Abstracts
OBJECTIVE:To describe the main undesirable side effects of glucocorticoid therapy, mechanisms of action and the necessary measures to minimize side effects. SOURCES: Author's experience, supplemented with papers published in MEDLINE. SUMMARY OF THE FINDINGS: The principles for minimizing undesirable side effects of glucocorticoid therapy include: a) only use glucocorticoids if they are essential; b) avoid the use of long-acting glucocorticoids, using short- and intermediate-acting glucocorticoids instead; c) keep treatment as short as possible, since treatment lasting 5 to 7 days shows fewer side effects and quick recovery of the hypothalamic-pituitary axis; d) use glucocorticoids with local activity preferentially, such as inhaled glucocorticoids; e) use in association with other drugs, especially with other more specific anti-inflammatory or immune suppressive drugs, promoting a synergistic effect in order to avoid the use of glucocorticoids or to reduce dosage and duration of glucocorticoid therapy; f) indicate the minimum effective dose, respecting individual sensitivity to glucocorticoids. CONCLUSION: In order to choose the best glucocorticoid schedule it is essential to understand the pharmacological characteristics and the biological action of glucocorticoids, allowing the most adequate indication, glucocorticoid dose, mode of administration and the duration of glucocorticoid therapy.
Glucocorticoids; side effects; sensitivity; receptor
OBJETIVO: Ressaltar os principais efeitos indesejáveis durante a corticoterapia, o mecanismo de seu desencadeamento e as medidas necessárias para minimizar os efeitos colaterais. FONTES DOS DADOS: Experiência do autor, complementada com trabalhos publicados no MEDLINE. SÍNTESE DOS DADOS: Os princípios para que se minimizem os efeitos indesejáveis da corticoterapia incluem: a) indicação rígida em que o uso do glicocorticóide seja essencial; b) evitar o uso de glicocorticóides de ação prolongada, preferindo glicocorticóide de ação curta ou intermediária; c) reduzir ao mínimo necessário a duração do tratamento, visto que tratamentos com duração entre 5 e 7 dias apresentam poucos efeitos colaterais e rápida recuperação do eixo hipotalâmico-hipofisário; d) preferir glicocorticóides de ação local, como glicocorticóides inalatórios; e) associação com outros fármacos, em especial outros antiinflamatórios ou imunossupressores mais específicos, buscando efeitos sinérgicos que permitam evitar o uso de glicocorticóides ou reduzir a dose e o tempo da corticoterapia; f) oferecer a menor dose necessária para o efeito desejado, respeitando a sensibilidade de cada indivíduo aos glicocorticóides. CONCLUSÕES: o conhecimento das características farmacológicas e ações biológicas dos glicocorticóides permite a melhor opção terapêutica quanto à indicação, dose, via de administração e duração da corticoterapia.
Glicocorticóide; efeito adverso; sensibilidade; receptor
REVIEW ARTICLE
Glucocorticoid therapy: minimizing side effects
Carlos Alberto Longui
Professor Chefe de Clínica adjunto, Unidade de Endocrinologia Pediátrica, Depto. Pediatria e Puericultura, Irmandade Santa Casa de Misericórdia de São Paulo, São Paulo, SP, Brazil. Professor adjunto, Laboratório de Medicina Molecular, Depto. Ciências Fisiológicas, Faculdade de Ciências Médicas, Santa Casa de São Paulo, São Paulo, SP, Brazil
Correspondence Correspondence: Carlos Alberto Longui Rua Prof. Artur Ramos 96, 2º andar, Jardim Paulistano CEP 01454-010 - São Paulo, SP - Brazil Email: carloslongui@msn.com
ABSTRACT
OBJECTIVE: To describe the main undesirable side effects of glucocorticoid therapy, mechanisms of action and the necessary measures to minimize side effects.
SOURCES: Author's experience, supplemented with papers published in MEDLINE.
