Abstracts
OBJECTIVE: To ascertain the effect of inhaled corticosteroid use on gain in height and weight of asthmatic pediatric outpatients. METHODS: A one-year prospective cohort study was carried out with 124 asthmatic children aged 3 to 16 years who were prescribed inhaled corticosteroids for at least 12 months, evaluating z-scores for height/age, weight/age, body mass index and parental target height for current age. Exclusion criteria were: birth weight less than 2,500 g, malnutrition, chronic diseases and systemic corticoid use for more than 7 consecutive days. RESULTS: The mean ± standard deviation for z-scores for initial and final height/age were 0.06±1.2 and 0.01±1.2, (95%CI 0.05-0.11), respectively; for initial and final weight/age z-scores they were 0.6±1.5 and 0.5±1.5 (95%CI 1.84-6.6), respectively. These figures did not differ significantly (p = 0.199 and p = 0.808). There was also no loss in stature when children were stratified into well and poorly controlled asthma or into pubescent and non-pubescent groups. CONCLUSIONS: In comparison with the NCHS (National Center for Health Statistics) growth curves, there was no compromise to the height or body weight of children/adolescents using inhaled corticosteroids for more than 1 year at the doses recommended for asthma prevention.
Corticosteroids; asthma; growth; children; adolescents
OBJETIVO: Verificar o efeito do uso de corticosteróides inalatórios no aumento estatural e ponderal de crianças asmáticas tratadas ambulatorialmente MÉTODOS: Foi realizado um estudo de coorte prospectivo de 1 ano, no qual 124 crianças asmáticas com 3 a 16 anos de idade que haviam recebido prescrição para uso de corticosteróides inalatórios há pelo menos 12 meses foram avaliadas quanto aos escore z altura/idade, peso/idade, índice de massa corporal e altura alvo parental estimada para a idade atual. Os critérios de exclusão foram: peso de nascimento menor que 2.500 g, desnutrição, doenças crônicas e uso de corticóide sistêmico por mais de 7 dias consecutivos. RESULTADOS: A média ± desvio padrão dos escores z altura/idade inicial e final foi, respectivamente, de 0,06±1,2 e 0,01±1,2, (IC95% 0,05-0,11); dos escores z peso/idade inicial e final foi de 0,6±1,5 e 0,5±1,5, respectivamente (IC95% 1,84-6,6). Esses valores não diferiram significativamente (p = 0,199 e p = 0,808). Quando estratificados em grupos bem e mal controlados da asma, púberes e não-púberes, também não houve perda estatural. CONCLUSÃO: Em relação às curvas NCHS (National Center for Health Statistics), não houve prejuízo na estatura e peso corporal de crianças/adolescentes que utilizaram corticosteróides inalatórios por mais de 1 ano nas doses preconizadas para prevenir asma.
Corticosteróides; asma; crescimento; crianças; adolescentes
ORIGINAL ARTICLE
Inhaled corticosteroid treatment and growth of asthmatic children seen at outpatient clinics
Elisete E. ArendI; Gilberto B. FischerII; Márcio DebiasiIII; Helena SchmidIV
IMestranda em Ciências Médicas: Pediatria, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brasil
IIDoutor. Professor titular, Fundação Faculdade Federal de Ciências Médicas de Porto Alegre (FFFCMPA), Porto Alegre, RS, Brasil. Responsável pela Disciplina de Doenças Respiratórias, Programa de Pós-Graduação de Pediatria, UFRGS, Porto Alegre, RS, Brasil. Chefe, Ambulatório de Atendimento ao Asmático, Hospital Materno-Infantil Presidente Vargas, Porto Alegre, RS, Brasil
IIIAcadêmico de Medicina, FFFCMPA, Porto Alegre, RS, Brasil. Bolsista, Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS)
IVPós-doutora, University of Michigan, Ann Harbor, USA. Doutora, Universidade de São Paulo (USP), São Paulo, SP, Brasil. Endocrinologista, professora titular, FFFCMPA, e UFRGS, Porto Alegre, RS, Brasil
Correspondence Correspondence: Helena Schmid Av. Felipe Neri, 296/301 CEP 90440-150 - Porto Alegre, RS - Brazil E-mail: hschmid@fffcmpa.tche.br
ABSTRACT
OBJECTIVE: To ascertain the effect of inhaled corticosteroid use on gain in height and weight of asthmatic pediatric outpatients.
