análise qualitativa.
Conclusão
O efeito terapêutico da IVIG para prevenção dos surtos pós-parto na EM não pôde ser claramente estabelecida nesta meta-análise.
esclerose múltipla; gestação; imunoglobulinas; Neurologia; revisão sistemática; meta-análise
Multiple sclerosis (MS) is a complex autoimmune and neurodegenerative disease affecting the central nervous system. The disease is more prevalent in young adults, predominantly in females, and can manifest with a variety of transient neurological signs and symptoms11. Huang WJ, Chen WW, Zhang X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp Ther Med. 2017 Jun;13(6):3163-6. https://doi.org/10.3892/etm.2017.4410
https://doi.org/10.3892/etm.2017.4410...
. Manifestations of MS reflect areas of demyelination in the central nervous system and frequently include unilateral vision loss, double vision, limb weakness, sensory abnormalities, incoordination, unsteadiness of gait, and/or sphincter dysfunction. Most often, the disease starts in a relapsing-remitting form and progresses to a neurodegenerative phase in which neurological disability accumulates without remission22. Jenkins TM, Thompson AJ. Diagnosing and managing multiple sclerosis. Practitioner. 2009 Sep;253(1721):25-30.. Since MS affects mostly women of reproductive age, it is expected that pregnancy issues will arise in daily practice33. Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015 Mar;29(3):207-20. https://doi.org/10.1007/s40263-015-0238-y
https://doi.org/10.1007/s40263-015-0238-...
. One of the main concerns regarding pregnancy in women with MS is the increased risk of a demyelinating relapse after delivery.
There is no specific recommendation to decrease this risk of relapses in the puerperium, but some groups advocate the use of intravenous human immunoglobulin (IVIG) as a prophylactic treatment for these patients. Although this treatment seems safe, conflicting results regarding its efficacy have been published. Furthermore, it is an expensive therapy costing in excess of US$15,000 per treated patient. The present study is a systematic review and meta-analysis on the efficacy of IVIG for prevention of postnatal relapses in women with MS.
METHODS
There was no review of confidential health information and the study was not interventional. Therefore, ethics committee approval was not required.
The authors individually searched for records using PubMed, Medline, EMBASE, Cochrane, SciELO, LILACS, and Google Scholar. The terms “multiple sclerosis” OR “MS” AND “pregnancy” OR “gestation” OR “partum” OR “post-partum” OR “puerperium” AND “immunoglobulin” were used in the search. References from selected papers were also searched for further papers of potential interest. Once eligible for full-text appraisal, articles were included in the qualitative review if they complied with the following inclusion criteria: a) contained data on immunoglobulin treatment and postpartum MS relapse; b) published after 1983, when Poser’s criteria for MS were established44. Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227-31. https://doi.org/10.1002/ana.410130302
https://doi.org/10.1002/ana.410130302...
; c) was a case series, observational or experimental study. Articles were excluded if they: a) were congress reports, banners or abstracts; b) were preclinical studies; c) did not present data on patients. If the study contained data comparing patients with controls (i.e. patients who did not receive immunoglobulin treatment), the study was included in the meta-analysis. It followed the strict Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines55. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010 Sep;25(9):603-5. https://doi.org/10.1007/s10654-010-9491-z
https://doi.org/10.1007/s10654-010-9491-...
, and the meta-analysis was performed using the Cochrane RevMan 5.
Risk of bias was assessed using the Newcastle-Ottawa checklist for case-control, cohort and cross-sectional studies66. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. https://doi.org/10.1016/j.jclinepi.2009.06.005
https://doi.org/10.1016/j.jclinepi.2009....
. The Newcastle-Ottawa scale assesses the risk of bias of nonrandomized studies in meta-analyses and is recommended by the Cochrane Collaboration77. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011 [access 2017 July 10 2017]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
http://www.ohri.ca/programs/clinical_epi...
. It ranges from zero for the highest risk to nine points (stars) for the lowest risk of bias in three dimensions: a) selection (4 points); b) comparability (2 points); and c) exposure or outcome (3 points) for case-control, cohort or cross-sectional studies. Scores of less than 7 were considered to indicate the possibility of high risk of bias77. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011 [access 2017 July 10 2017]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
http://www.ohri.ca/programs/clinical_epi...
. For randomized clinical trials, the Cochrane Collaboration tool for assessing the risk of bias was used. The following elements were reviewed: a) random sequence generation; b) allocation concealment; c) blinding of participants and personnel; d) blinding of outcome assessment; e) incomplete outcome data; and f) selective reporting. Studies were judged as presenting low, unclear or high risk, as set out by Higgins and Green88. Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 London: The Cochrane Collaboration; 2011 [access 2017 December 10]. Available from: http://handbook-5-1.cochrane.org/
http://handbook-5-1.cochrane.org/...