SUMMARY OF THE FINDINGS: The principles for minimizing undesirable side effects of glucocorticoid therapy include: a) only use glucocorticoids if they are essential; b) avoid the use of long-acting glucocorticoids, using short- and intermediate-acting glucocorticoids instead; c) keep treatment as short as possible, since treatment lasting 5 to 7 days shows fewer side effects and quick recovery of the hypothalamic-pituitary axis; d) use glucocorticoids with local activity preferentially, such as inhaled glucocorticoids; e) use in association with other drugs, especially with other more specific anti-inflammatory or immune suppressive drugs, promoting a synergistic effect in order to avoid the use of glucocorticoids or to reduce dosage and duration of glucocorticoid therapy; f) indicate the minimum effective dose, respecting individual sensitivity to glucocorticoids.
CONCLUSION: In order to choose the best glucocorticoid schedule it is essential to understand the pharmacological characteristics and the biological action of glucocorticoids, allowing the most adequate indication, glucocorticoid dose, mode of administration and the duration of glucocorticoid therapy.
Keywords: Glucocorticoids, side effects, sensitivity,receptor.
Introduction
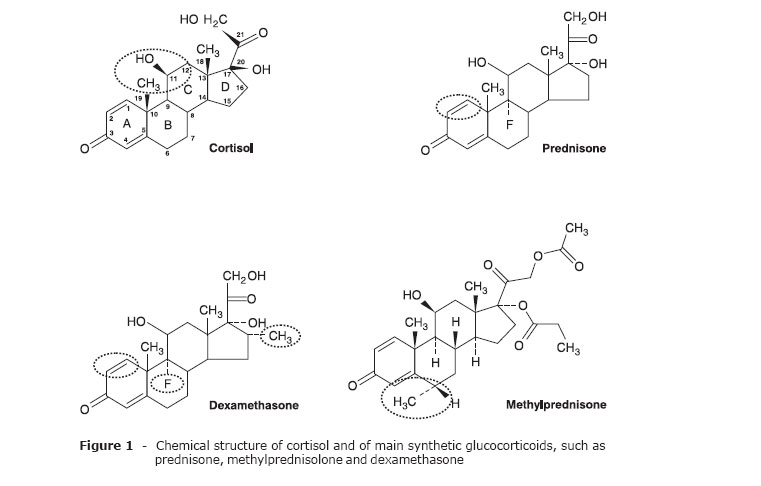
During the 1930s and 1940s, several studies showed the effects of adrenocorticoid hormones on the balance of electrolytes (mineralocorticoids), as well as on the metabolism of carbohydrates (glucocorticoids).1,2 Cortisol was synthesized in 1946, and in 1948 it was first used by Hench in the treatment of rheumatoid arthritis. Unfavorable side effects arose subsequently, limiting the therapeutic use of glucocorticoids. In the 1950s, changes in the structure of cortisol resulted in the manufacture of new drugs, such as prednisone and prednisolone. Subsequent structural modifications of synthetic steroids (Figure 1) enhanced glucocorticoid potency and extended the duration of effect (Table 1), and also provided drugs with different affinities and binding time-courses to the glucocorticoid receptor (GR).
These new characteristics resulted in additional complications related to longer plasmatic half-life (necessary time-course to reduce initial plasma levels of the drug by 50%) and longer biologic half-life (residence time of the drug within the tissue, reflecting the duration of action or therapeutic effect). These compounds also present variations in hepatic metabolization (hydroxilation, glucoronidation, sulphatation) and in renal excretion of inactive metabolites (20% in the free nonconjugate form). Glucocorticoids are lipophilic steroids with bioavailability between 60 and 100% when administered orally. Most are succinate or phosphate esters, whose conversion to the active form occurs between 5 and 30 min after intravenous injection. Plasmatic concentration depends mostly on the capacity to bind to serum proteins, such as transcortin e albumin.
Pediatricians must also be alert to possible drug interactions (Table 2) for glucocorticoids, which can aggravate intrinsic diseases or bring on potentially preventable side effects.