METHODS: A one-year prospective cohort study was carried out with 124 asthmatic children aged 3 to 16 years who were prescribed inhaled corticosteroids for at least 12 months, evaluating z-scores for height/age, weight/age, body mass index and parental target height for current age. Exclusion criteria were: birth weight less than 2,500 g, malnutrition, chronic diseases and systemic corticoid use for more than 7 consecutive days.
RESULTS: The mean ± standard deviation for z-scores for initial and final height/age were 0.06±1.2 and 0.01±1.2, (95%CI 0.05-0.11), respectively; for initial and final weight/age z-scores they were 0.6±1.5 and 0.5±1.5 (95%CI 1.84-6.6), respectively. These figures did not differ significantly (p = 0.199 and p = 0.808). There was also no loss in stature when children were stratified into well and poorly controlled asthma or into pubescent and non-pubescent groups.
CONCLUSIONS: In comparison with the NCHS (National Center for Health Statistics) growth curves, there was no compromise to the height or body weight of children/adolescents using inhaled corticosteroids for more than 1 year at the doses recommended for asthma prevention.
Key words: Corticosteroids, asthma, growth, children, adolescents.
Introduction
Inhaled corticoids (IC) have been used with success1 for more than 2 decades for the prevention of persistent moderate and severe asthma,2 reducing hospital admissions, visits to emergency services, oral corticoid (OC) bronchodilator usage and frequency of symptoms.1 Nevertheless, adverse effects linked to their use have been described, dependent on dose, length of treatment, method of administration and mode of withdrawal - abrupt or gradual.2,3
All chronic diseases, including asthma, can affect growth.4,5 A review article4 listed early onset of asthma, disease severity, hypoxemia, chronic anorexia, corticoid use and low socioeconomic status as possible factors responsible for growth restriction.
Several different studies have been conducted to investigate the effect of IC on growth.6 One of these7 found short stature in children with asthma and rhinitis was associated with birth weight below 2,500 g and prolonged disease duration rather than with corticoid use; others have found links between growth restriction and OC use,8 type of IC, method of administration (during the first year of use)9 and high doses10 of IC.
Possible risk factors for restricted height in children considered normal that have been described include: low maternal educational level, per capita income less than or equal to 0.5 national minimum wages, short maternal and paternal stature and short stature and low weight at birth.11-14
The objective of this study was to investigate the possibility that IC influences the height and weight gain of asthmatic children in chronic treatment at two clinics on the public health system in Porto Alegre (Rio Grande do Sul state) that provide medication free of charge.