. Similarly, case series were assessed in accordance with the prescriptions of the National Institute of Health99. U.S. Department of Health & Human Services. National Heart, Lung and Blood Institute. Study quality assessment tools: quality assessment tool for case series studies. Bethesda: National Heart, Lung and Blood Institute; [s.d.] [accessed 2017 July 10]. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series
https://www.nhlbi.nih.gov/health-pro/gui...
. These scales were used in conjunction with the qualitative review to holistically review the strength of each study.
Data were dichotomized as patients who received immunoglobulin treatment versus those who did not; and as patients who relapsed in the first six months postpartum vs. those who did not. Data were converted into odds ratios and, in the expectation of a small sample size, the Mantel-Haenszel procedure was used to weight the data. A fixed-effect model was selected based on the Cochran Q measure of heterogeneity. From the odds ratio, the unadjusted number needed to treat (NNT) was also calculated. Tau22. Jenkins TM, Thompson AJ. Diagnosing and managing multiple sclerosis. Practitioner. 2009 Sep;253(1721):25-30. and I2were used to further evaluate heterogeneity. Data were then collated into summary tables and forest plots to assess the individual study characteristics and potential therapeutic effects of the intervention.
RESULTS
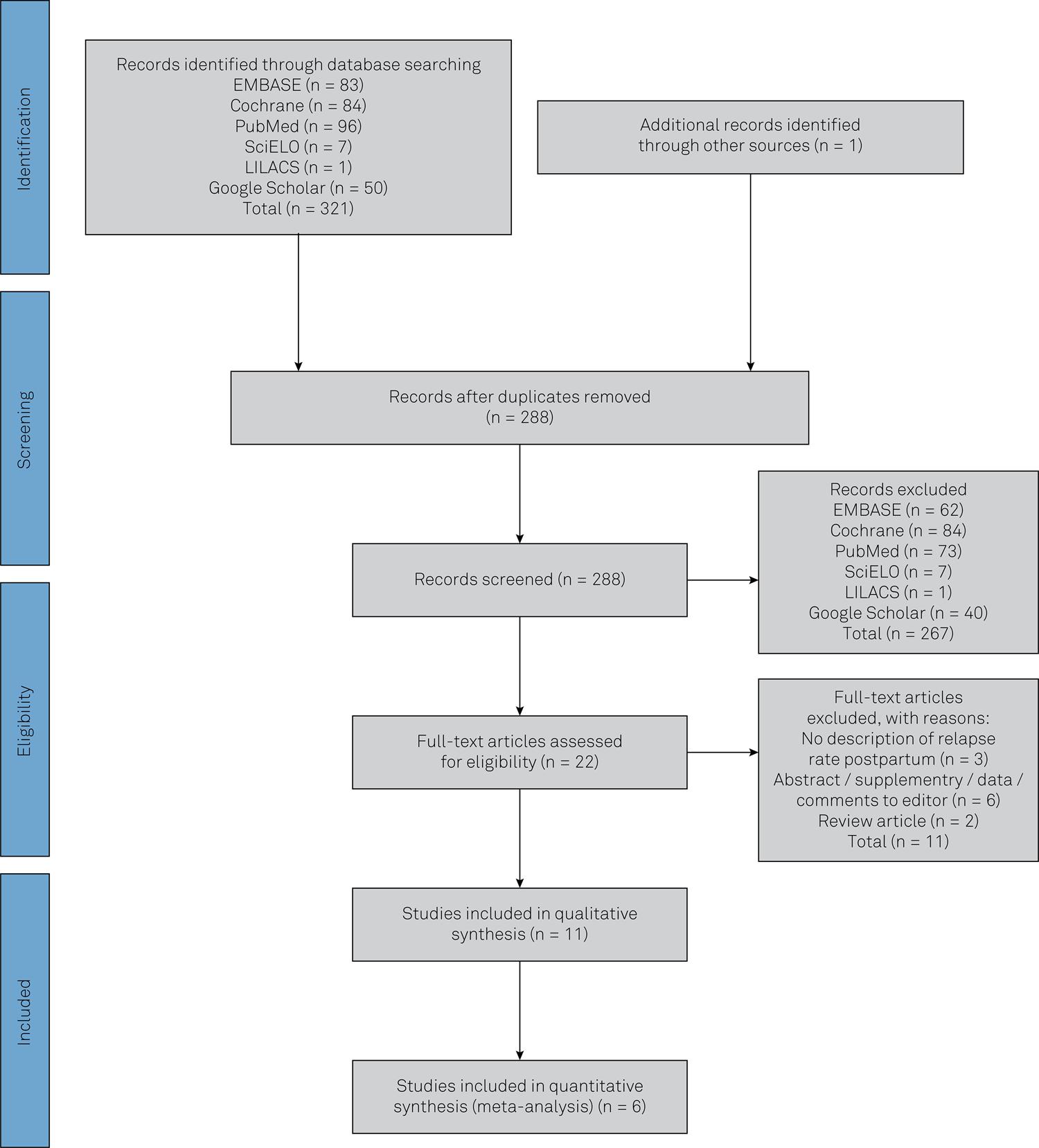
The results from the search strategy, screening and review of eligibility are summarized in Figure 1. The initial search returned 321 papers. After removal of duplicates, 288 papers were selected for abstract review. There were 22 eligible articles, but 11 did not fulfill the criterion of using original data from patients. Therefore, the systematic review included 11 articles for assessment1010. Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
https://doi.org/10.1007/BF00878527...