Definitions and concepts
Some definitions and concepts3 are essential for understanding therapeutic effects and possible undesirable side effects of glucocorticoids:
-Duration of action: short (up to 12 h); intermediate (12-36 h); long (> 36 h);
-Duration of treatment: short term (< 10 days); intermediate term (10-30 days); long term (> 30 days);
-Therapeutic schedule: single dose (morning or evening); fractionated dose (2-4 times per day); alternate daily dose (every other day); mini-pulse therapy (2.5 mg/kg methylprednisolone); pulse therapy (10-20 mg/kg methylprednisolone);
-Therapeutic dose: replacement (7-10 mg/m2/day hydrocortisone); low (< 5 mg prednisone/m2/day; saturation of < 50% of receptors); medium (5-20 mg prednisone/m2/day; saturation between 50-100% of receptors); high (> 20 mg prednisone/m2/day; saturation of 100% of receptors). Very high doses (> 50 mg prednisone/m2/day) and pulse therapy (> 150 mg prednisolone/m2) present additional nongenomic effects;
-Stress dose: mild and moderate stress (2 x replacement dose, via an oral, intramuscular or intravascular route); severe stress (5 x substitutive dose, intramuscular or intravascular); shock (10-15 x substitutive dose, intravascular bolus, followed by continuous maintenance).
Therapeutic indications
Glucocorticoids have a broad spectrum of therapeutic indications1-3 and may be administered as replacement therapy in cases of adrenocorticoid insufficiency or in diseases such as Cushing' s syndrome. They may also be used in the acute treatment of both hypoglycemia and hypercalcemia. They can induce cell maturation (type II pneumocyte), cell differentiation (neural crest lineages) or even cell death (apoptosis), thus allowing their use in the treatment of tumors, especially hematopoietic lineage tumors. However, glucocorticoids play central roles in the treatment of diseases involving immune and inflammatory mechanisms.
Hypoglycemia
Glucocorticoids have a hyperglycemic effect as they provide a combined action both reducing peripheral use of glucose (as they reduce insulin sensitivity) and increasing glucose production by stimulating both glucogenolysis and gluconeogenesis. These effects are associated with muscular proteolysis and with heterogeneous lipid metabolism abnormalities, combining areas of lipolysis and lipogenesis (especially visceral).
Hypercalcemia
Glucocorticoids can reduce calcemia. Acutely, they induce calcium redistribution from intravascular to intracellular space. In the medium and long term, they reduce osteoblastic activity, intestinal absorption and renal calcium resorption.
Tumors
Glucocorticoids can modulate cell proliferation,4 as they reduce the expressions of the heterodimer jun-fos and c-myc, among other transcription factors that decide on cell overlife and multiplication, determining the pause between G1 phase and S phase of the cell cycle. They can also induce apoptotic cell death, culminating in the activation of proteins with nuclear activity involved in the degradation of DNA, RNA and other structural cell proteins.
Inflammatory response and immunomodulation
They are the main indications for glucocorticoids.5,6 Glucocorticoid action on the immune system takes place at various points, culminating in deviation toward a T helper 2 (Th2) type response, with anti-inflammatory characteristics dependent on the increase of cytokines such as interleukins IL, IL4, IL5, IL6, IL10, IL13, and in the granulocyte-macrophage colony-stimulating factor (GMSF). It also induces transforming growth factor (TGFb) secretion, which is able to reduce lymphocyte T activation and cell proliferation. Glucocorticoids are able to inhibit pro-inflammatory cytokines, such as interleukins IL2 and IL12, interferon gamma (INFy) and tumor necrosis factor (TNFa), as well as adhesion molecules such as lipocortin-1, vascular adhesion molecules (VCAM-1) and intercellular adhesion molecules (ICAM), and also enzymes, such as inducible nitric oxide synthase (INOS), cyclooxygenase (COX2) and phospholipase (PLA2). One of the main mechanisms of modulating action of glucocorticoids on the inflammatory process acts on the expression rate of transcription factors, such as nuclear factor kappa B (NFkB), inhibitory protein for NFkB (IkB), and IkB protein kinase (IKK). Nongenomic glucocorticoid effects are also present, determining histamine decrease of action, and reduction in prostaglandin synthesis (reducing phospholipase A2) and in plasminogen activation. In appreciating these mechanisms, it is important to understand the molecular mechanisms of glucocorticoid action (Figure 2) and its main interactions in cellular transduction pathways.7 This is the only possible way to understand the main implications of glucocorticoid use in the death or proliferation of cells (Figure 3), as well as in the modulation of inflammatory response (Figure 4).8
Undesirable side effects
There is a long list of undesirable side effects1,2 associated with corticotherapy, usually related to duration of treatment and use of longer-acting glucocorticoids.