Patients and methods
This was a prospective cohort study. The object of the study was the effect of the use of IC in asthmatic children and adolescents and outcomes were height and weight gain. During the period from October 2002 to October 2003 all asthmatic children and adolescents were invited to participate in the study if they were aged 3 to 16 years of age, had 1 year or more of IC treatment and attended the asthma clinic at the Hospital Materno-Infantil Presidente Vargas or the Posto IAPI, both part of the municipal health system of Porto Alegre. Children aged less than 9 years had been prescribed IC for at least 1 year and those aged 9 or more for at least 2 years. Patients were excluded if their birth weights had been < 2,500 g, they were malnourished (weight/age index < -2 z-scores ), had other chronic diseases or were on continuous OC. The medical records of patients enrolled were reviewed from the date they had registered at the clinic and the following details recorded: initial height and weight, the IC prescribed, nasal corticoid (NC) usage, dose, duration of use, the use of other drugs and the number and severity of asthma crises. On enrollment one guardian of each patient was interviewed for collection of social data (income, parents' education and living conditions) and clinical data (number of asthma crises, previous hospitalizations, visits to emergency, diseases and, at every follow-up appointment, corticoid use and dosage). Children consulted at intervals of 1 to 3 months. Questionnaires were filled out by three previously trained interviewers in order to maintain uniformity of information. Puberty was evaluated using the Tanner classification.15 Compliance with treatment was assessed by verification of the number of corticoid vials used. Every child enrolled on the study had their height measured barefoot, standing erect against a flat vertical surface with heels together, using the same stadiometer (brand Seca, wall mounted, with precision to 0.1 cm), by the same people (the interviewer, one author of this article and an academic nutritionist). Body weight was measured on Filizola scales. The anthropometric profile of the participants was obtained in accordance with World Health Organization (WHO) guidelines,16 with variations from normal established by comparison with the NCHS curves, z-score values and mid-parental targets. When z-scores for height (HAZ) and weight (WAZ) were calculated, short stature was defined as HAZ < -2, overweight as body mass index (BMI) z-score from +1 to +2, obesity as BMI z-score > +2. Body mass indices [weight (kg)/height (m2)] were also compared with the NCHS curves, taking account of age and sex of participants.
Mid-parental target heights were also calculated for all participants. This represents the height that 95% of the children of a couple should attain in adulthood.17,18 The following formulae were used to calculate mid-parental targets:
Girls: Mother's height + (father's height - 13 cm)
2
Boys: Father's height + (mother's height + 13 cm)
2
This target was used to find the point on the NCHS curves that corresponded to the current age of the patient on the percentile of their target height. Parents' heights were determined using the same stadiometer described above or, in the cases of 38 fathers and two mothers, from the mean of three measurements taken at home by the parent who attended the consultations and who had been trained to measure the parent who did not attend.
Children were defined as having poorly-controlled asthma if they presented two or more crises requiring OC during the study year, if they had to attend emergency or if they required hospital admission.19 Nasal and IC were compared in the same manner and their topical power calculated relative to beclomethasone. For example, if 200 µg/day fluticasone was actually given, this was defined as equivalent to 400 µg/day beclomethasone, according to the topical power table of the Asthma Consensus.2 The nasal IC used in this study were beclomethasone (250 µg/shot), fluticasone (50 µg/shot) and budesonide (50 µg/shot) and triamcinolone was also given. Compliance with treatment was reported by parents and confirmed by their requesting replacement medication provided by the state. The mean IC dose used was calculated at each visit to take account of possible changes in medication or doses. The mean daily use for the year was calculated from these data.
Ethical considerations
The study protocol was approved by the Research Ethics Committee at the Hospital Presidente Vargas, and permission was received from the Posto IAPI. The study was considered of minimal risk and informed consent was given by parents or guardians (98.6% agreed).
Statistical analysis
The sample size was estimated at 73 children based on a calculation designed to allow the detection of a 1 cm difference in mean height, with standard difference deviation of 3 cm, alpha of 0.05 and power of 80%. Quantitative variables were described as mean and standard deviation or median and percentiles. Qualitative variables were expressed as absolute and relative frequencies. The Kolmogorov-Sminov test was applied to verify normality of the data. The comparison between HAZ at enrollment and at follow-up was performed using the t test for paired samples. In comparisons that also involved patients' first consultations at the clinic, analysis of variance (ANOVA) for repeated measurements was used. Quantitative dichotomous variables were compared with the t test for independent samples and ANOVA was applied to polytomous quantitative variables. Associations between dichotomous variables were analyzed using either the chi-square test or Fisher's exact test. Associations between quantitative variables were evaluated using either Pearson's (normal distribution) or Spearman's (asymmetrical distribution) correlation coefficients. The association between parental target height adjusted for current age (PHAA) and HAZ was assessed using Pearson's correlation coefficient. The level of significance adopted was 5%, and analysis was performed using SPSS version 10.0.