,1111. Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004 Sep;251(9):1133-7. https://doi.org/10.1007/s00415-004-0495-z
https://doi.org/10.1007/s00415-004-0495-...
,1313. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007 Aug;13(7):900-8. https://doi.org/10.1177/1352458506075654
https://doi.org/10.1177/1352458506075654...
,1414. Orvieto R, Achiron R, Rotstein Z, Noy S, Bar-Hava I, Achiron A. Pregnancy and multiple sclerosis: a 2-year experience. Eur J Obstet Gynecol Reprod Biol. 1999 Feb;82(2):191-4. https://doi.org/10.1016/S0301-2115(98)00231-0
https://doi.org/10.1016/S0301-2115(98)00...
,1515. Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk S, Schwödiauer V et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008 Jul;118(1):24-8. https://doi.org/10.1111/j.1600-0404.2007.00978.x
https://doi.org/10.1111/j.1600-0404.2007...
,1616. Hellwig K, Beste C, Schimrigk S, Chan A. Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disorder. 2009 Jan;2(1):7-11. https://doi.org/10.1177/1756285608100416
https://doi.org/10.1177/1756285608100416...
,1717. Durelli L, Ricci A, Verdun E. Immunoglobulin treatment of multiple sclerosis: future prospects. Neurol Sci. 2003 Oct;24(0 Suppl 4):S234-8. https://doi.org/10.1007/s10072-003-0085-3
https://doi.org/10.1007/s10072-003-0085-...
,1818. Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000.
https://doi.org/10.2165/11538960-0000000...
,1919. Fragoso YD, Adoni T, Alves-Leon SV, Azambuja ND Jr, Barreira AA, Brooks JB et al. Postpartum treatment with immunoglobulin does not prevent relapses of multiple sclerosis in the mother. Health Care Women Int. 2015;36(10):1072-80. https://doi.org/10.1080/07399332.2014.948627
https://doi.org/10.1080/07399332.2014.94...
,2020. Vukusic S, Durand-Dubief F, Benoit A, Marignier R, Frangoulis B, Confavreux C. Natalizumab for the prevention of post-partum relapses in women with multiple sclerosis. Mult Scler. 2015 Jun;21(7):953-5. https://doi.org/10.1177/1352458514554056
https://doi.org/10.1177/1352458514554056...
,2121. Brandt-Wouters E, Gerlach OH, Hupperts RM. The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet. 2016 Aug;134(2):194-6. https://doi.org/10.1016/j.ijgo.2016.01.008
https://doi.org/10.1016/j.ijgo.2016.01.0...
, although only in six of them was it possible to compare the effect of the drug on the outcome1010. Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
https://doi.org/10.1007/BF00878527...
,1111. Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004 Sep;251(9):1133-7. https://doi.org/10.1007/s00415-004-0495-z
https://doi.org/10.1007/s00415-004-0495-...
,11. Huang WJ, Chen WW, Zhang X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp Ther Med. 2017 Jun;13(6):3163-6. https://doi.org/10.3892/etm.2017.4410
https://doi.org/10.3892/etm.2017.4410...
44. Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227-31. https://doi.org/10.1002/ana.410130302
https://doi.org/10.1002/ana.410130302...
,1515. Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk S, Schwödiauer V et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008 Jul;118(1):24-8. https://doi.org/10.1111/j.1600-0404.2007.00978.x
https://doi.org/10.1111/j.1600-0404.2007...
,1616. Hellwig K, Beste C, Schimrigk S, Chan A. Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disorder. 2009 Jan;2(1):7-11. https://doi.org/10.1177/1756285608100416
https://doi.org/10.1177/1756285608100416...
,1818. Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000.
https://doi.org/10.2165/11538960-0000000...
. For the remaining five, data were analyzed and presented using qualitative synthesis1313. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007 Aug;13(7):900-8. https://doi.org/10.1177/1352458506075654
https://doi.org/10.1177/1352458506075654...
,1717. Durelli L, Ricci A, Verdun E. Immunoglobulin treatment of multiple sclerosis: future prospects. Neurol Sci. 2003 Oct;24(0 Suppl 4):S234-8. https://doi.org/10.1007/s10072-003-0085-3
https://doi.org/10.1007/s10072-003-0085-...