Alterations in fat distribution
Centripetal obesity, moon facies, buffalo hump, supraclavicular fat deposition.
Musculoskeletal system
Osteoporosis, bone fractures, weakness, myopathy, proximal muscle atrophy; aseptic necrosis of femoral and humoral heads.
Hypophyseal/gonadal dysfunction
Menstrual disorders, decreased libido, impotence, hypothyroidism, growth failure and short stature (children).
Cutaneous manifestations
Violaceous striae, plethora, hyperpigmentation, hirsutism or hypertrichosis, acne, ecchymosis.
Endocrine-metabolic system
Suppression of HPA axis, growth failure (in children), carbohydrate intolerance (insulin resistance, hyperinsulinemia, abnormal glucose tolerance, diabetes mellitus); cushingoid features (moon facies, facial plethora, central obesity, buffalo hump, acne, thin and fragile skin, violaceous striae), menstrual disorders, impotence, hypokalemia; metabolic alkalosis; renal calculosis.
Gastrointestinal system
Gastric irritation, peptic ulcer; acute pancreatitis (rare); fatty infiltration of liver and hepatomegaly (rare).
Hemopoietic system
Leucocytosis (with neutrophilia); lymphocytopenia; eosinopenia; monocytopenia.
Imunne system
Suppression of delayed hypersensitivity; suppression of the primary antigen response; suppression of the T helper 1 (Th1) lymphocyte function and Th2 predominance.
Ophthalmic
Posterior subcapsular cataracts (more common in children); elevated intraocular pressure; glaucoma; central serous choroidopathy.
Neuropsychiatric disorders
Sleep disturbances and insomnia; irritability; euphoria and depression; mania and psychosis; pseudotumor cerebri (benign increase of intracranial pressure).
Renal system
Nephrocalcinosis; nephrolithiasis; uricosuria; euphoria (emotional lability), insomnia, psychosis.
Cardiovascular system
Arterial hypertension; myocardial infarction (rare); cerebrovascular accident (rare).
Most common signs and symptoms of endogenous Cushing's syndrome
Arterial hypertension; acne and hirsutism; menstrual disorders; striae; ecchymosis; plethora.
Virtually exclusive signs and symptoms of exogenous administration
Intracranial hypertension; glaucoma; cataracts; aseptic bone necrosis; pancreatitis; panniculitis.
Signs and symptoms of both conditions
Obesity; osteoporosis; myopathy; carbohydrate intolerance; psychiatric manifestations; poor wound healing.
A summary of the main undesirable side effects is shown in Table 3.
Among the undesirable side effects of glucocorticoids in children and adolescents, we can point out the following:
-Suppression of hormone axis, especially HPA axis, with serious implications during abrupt or inadvertent glucocorticoid withdrawal.
-Vascular effects including endothelial abnormalities and vascular permeability and tonus alterations, which, considered together, are involved in the appearance of arterial hypertension and features of vasculitis.
-Neurological alterations associated with central nervous system (CNS) vasculitis and interference with neurotransmitters involved in appetite, sleep and behavioral alterations frequently observed during corticotherapy.
-Growth retardation: glucocorticoids produce important suppressing effects on the somatothrophic axis. In long term treatments, they reduce the hypophyseal secretion of the growth hormone (GH) and its capacity to generate IGF-I in hepatic and osteocartilaginous levels; they also promote increase in IGF-I transport proteins, such as IGFBP1, reducing IGF-I bioavailability. As a final effect, we can observe reduction of IGF-I local concentration and GH action on growth cartilage. These effects are aggravated by the inhibiting action of glucocorticoids on the growth cartilage, preventing maturation of resting cells (GH dependent) and cell division in the proliferative layer (IGF-I dependent).