Results
One hundred and forty patients took part in the study. One hundred and twenty four of these were followed for 1 year (88.5%). Patients were defined as lost to the study if it was not possible to measure their height after 1 year of follow-up, because they did not attend return appointments (15 cases) and children who were not compliant with treatment, identified by the fact that fresh IC supplies were not required on more than one occasion (one case). There were, therefore, 16 losses, in 15 of these cases it was possible to contact the family by telephone and non-attendance was explained by the disappearance of symptoms.
The mean age of the 124 cases that were followed-up for 1 year was 8.6 years and 81 children were less than 10 years old. The majority of the children were from low-income families and mean maternal schooling was 6.9 years. There were 74 boys, 59 children were pubescent in 77 cases asthma was well controlled. The distribution of patients by type of corticoid was as follows: aerosol - beclomethasone (47), budesonide (43) and fluticasone (9); in dry powder - beclomethasone (15). The remaining patients were given mixed medication and were excluded from the analysis of corticoid type . Converted to beclomethasone-equivalent topical power, mean doses ± standard deviation (SD) for the follow-up year were: IC + NC - 594.04±264.53 µg/day; IC - 494.17±314.48 µg/day; OC - 172.35±218.04 mg/year; OC per cycle - 85.28±82.75 mg/cycle. Overall, 70.9% of the children were given the equivalent of up to 600 mg/day of beclomethasone, and 29.1% were given the equivalent of more than 600 mg/day. These and other characteristics are presented in Table 1. Mean ± SD for the time between first consultation at the asthma clinic and enrollment on the study was 31.43±20.51 months.
Once the figures for overall patient height on the date of the first consultation within the study period were assessed, it was observed that mean height was greater than the mean adjusted target parental height (p < 0.001) and was similar to the 50th percentile of the NCHS curve (Table 2). Analysis of HAZ returned a mean figure of 0.04±1.14 for date of registration at the clinic, similar to the 0.06±1.20 for the date of the first visit during the study period (p = 0.800).
Mean overall height and BMI gain during the year were 5.9 cm (p < 0.001) and 0.5 kg/m2 (p < 0.001), respectively, as shown in Tables 2 and 3.
Height increase was the result of increased age since HAZ was not significantly different between the two assessments, baseline and 1 year (p = 0.199). At enrollment 3 (2.4%) patients had HAZ < -2, i.e. they had short stature, and, at 1-year follow-up, this number had increased to five (4.0%). These values do not differ significantly (p = 0.5).
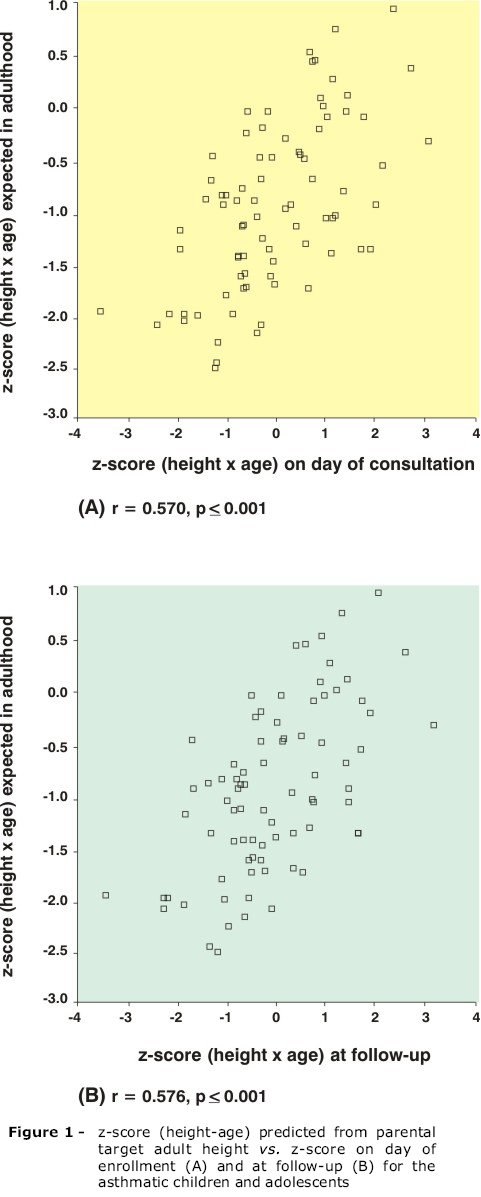
The patients' HAZ correlated with their PHAA, both on the day of enrollment and after 1 year, demonstrating that the patients studied grew in line with their genetic potential, despite the use of corticoids (Figure 1).