,1818. Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000.
https://doi.org/10.2165/11538960-0000000...
,1919. Fragoso YD, Adoni T, Alves-Leon SV, Azambuja ND Jr, Barreira AA, Brooks JB et al. Postpartum treatment with immunoglobulin does not prevent relapses of multiple sclerosis in the mother. Health Care Women Int. 2015;36(10):1072-80. https://doi.org/10.1080/07399332.2014.948627
https://doi.org/10.1080/07399332.2014.94...
,2121. Brandt-Wouters E, Gerlach OH, Hupperts RM. The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet. 2016 Aug;134(2):194-6. https://doi.org/10.1016/j.ijgo.2016.01.008
https://doi.org/10.1016/j.ijgo.2016.01.0...
.
Flowchart of study selection for the systematic review and meta-analyses following the strict Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol55. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010 Sep;25(9):603-5. https://doi.org/10.1007/s10654-010-9491-z
https://doi.org/10.1007/s10654-010-9491-... .
The 11 articles did not have uniform methodology and some used historical controls, including one from 20162121. Brandt-Wouters E, Gerlach OH, Hupperts RM. The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet. 2016 Aug;134(2):194-6. https://doi.org/10.1016/j.ijgo.2016.01.008
https://doi.org/10.1016/j.ijgo.2016.01.0...
with over 30-year-old data as the historical control group2222. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998 Jul;339(5):285-91. https://doi.org/10.1056/NEJM199807303390501
https://doi.org/10.1056/NEJM199807303390...
. Inclusion of patients exposed to other potential risk-protection factors also varied among the studies, thus making interpretation of the results particularly difficult. Dose and therapeutic scheme were not uniform among the studies. Follow-up periods also varied among studies and were typically six, nine or 12 months.
In total, there were 380 patients who received immunoglobulin after pregnancy to reduce the number of postpartum relapses. The mean age ± standard deviation of these patients was 29.5 ± 4.5 years and the mean MS duration was 5.0 ± 3.3 at the time of pregnancy. Disability was assessed using the Expanded Disability Scale Score2323. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444-52. https://doi.org/10.1212/WNL.33.11.1444
https://doi.org/10.1212/WNL.33.11.1444...
, which ranges from 0 (normal neurological examination) to 10 (death due to MS) with increments of 0.5 points. The mean Expanded Disability Scale Score at the beginning of pregnancy, reported by seven studies, was 2.3 ± 1.1. Clinical and demographic data did not differ substantially across the qualitative review. The studies were conducted in various locations: one multinational study in nine European countries1313. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007 Aug;13(7):900-8. https://doi.org/10.1177/1352458506075654
https://doi.org/10.1177/1352458506075654...
, three each in Israel1010. Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
https://doi.org/10.1007/BF00878527...
,1111. Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004 Sep;251(9):1133-7. https://doi.org/10.1007/s00415-004-0495-z
https://doi.org/10.1007/s00415-004-0495-...
,1414. Orvieto R, Achiron R, Rotstein Z, Noy S, Bar-Hava I, Achiron A. Pregnancy and multiple sclerosis: a 2-year experience. Eur J Obstet Gynecol Reprod Biol. 1999 Feb;82(2):191-4. https://doi.org/10.1016/S0301-2115(98)00231-0
https://doi.org/10.1016/S0301-2115(98)00...
and Germany1212. Haas J. High dose IVIG in the post-partum period for prevention of exacerbations in MS. Mult Scler. 2000 Oct;6 Suppl 2:S18-20.,1515. Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk S, Schwödiauer V et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008 Jul;118(1):24-8. https://doi.org/10.1111/j.1600-0404.2007.00978.x
https://doi.org/10.1111/j.1600-0404.2007...
,11. Huang WJ, Chen WW, Zhang X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp Ther Med. 2017 Jun;13(6):3163-6. https://doi.org/10.3892/etm.2017.4410
https://doi.org/10.3892/etm.2017.4410...
66. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. https://doi.org/10.1016/j.jclinepi.2009.06.005
https://doi.org/10.1016/j.jclinepi.2009....
, two in Brazil1818. Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000.
https://doi.org/10.2165/11538960-0000000...
,1919. Fragoso YD, Adoni T, Alves-Leon SV, Azambuja ND Jr, Barreira AA, Brooks JB et al. Postpartum treatment with immunoglobulin does not prevent relapses of multiple sclerosis in the mother. Health Care Women Int. 2015;36(10):1072-80. https://doi.org/10.1080/07399332.2014.948627
https://doi.org/10.1080/07399332.2014.94...
, and one each in France2020. Vukusic S, Durand-Dubief F, Benoit A, Marignier R, Frangoulis B, Confavreux C. Natalizumab for the prevention of post-partum relapses in women with multiple sclerosis. Mult Scler. 2015 Jun;21(7):953-5. https://doi.org/10.1177/1352458514554056
https://doi.org/10.1177/1352458514554056...