-Bone mass: bone mass loss is one of the main chronic complications in treatment with glucocorticoids, due to unbalanced bone turnover caused by reduction in bone synthesis and increase in bone resorption. A small amount of bone synthesis depends on decreased calcium availabilty and decreased glucocorticoid-induced osteoblastic activity. The increase in bone resorption is secondary to the increase in osteoclastic number and adhesivity, as well as to the increase in the secretion of the parathyroid hormone (PTH). Glucocorticoids reduce osteoblasts overlife and interfere in the osteoblast signaling to the osteoclast, preventing adequate bone rebuilding where bone resorption is more active.
-Obesity and metabolic syndrome:9-11 increased appetite and predominantly visceral lipogenesis are common findings in patients using glucocorticoids, partially related to insulin resistance. The clinical features of iatrogenic Cushing's syndrome have much in common with the metabolic syndrome features (centripetal obesity, glucose intolerance or diabetes mellitus, dyslipidemia and arterial hypertension, increasing the risk of cardiovascular events).
-Imunossuppression: anti-inflammatory and immunomodulating effects are among the main therapeutic tools offered by glucocorticoids, as they reduce antigen exposition to the immune system, diminish release of pro-inflammatory cytokines and efficaciously eliminate the aggressor agent and the infected cells. Therefore, when this is a long or intense action, we are at increased risk of developing infections, both in number and severeness.
-Disproportionate cell death: part of the therapeutic efficacy of glucocorticoids is due to their capability of reducing cell proliferation rate and inducing apopotic cell death. This is an important mechanism against neoplastic cells, however these are unspecified effects and can also reach non-neoplastic cells. These actions can be observed in hematopoietic cells, collagen- and elastin- producing cells, epidermic cells, digestive and respiratory tract mucosal cells, muscle cells, etc. Thus, common findings are lymphopenia, thin skin with striae, mucosal eruptions, peripheral myopathy, dilated myocardiopathy, etc.
Minimizing side effects
The general principles that should be followed to minimize undesirable side effects of corticotherapy include: a) strict indication for the use of glucocorticoids must prove essential; b) avoiding the use of long-acting glucocorticoids, using short- and intermediate-acting glucocorticoids, such as hydrocortisone and prednisone or prednisolone; c) shortening the treatment to the minimum necessary duration, as 5- to 7-day treatments show few side effects and quick recovery of hypothalamic-hypophyseal axis; d) preferring glucocorticoids with local activity, such as inhaled glucocorticoids (Table 4); e) association with other pharmaceuticals, especially with other more specific anti-inflammatories or immunossupressors, aiming at synergic effects in order to avoid the use of glucocorticoids or to reduce dosage and duration of corticotherapy; f) offering the minimum effective dose, respecting the individual patient sensitivity to glucocorticoids. Clinical experience shows great variation in this sensitivity.12-14 We are provided with a broad spectrum, from complete resistance to the effects of glucocorticoids, even following high-dose long-term administration, to features of hypersensitivity with massive cell death. In vivo and in vitro tests have been carried out with the purpose of recognizing individual patient sensitivity to glucocorticoids, thus allowing adequate adjustment of dose.
Even optimizing corticotherapy, some undesirable side effects will be present and need specific measures:
-Growth: reduction in longitudinal growth is a frequent corticotherapy complication and depends on the action of the glucocorticoids on the somatothrophic axis. The use of the GH has been able to revert or prevent low growth speed in patients with renal insufficiency (pre- or post-transplant), nephrotic syndrome or chronic inflammatory diseases, such as juvenile rheumatoid arthritis. In these situations, the GH is able to increase hepatic generation of IGF-I and IGFBP3, increase bone IGF-I concentration and antagonize the local glucocorticoid effects on the growth cartilage level.
-Bone mass: osteopenia or osteoporosis are possible complications during chronic administration of glucocorticoids. Prevention should be attempted, respecting the general principles of corticotherapy. Additional calcium supplement or concomitant use with vitamin D have been approved for children and adolescents receiving treatment, but their efficacy is discussible, and they might cause hypercalciuria and urinary tract calculosis. Adequate exercise should be highly recommended as an indispensable weapon against bone and muscle loss. The use of bisphosphonates, decreasing excessive bone resorption, is a viable palliative alternative for a limited period of time. The use of the GH can minimize catabolic effect on bones and prevent bone mass loss.