The possibility that IC dosage would correlate with HAZ was also considered and assessed. There was no correlation between HAZ and total IC and nasal doses given (r = -0.08; p = 0.4), nor with total doses of OC (r = 0.02; p = 0.84).
When the sample was broken down into groups "pubescent vs. prepubescent," "controlled vs. uncontrolled asthma" and "male vs. female sex", there were also no significant difference in height gain (data not shown).
Overall BMI for the patients on the date of their first visit within the study (mean ± SD) was 18.2±3.16 (Table 3) and, 1 year later it was 18.7±3.5. When classified according to the NCHS BMI curves, there were 19 (15.3%) overweight children (BMI z-score > 2) on enrollment and 1 year later there were 15 (12.1%), which difference was not statistically significant (p = 0.424).
The BMI increased significantly over the year, for pubescent (p = 0.026) and prepubescent children (p < 0.001), for controlled (p = 0.001) and uncontrolled asthma cases (p = 0.013), for those on beclomethasone powder (p = 0.023) and those on other types of corticoid (p < 0.001). These increases are therefore probably related to age since the number of children with WAZ that deviated from normal did not change from enrollment to the end of the study, and neither did the mean ± SD of WAZ differ significantly (initial WAZ = 0.6±1.5; final WAZ = 0.5±1.5; 95%CI 1.84-6.6; p = 0.808).
When patients were split into groups according to the type of inhaled corticoid, it was observed that the mean BMI for patients in different groups was similar at enrollment (ANOVA: p = 0.501) and follow-up (ANOVA: p = 0.671).
The HAZ scores for children/adolescents in the different groups also did not differ from enrollment to 1 year, with the exception of the group given beclomethasone powder, which exhibited a significant reduction at follow-up (initial z score = 0.17±1.26; final z score = 0.00±1.16; 95%CI 0.05-0.29; p = 0.010). When the mean heights of this group for the year were compared with their mean mid-parental targets, this difference was not observed (p = 0.267).
Discussion
Research into the growth of asthmatics using corticoid therapy has returned conflicting results.4,10,20,21 Intervention studies, such as randomized clinical trials, are the most appropriate form of study for assessing cause and effect. However, in the case of asthma such studies have ethical limitations since there is no question of the benefits of IC.4
In the current study 124 patients who used IC were followed for 1 year and exhibited growth or final stature similar to what is observed with individuals in the same age groups.22 These findings are in agreement with several different studies of long-term IC use.20,21,23 Similarly, Allen et al.8 performed a meta-analysis of 810 asthmatics treated with OC or IC, observing correlation with growth restriction only in the case of OC use. In contrast, McCowan et al.10 demonstrated less growth when high doses of IC were given.
In the current study an increase in stature of 5.9 cm/year was observed, which is an identical figure to that reported by Agertoft & Pedersen20 for patients of a similar age group using IC (budesonide) for 9.2 years. In common with what was observed by Kovalhuk et al.,24 in the current study there was no growth restriction related to uncontrolled asthma, IC duration or dosage, parents' schooling or family income, although some studies have shown reduced height gain related to severity of asthma.21
According to Luo et al.,25 95% of the population reach an adult height that is within 9-10 cm above or below the height predicted from their parents' heights (mid-parental target height). When data from the current study was analyzed, comparing the heights of the children and adolescents with their parental target heights, it was observed that the IC had not provoked growth restriction, which is in agreement with a study by Agertoft & Pedersen.20 On the other hand, both at the first visit and at 1 year follow-up, the mean heights of the children participating in this study were above those predicted from their parental target heights, in accordance with a tendency that has been reported for many populations worldwide and which, apparently, reflects the improved socioeconomic conditions of these populations when compared with those of their parents, just one generation earlier.26,27 Therefore, it can be stated that there is no compromise to the height of the children studied after more than 1 year of IC use. On the contrary, these children are following the tendency to reach an average height above that of their parents and to grow in line with their genetic potential (when the possibility of a correlation between HAZ and height of parents was evaluated, it was observed that there was a positive correlation).