, the Netherlands2121. Brandt-Wouters E, Gerlach OH, Hupperts RM. The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet. 2016 Aug;134(2):194-6. https://doi.org/10.1016/j.ijgo.2016.01.008
https://doi.org/10.1016/j.ijgo.2016.01.0...
and Italy1717. Durelli L, Ricci A, Verdun E. Immunoglobulin treatment of multiple sclerosis: future prospects. Neurol Sci. 2003 Oct;24(0 Suppl 4):S234-8. https://doi.org/10.1007/s10072-003-0085-3
https://doi.org/10.1007/s10072-003-0085-...
. After further analyses of the articles, the French case series was excluded for the lack of specific data on IVIG2020. Vukusic S, Durand-Dubief F, Benoit A, Marignier R, Frangoulis B, Confavreux C. Natalizumab for the prevention of post-partum relapses in women with multiple sclerosis. Mult Scler. 2015 Jun;21(7):953-5. https://doi.org/10.1177/1352458514554056
https://doi.org/10.1177/1352458514554056...
.
Qualitative results
In 1996, Achiron et al.1010. Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
https://doi.org/10.1007/BF00878527...
published a pilot cohort study assessing the effects of IVIG at 0.4g/per day for five consecutive days during the first week after gestation and then with follow-up administration at six and 12 weeks. Three patients received this dosing regimen and none of them relapsed during the first six months after gestation, while 12 non-IVIG treated pregnancies relapsed over this period. It was concluded that postpartum IVIG treatment might be a useful therapeutic strategy for preventing acute childbirth-associated exacerbations in MS patients. In a subsequent retrospective cohort study on 108 patients, the same authors reviewed three separate groups: group I received no treatment; group II received IVIG 0.4 g/kg/day for five consecutive days in the first week post-gestation, with additional booster doses at six and 12 weeks; and group III received the same therapeutic scheme, but during the entire gestation and postpartum period1111. Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004 Sep;251(9):1133-7. https://doi.org/10.1007/s00415-004-0495-z
https://doi.org/10.1007/s00415-004-0495-...
. This study demonstrated a noteworthy therapeutic effect from IVIG treatment for reduction of the relapse rate among MS patients during pregnancy and the postpartum period, relative to the non-treated group. No significant side effects were observed in both studies, either for patients or for newborns, during or after pregnancy. Both studies from Achiron et al. were classified as being of high quality.
A discussion by Haas1212. Haas J. High dose IVIG in the post-partum period for prevention of exacerbations in MS. Mult Scler. 2000 Oct;6 Suppl 2:S18-20. reinforced the information provided by Achiron et al.’s first study and proposed a large European cohort of research on the potential role of IVIG in the prevention of postnatal exacerbations in MS. This cohort is discussed in the qualitative analyses below1313. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007 Aug;13(7):900-8. https://doi.org/10.1177/1352458506075654
https://doi.org/10.1177/1352458506075654...
.
In the cohort of Orvieto et al.1414. Orvieto R, Achiron R, Rotstein Z, Noy S, Bar-Hava I, Achiron A. Pregnancy and multiple sclerosis: a 2-year experience. Eur J Obstet Gynecol Reprod Biol. 1999 Feb;82(2):191-4. https://doi.org/10.1016/S0301-2115(98)00231-0
https://doi.org/10.1016/S0301-2115(98)00...
, 14 patients received the same therapeutic scheme as described in the Achiron et al. pilot study1010. Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
https://doi.org/10.1007/BF00878527...
. None of the 14 patients experienced childbirth-associated exacerbations during the first six months after delivery. No significant adverse events were reported. However, the comparability of the non-treated cohort to the treated cohort was uncertain and the study’s conclusions were considered to be of low quality.
In a study by Hellwig et al.1515. Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk S, Schwödiauer V et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008 Jul;118(1):24-8. https://doi.org/10.1111/j.1600-0404.2007.00978.x
https://doi.org/10.1111/j.1600-0404.2007...
, a survey was used to retrospectively screen 73 MS patients (corresponding to 88 pregnancies). From this sample, 14 patients received postnatal IVIG, although the treatment regimen was not described. Five of the patients had a relapse in the initial six months following delivery, while in their entire sample (including the five IVIG patients above) there were a total of 44 exacerbations over the six months postpartum. No further information was provided on the patients who received IVIG. This study’s recommendation for using IVIG was considered to be of low strength.