-Insulin resistance and metabolic syndrome: increased appetite associated with glucocorticoid-induced metabolic alterations sets a tendency toward weight gain, aggravating insulin resistance induced by the medication. Preventive and therapeutic measures to fight obesity should be considered as soon as treatment with glucocorticoids is indicated. Strict surveillance on glucose tolerance is necessary, especially in adolescents.
Glucocorticoid withdrawal
Weaning patients from corticotherapy should be a planned step, since inadequate glucocorticoid withdrawal can reactivate the disease under therapy or trigger an adrenal insufficiency crisis due to long suppression of the HPA axis (anorexia, fatigue, nausea, abrupt weight loss, arthralgia, muscle weakness and myalgia, arterial hypotension and hypoglycemia).
In short term treatments (< 10 days), irrespective of dosage or type of corticoid, cessation of corticotherapy should be abrupt, shortening total length of therapy and diminishing side effects.
In intermediate term treatments (10-30 days), glucocorticoids should be withdrawn over a period of 2 weeks, with dose reduction every 4 days.
In long term treatments, some principles for dose reduction should be observed prior to medication withdrawal: a) switch to short- or intermediate-acting glucocorticoids; b) reduce number of doses, aiming at once-a-day dosing in the morning; c) gradual glucocorticoid dose reduction (protocol by Samuels, Table 5).
In the end of the protocol for dose reduction, HPA axis testing can be performed with morning dosage of serum cortisol. Rates greater than 10 mcg/dL indicate adequate recovery of the axis and allow glucocorticoid withdrawal. Rates less than 5 mcg/dL indicate suppressed axis and need of dose reduction, with an additional waiting period of 2-4 weeks before cessation. Cortisol concentration kept between 5 and 10 mcg/dL might require adrenocorticothrophic hormone stimulation test (Synacthen, 1 mcg/m2 intravascular bolus) to ensure adrenal recovery and adequate endogenous cortisol production in the face of stressful situations.
References
- 1. Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill; 2007. p. 1587-612.
- 2. Guyton AC, Hall JE. Textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders: 2006.
- 3. Faria CD, Longui CA. Esteroidogênese adrenal. In: Monte O, Longui CA, Calliari E, Kochi C, editores. Endocrinologia para o pediatra. 3Ş ed. Rio de Janeiro: Atheneu; 2006. p. 253-68.
- 4. Longui CA, Santos MC, Formiga CB, Oliveira DV, Rocha MN, Faria CD, et al. Antiproliferative and apoptotic potencies of glucocorticoids: nonconcordance with their antiinflammatory and immunosuppressive properties. Arq Bras Endocrinol Metabol. 2005;49:378-83.
- 5. Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125:697-706.
- 6. Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125-63.
- 7. Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions: an introduction. Ann N Y Acad Sci. 2004;1024:1-8.
- 8. Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death-a new approach to cancer therapy. J Clin Invest. 2005;115:2625-32.
- 9. Paterson JM, Morton NM, Fievet C, Kenyon CJ, Holmes MC, Staels B, et al. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci USA. 2004;101:7088-93.
- 10. Duclos M, Marquez Pereira P, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157-66.
- 11. Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85:457-67.
- 12. Longui CA, Giusti MM, Calliari LE, Katiki T, Kochi C, Monte O. Partial glucocorticoid resistance in obese children detected by very low dose dexamethasone suppression test. J Pediatr Endocrinol Metab. 2003;16:1277-82.
- 13. Hindmarsh PC, Brook CG. Single dose dexamethasone suppression test in children: dose relationship to body size. Clin Endocrinol (Oxf). 1985;23:67-70.
- 14. Melo MR, Faria CD, Melo KC, Rebouças NA, Longui CA. Real-time PCR quantitation of glucocorticoid receptor alpha isoform. BMC Mol Biol. 2004;5:19.
Publication Dates
-
Publication in this collection
17 Dec 2007 -
Date of issue
Nov 2007