It is of interest to note that, in the current study, patients using beclomethasone powder exhibited a reduced height gain over 1 year. It should be emphasized that this project was not designed to detect the effects of different types of corticoids and the sample size was not calculated with this objective in mind. Other studies have observed that IC in powder has an influence on growth.9,28
While the sample size of this study was small with respect of the different types of IC, the findings exhibit a tendency that is similar to the studies cited above. The oral bioavailability, systemic absorption and hepatic metabolism of IC in powder contributes to a potential systemic effect.29 Taking into account the fact that IC are offered in preparations with varying bioavailability, it should be possible to detect differences in growth provoked by doses with similar anti-inflammatory power given to children, but with differences in the form of presentation (for example, in powder or aerosol).30 The fact that different doses and formulations of IC were employed, creating the need to find a measure of equivalence, is a limitation to the current study. Nevertheless, in published literature the same type of estimate has been used and the results are comparable, particularly in the cases of the most-used drugs such as budesonide and beclomethasone.29
While the primary objective of the current study was not to evaluate the effect of IC on BMI either, in contrast with other authors,31 no significant increase in WAZ was observed after 1 year, suggesting that the systemic effects of IC are not important.
Conclusions
This follow-up study of asthmatic children on IC, did not demonstrate any compromise to height gain after 1 year of observation, but actually detected mean growth above what would be expected based on mid-parental targets. This tendency has been described for normal children living in first world countries. The observed heights reinforce the idea that the negative influence of treatment with IC on final height is absent, although, in order to confirm this these patients must be followed for a longer period, i.e. until adulthood. The observation of similar weight/age at the 1 year follow-up appointment also suggests little or no systemic effect related to IC use. On the other hand, it is impossible to rule out the possibility that compliance with treatment was not adequate. Furthermore, in the current study a tendency was observed towards reduced growth with beclomethasone powder. Further studies of this particular IC presentation are necessary to confirm this tendency.
Acknowledgements
We are grateful to the statisticians Alan Birck and Céres Andréia de Oliveira, for the data analysis and to the nutritionist Rúbia Grando Rebelato for helping with data collection.
References
Manuscript received Sep 08 2005, accepted for publication Feb 01 2006.
- 1. The Childhood Asthma Management Program Research Group. Long-term effects of budesonida or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-63.
- 2. III Consenso Brasileiro no Manejo da Asma. J Pneumol. 2002;28:S1-28.
- 3. Allen DB. Inhaled steroids for children: effects on growth, bone and adrenal function. Endocrinol Metab Clin N Am. 2005;34:555-64.
- 4. Monteiro Antonio MARG, Ribeiro JD, Toro AADC, Pidrabuena AE, Morcillo AM. O crescimento de crianças com asma. J Pneumol. 2003;29:36-42.
- 5. Balfour-Lynn L. Growth and childhood asthma. Arch Dis Child. 1986;61:1049-55.
- 6. Fortkamp E, Brown MA. Corticosteróides inalatórios e crescimento. J Pediatr (Rio J). 2000;76:263-74.
- 7. Sant'Anna CA, Sole D, Naspitz CK. Short stature in children with respiratory allergy. Pediatr Allergy Immunol. 1996;7:187-92.
- 8. Allen DB, Mullen M, Mullen B. A meta-analysis of the effect of oral and inhaled corticosteroids in growth. J Allergy Clin Immunol. 1994;93:967-76.
- 9. Sharek PJ, Bergman DA. The effect of inhaled steroids on the linear growth of children with asthma: a meta-analysis. Pediatrics. 2000;106:E8.