In a later study (combined cohort and case-control), Hellwig et al.1616. Hellwig K, Beste C, Schimrigk S, Chan A. Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disorder. 2009 Jan;2(1):7-11. https://doi.org/10.1177/1756285608100416
https://doi.org/10.1177/1756285608100416...
studied the effects of IVIG, no treatment and conventional disease-modifying therapy in 124 pregnancies. Respectively, group I received one of three different treatment regimens (i.e. 10 g IVIG three days following delivery, 10 g IVIG every four weeks for six months after delivery or 20 g IVIG every four weeks after birth); group II did not receive treatment postpartum; group III received conventional disease-modifying treatment two weeks after birth (interferon beta or glatiramer acetate). The use of IVIG was not shown to be significantly superior to the other alternatives. The study’s risk of bias was considered to be low.
Durelli et al.1717. Durelli L, Ricci A, Verdun E. Immunoglobulin treatment of multiple sclerosis: future prospects. Neurol Sci. 2003 Oct;24(0 Suppl 4):S234-8. https://doi.org/10.1007/s10072-003-0085-3
https://doi.org/10.1007/s10072-003-0085-...
reported on a study of six patients treated with IVIG following birth. The dosing regimen was not explicitly stated and the control group was historical2222. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998 Jul;339(5):285-91. https://doi.org/10.1056/NEJM199807303390501
https://doi.org/10.1056/NEJM199807303390...
. This study’s conclusions were considered to be of low strength.
In a case series by Fragoso et al.1818. Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000.
https://doi.org/10.2165/11538960-0000000...
, 11 patients who received glatiramer acetate throughout pregnancy were described. In this sample, four patients received IVIG post-delivery. Dosing regimens were not specified. Two of the patients receiving postpartum IVIG had a relapse within six months. The risk of bias for the study was low, but any conclusions were limited due to the sample size and study design. In a later case-control study by Fragoso et al.1919. Fragoso YD, Adoni T, Alves-Leon SV, Azambuja ND Jr, Barreira AA, Brooks JB et al. Postpartum treatment with immunoglobulin does not prevent relapses of multiple sclerosis in the mother. Health Care Women Int. 2015;36(10):1072-80. https://doi.org/10.1080/07399332.2014.948627
https://doi.org/10.1080/07399332.2014.94...
, 87 puerperal patients with MS received IVIG and their relapse rate was compared with the rate among another 47 untreated women. Dosing regimens were not described. In the postpartum follow-up, there were no differences in relapse rates between the two groups. The risk of bias for this study was considered to be low.
Vukusic et al.2020. Vukusic S, Durand-Dubief F, Benoit A, Marignier R, Frangoulis B, Confavreux C. Natalizumab for the prevention of post-partum relapses in women with multiple sclerosis. Mult Scler. 2015 Jun;21(7):953-5. https://doi.org/10.1177/1352458514554056
https://doi.org/10.1177/1352458514554056...
reported on a small case series of retrospective data. They described six patients treated with natalizumab within the first month postpartum. The authors commented on IVIG as an inefficient alternative for controlling postnatal relapses but do not present their data on this therapy. The conclusions from the study were considered to be of low strength and the article was excluded from the analyses.
The dose comparison study of IVIG in postpartum MS (the GAMPP study) was a multinational, multicenter, randomized double-blind clinical trial in which two groups of different IVIG doses were compared in 173 pregnancies1313. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007 Aug;13(7):900-8. https://doi.org/10.1177/1352458506075654
https://doi.org/10.1177/1352458506075654...
. Group I received 150 mg/kg IVIG on day one and then placebo infusions on day two and day three postpartum, while group II received 450, 300 and 150 mg/kg on days one, two and three respectively. After this regimen, both groups received 150 mg/kg five times at four-week intervals. In the first three months postpartum, there were no significant differences in relapse rates between the two groups. The risk of bias for the study was considered to be low. The lack of a “no treatment control group” limited the strength of the conclusions.
Finally, Brand-Wouters et al.2121. Brandt-Wouters E, Gerlach OH, Hupperts RM. The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet. 2016 Aug;134(2):194-6. https://doi.org/10.1016/j.ijgo.2016.01.008
https://doi.org/10.1016/j.ijgo.2016.01.0...
retrospectively studied puerperal patients who received 10g IVIG for three consecutive days followed by monthly IVIG doses for five months. In total, 42 pregnancies were evaluated and compared with the PRISMS study2222. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998 Jul;339(5):285-91. https://doi.org/10.1056/NEJM199807303390501
https://doi.org/10.1056/NEJM199807303390...
. However, the historical PRISMS cohort was over three decades old, which limited the strength of conclusions from this study. The authors showed that the relapse rate in the first three to nine months postpartum was low. This series was considered to present a low risk of bias, but there was no concomitant control group for this series, significantly reducing the strength of conclusions.
Quantitative review
A total of five articles were excluded from the quantitative review for one or more of the following reasons: a) they did not have a no-treatment group as a control; b) results were reported only as annual relapse rates and not the number of relapses during the six months after childbirth; or c) no outcome was reported. Thus, six articles were included in this meta-analysis1010. Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
https://doi.org/10.1007/BF00878527...
,1111. Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004 Sep;251(9):1133-7. https://doi.org/10.1007/s00415-004-0495-z
https://doi.org/10.1007/s00415-004-0495-...
,1414. Orvieto R, Achiron R, Rotstein Z, Noy S, Bar-Hava I, Achiron A. Pregnancy and multiple sclerosis: a 2-year experience. Eur J Obstet Gynecol Reprod Biol. 1999 Feb;82(2):191-4. https://doi.org/10.1016/S0301-2115(98)00231-0
https://doi.org/10.1016/S0301-2115(98)00...
,1515. Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk S, Schwödiauer V et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008 Jul;118(1):24-8. https://doi.org/10.1111/j.1600-0404.2007.00978.x
https://doi.org/10.1111/j.1600-0404.2007...
,1616. Hellwig K, Beste C, Schimrigk S, Chan A. Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disorder. 2009 Jan;2(1):7-11. https://doi.org/10.1177/1756285608100416
https://doi.org/10.1177/1756285608100416...
,1818. Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000.
https://doi.org/10.2165/11538960-0000000...
. Again, the methods for inclusion of patients and dose schemes were not uniform.
In this quantitative data analysis, there were a total of 72 patients who received IVIG for the prevention of postpartum relapses. From this group, 11 relapsed within the first six months. The control group consisted of 132 patients who did not receive immunoglobulin treatment, and from this group, 65 relapsed during the first six months after delivery. The odds of relapse postpartum within the first six months after delivering the baby were 68% lower in the treatment group than in the no-treatment group: 0.32 [95% CI 0.15-0.67, p = 0.003, I2 = 20%, Q = 3.74 (p = 0.29)]. In the second analysis, the odds of a relapse in the first six months after delivery were 59% lower in the postpartum IVIG treatment group than in the control group: 0.41 [95% CI 0.25-0.70, p ≤ = 0.001, I2 = 20%, Q = 7.53 (p = 0.27)]. Figure 2 summarizes the meta-analysis data on IVIG versus no treatment or versus other therapeutic alternatives. The unadjusted NNTs for the two analyses were 6.3 and 5.8 respectively.
Meta-analyses on data referring to the number of relapses per pregnancy during first six months for patients who received IVIG postpartum versus other therapeutic options or no-treatment control groups.
DISCUSSION
MS is a chronic neurological condition that will accompany the patient for his/her life. Because of its onset at a fertile age and its predominance among females, the issue of pregnancy and MS is of great importance and requires a multidisciplinary approach33. Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015 Mar;29(3):207-20. https://doi.org/10.1007/s40263-015-0238-y
https://doi.org/10.1007/s40263-015-0238-...
. For obvious ethical reasons, there are no prospective double-blind clinical trials on pregnant women with MS. Therefore, our knowledge on the management of these patients comes from retrospective observational data and meta-analyses on published results.
A systematic review and meta-analysis on the use of IVIG as a preventive measure against postnatal relapses of MS was long overdue. This procedure has been routinely recommended by some doctors, although it is expensive and may delay the release of the new mother from hospital. If it were demonstrated that IVIG would avoid relapses, the issues of expense and increased hospitalization would no longer deter recommendations for its use. However, this systematic review with quantitative and qualitative analyses of published data did not establish any advantage of using IVIG. In fact, the methodologies used in previous studies have varied widely among researchers. The range of doses, therapeutic schemes, timing of treatment and assessment of relapses over time, and the low numbers of patients involved in the 11 studies selected presented serious limitations in reaching conclusions and consensus. Moreover, even the most optimistic NNT value established by the present study showed that over US$87,000 must be invested to avoid one relapse during the postnatal period of one patient with MS.
In conclusion, the therapeutic effect of IVIG for prevention of postnatal relapses in MS could not be clearly established in this systematic review and meta-analysis.
References
-
1Huang WJ, Chen WW, Zhang X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp Ther Med. 2017 Jun;13(6):3163-6. https://doi.org/10.3892/etm.2017.4410
» https://doi.org/10.3892/etm.2017.4410 -
2Jenkins TM, Thompson AJ. Diagnosing and managing multiple sclerosis. Practitioner. 2009 Sep;253(1721):25-30.