- 10. McCowan C, Neville RG, Thomas GE, Crombie IK, Clark RA, Rickettts IW, et al. Effect of asthma and its treatment on growth: four year follow up of cohort of children from general practices in Tayside, Scotland. BMJ. 1998;316:668-72.
- 11. Guimarães LV, Latorre MRDO, Barros MBA. Fatores de risco para ocorrência de déficit estatural em pré-escolares. Cad Saude Publica. 1999;15:605-15.
- 12. Amigo H, Bustos P, Radrigan ME. Is there a relation between parent's short height and their children's? Rev Med Chile. 1997;125:863-8.
-
13Pesquisa Nacional de Saúde e Nutrição (PNSN). Perfil de crescimento da população brasileira de 0-25 anos. Brasília: Secretaria de Política da Saúde, Instituto Nacional de Alimentação e Nutrição; 1990.
- 14. Aerts D, Drachler ML, Giugliani ER. Determinantes de atraso de crescimento no sul do Brasil. Cad Saude Publica. 2004;20:1182-90.
- 15. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height velocity, weight velocity and stages of puberty. Arch Dis Child. 1976;51:170-9.
- 16. Gorstein J, Sullivan K, Yip R, Onis M, Trowbridge F, Fajans P, et al. Issues in the assessment of nutritional status using anthropometry. Bull World Health Organ. 1994;72:273-83.
- 17. Zeferino AMB, Barros Filho AA, Bertiol H, Barbieri MA. Acompanhamento do crescimento. J Pediatr (Rio J). 2003;79:S23-32.
- 18. Tanner JM. The use and abuse of growth standards. In: Falkner F, Tanner JM, editors. Human growth. 2nd ed. New York: Plenum; 1986. vol.3, p. 95-112.
- 19. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJH, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836-44.
- 20. Agertoft L, Pedersen S. Effects of long term treatment with inhaled budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-9.
- 21. Van Bever HP, Desager K, Lijssens N, Weyler JJ, Du Caju MVL. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-75.
- 22. National Center for Health Statistic. 2000 CDC growth charts: United States. http://www.cdc.gov/growthcharts/ Acesso: 03/2002.
- 23. Silverstein MD, Yunginger JW, Reed CE. Attained adult height after childhood asthma: effect of glicocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-7.
- 24. Kovalhuk LCS, Rosário NA, Lacerda L, Gabardo J. Avaliação do crescimento linear de crianças asmáticas em uso de corticóides. Rev Bras Allerg Immunopatol. 1999;22:41-56.
- 25. Luo ZC, Albertsson-Wikland K, Karlberg J. Target height as predicted by parental heights in a population based study. Pediatr Res. 1998;44:563-71.
- 26. Smith AS, Norris BJ. Changes in body size of UK and US children over past three decades. Ergonomics. 2004;47:1195-207.
- 27. França Junior I, da Silva GR, Monteiro CA. Secular trends of height in adulthood of children born in the city of São Paulo, Brazil, from 1950-1976. Rev Saude Publica. 2000;34:102-7.
- 28. Wolthers OD, Heuck C. Assessment of the relation between short and intermediate term growth in children with asthma treated with inhaled glicocorticoids. Allergy. 2004;59:1193-7.
- 29. Liptworth BJ. Systemic adverse effects of inhaled corticosteroid therapy. Arch Intern Med. 1999;159:941-55.
- 30. Hubner M, Pharm B, Hochhaus G, Derendorf H. Comparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroids. Immunol Allergy Clin N Am. 2005;25:469-88.
- 31. Wickens K, Barry D, Friezema A, Rhodius R, Bone N, Purdie G, et al. Obesity and asthma in 11-12 years old New Zealand children in 1989 and 2000. Thorax. 2005;60:7-12.
Correspondence:
Publication Dates
-
Publication in this collection
10 July 2006 -
Date of issue
June 2006
History
-
Received
08 Sept 2005 -
Accepted
01 Feb 2006