-
3Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015 Mar;29(3):207-20. https://doi.org/10.1007/s40263-015-0238-y
» https://doi.org/10.1007/s40263-015-0238-y -
4Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227-31. https://doi.org/10.1002/ana.410130302
» https://doi.org/10.1002/ana.410130302 -
5Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010 Sep;25(9):603-5. https://doi.org/10.1007/s10654-010-9491-z
» https://doi.org/10.1007/s10654-010-9491-z -
6Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 Oct;62(10):1006-12. https://doi.org/10.1016/j.jclinepi.2009.06.005
» https://doi.org/10.1016/j.jclinepi.2009.06.005 -
7Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011 [access 2017 July 10 2017]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
» http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp -
8Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 London: The Cochrane Collaboration; 2011 [access 2017 December 10]. Available from: http://handbook-5-1.cochrane.org/
» http://handbook-5-1.cochrane.org/ -
9U.S. Department of Health & Human Services. National Heart, Lung and Blood Institute. Study quality assessment tools: quality assessment tool for case series studies. Bethesda: National Heart, Lung and Blood Institute; [s.d.] [accessed 2017 July 10]. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series
» https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series -
10Achiron A, Rotstein Z, Noy S, Mashiach S, Dulitzky M, Achiron R. Intravenous immunoglobulin treatment in the prevention of childbirth-associated acute exacerbations in multiple sclerosis: a pilot study. J Neurol. 1996 Jan;243(1):25-8. https://doi.org/10.1007/BF00878527
» https://doi.org/10.1007/BF00878527 -
11Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004 Sep;251(9):1133-7. https://doi.org/10.1007/s00415-004-0495-z
» https://doi.org/10.1007/s00415-004-0495-z -
12Haas J. High dose IVIG in the post-partum period for prevention of exacerbations in MS. Mult Scler. 2000 Oct;6 Suppl 2:S18-20.
-
13Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007 Aug;13(7):900-8. https://doi.org/10.1177/1352458506075654
» https://doi.org/10.1177/1352458506075654 -
14Orvieto R, Achiron R, Rotstein Z, Noy S, Bar-Hava I, Achiron A. Pregnancy and multiple sclerosis: a 2-year experience. Eur J Obstet Gynecol Reprod Biol. 1999 Feb;82(2):191-4. https://doi.org/10.1016/S0301-2115(98)00231-0
» https://doi.org/10.1016/S0301-2115(98)00231-0 -
15Hellwig K, Brune N, Haghikia A, Müller T, Schimrigk S, Schwödiauer V et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand. 2008 Jul;118(1):24-8. https://doi.org/10.1111/j.1600-0404.2007.00978.x
» https://doi.org/10.1111/j.1600-0404.2007.00978.x -
16Hellwig K, Beste C, Schimrigk S, Chan A. Immunomodulation and postpartum relapses in patients with multiple sclerosis. Ther Adv Neurol Disorder. 2009 Jan;2(1):7-11. https://doi.org/10.1177/1756285608100416
» https://doi.org/10.1177/1756285608100416 -
17Durelli L, Ricci A, Verdun E. Immunoglobulin treatment of multiple sclerosis: future prospects. Neurol Sci. 2003 Oct;24(0 Suppl 4):S234-8. https://doi.org/10.1007/s10072-003-0085-3
» https://doi.org/10.1007/s10072-003-0085-3 -
18Fragoso YD, Finkelsztejn A, Kaimen-Maciel DR, Grzesiuk AK, Gallina AS, Lopes J et al. Long-term use of glatiramer acetate by 11 pregnant women with multiple sclerosis: a retrospective, multicentre case series. CNS Drugs. 2010 Nov ;24(10):969-76. https://doi.org/10.2165/11538960-000000000-00000
» https://doi.org/10.2165/11538960-000000000-00000 -
19Fragoso YD, Adoni T, Alves-Leon SV, Azambuja ND Jr, Barreira AA, Brooks JB et al. Postpartum treatment with immunoglobulin does not prevent relapses of multiple sclerosis in the mother. Health Care Women Int. 2015;36(10):1072-80. https://doi.org/10.1080/07399332.2014.948627
» https://doi.org/10.1080/07399332.2014.948627 -
20Vukusic S, Durand-Dubief F, Benoit A, Marignier R, Frangoulis B, Confavreux C. Natalizumab for the prevention of post-partum relapses in women with multiple sclerosis. Mult Scler. 2015 Jun;21(7):953-5. https://doi.org/10.1177/1352458514554056
» https://doi.org/10.1177/1352458514554056 -
21Brandt-Wouters E, Gerlach OH, Hupperts RM. The effect of postpartum intravenous immunoglobulins on the relapse rate among patients with multiple sclerosis. Int J Gynaecol Obstet. 2016 Aug;134(2):194-6. https://doi.org/10.1016/j.ijgo.2016.01.008
» https://doi.org/10.1016/j.ijgo.2016.01.008 -
22Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998 Jul;339(5):285-91. https://doi.org/10.1056/NEJM199807303390501
» https://doi.org/10.1056/NEJM199807303390501 -
23Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444-52. https://doi.org/10.1212/WNL.33.11.1444
» https://doi.org/10.1212/WNL.33.11.1444
Publication Dates
-
Publication in this collection
June 2018
History
-
Received
18 Jan 2018 -
Accepted
28 Feb 2018