Abstracts
OBJECTIVE: To determine the impact of speech therapy on asthma and allergic rhinitis control in mouth breathing children and adolescents. METHODS: This was a quasi-experimental randomized study of 24 mouth breathing patients with asthma and allergic rhinitis, aged from 6 to 15 years. All patients were taking beclomethasone diproprionate through oral inhalation at the start of the study. At enrollment on the study, oral inhalation was substituted with exclusively nasal inhalation and 1 month later half of the patients began speech therapy. They attended 16 speech therapy sessions in 8 weeks and continued taking beclomethasone dipropionate through exclusively nasal inhalation (BDT group). The comparison group received only beclomethasone diproprionate through exclusively nasal inhalation (BDI group). Both groups were assessed five times. Clinical scores were calculated for allergic rhinitis and asthma, an adapted version of the Marchesan orofacial myofunctional assessment protocol was applied, and parents/guardians' observations were recorded, in addition to spirometry measurements of peak inspiratory and peak expiratory flow. RESULTS: There were significant improvements in the BDT group: clinical asthma score at T5 (p = 0.046); peak inspiratory flow at T4 (p = 0.030); peak expiratory flow at T3 (p = 0.008); breathing mode and lip position (p = 0.000) from T3 onwards; and parents/guardians' observations at T2, T4, and T5 (p = 0.010; p = 0.027; p = 0.030). CONCLUSIONS: Speech therapy in combination with beclomethasone diproprionate through exclusively nasal inhalation resulted in earlier and longer-lasting clinical and functional control of asthma, allergic rhinitis, and mouth breathing than was achieved in the group that only took beclomethasone diproprionate.
Myofunctional therapy; asthma; allergic rhinitis; mouth breathing
OBJETIVO: Detectar o impacto do tratamento fonoaudiológico no controle da asma e da rinite alérgica em crianças e adolescentes respiradores orais. MÉTODOS: Trata-se de um estudo quase-experimental; foram randomizados 24 pacientes com asma, rinite alérgica e respiração oral, idade de 6 a 15 anos. Todos os pacientes usavam dipropionato beclometasona inalação oral. No momento em que aceitaram participar da pesquisa, a inalação oral foi substituída pela inalação exclusivamente nasal na inspiração e, após 1 mês, associou-se ao tratamento fonoaudiológico em metade dos pacientes. Esses receberam 16 sessões de tratamento fonoaudiológico em 8 semanas, além do dipropionato de beclometasona inalação exclusivamente nasal (grupo DBF). O grupo de comparação recebeu somente dipropionato beclometasona inalação exclusivamente nasal (grupo DBI). Os dois grupos foram avaliados em cinco tempos. Utilizou-se o escore clínico da rinite alérgica, da asma, o protocolo de avaliação miofuncional orofacial adaptado de Marchesan (2003), a observação dos responsáveis, dados de espirometria, de pico de fluxo inspiratório e de pico de fluxo expiratório. RESULTADOS: Houve melhora significativa do grupo DBF: escores clínicos da asma no tempo 5 (p = 0,046); valores do pico de fluxo inspiratório no tempo 4 (p = 0,030); pico de fluxo expiratório no tempo 3 (p = 0,008); modo respiratório e postura de lábios (p = 0,000) a partir do tempo 3; observação dos responsáveis, no tempo 2, tempo 4 e tempo 5 (p = 0,010; p = 0,027; p = 0,030). CONCLUSÕES: O tratamento fonoaudiológico associado ao dipropionato beclometasona por inalação exclusivamente nasal promoveu um controle clínico e funcional mais precoce e duradouro da asma, da rinite alérgica e da respiração oral entre os grupos estudados.
Terapia miofuncional; asma; rinite alérgica; respiração bucal
ORIGINAL ARTICLE
The impact of speech therapy on asthma and allergic rhinitis control in mouth breathing children and adolescents
Silvia M. A. CampanhaI; Maria J. F. FontesII; Paulo A. M. CamargosIII; Lincoln M. S. Freire (in memoriam)IV
IMestre, Ciências da Saúde, Instituto de Previdência dos Servidores do Estado de Minas Gerais, Brazil, Professora, Graduação, Fonoaudiologia, Faculdade de Estudos Administrativos, Centro de Gestão Empreendedora, Brazil
IIProfessora adjunta, Pediatria e Pneumologia Pediátrica, Faculdade de Medicina, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil, Professora, Pós-Graduação, Ciências da Saúde da Criança e do Adolescente, Faculdade de Medicina, UFMG, Belo Horizonte, MG, Brazil
IIIProfessor titular, Departamento de Pediatria, Faculdade de Medicina, UFMG, Belo Horizonte, MG, Brazil, Coordenador, Unidade de Pneumologia Pediátrica, Hospital das Clínicas, UFMG, Belo Horizonte, MG, Brazil
IVDoutor, Presidente, Academia Mineira de Pediatria, Membro, Colegiado de Pós-Graduação, UFMG, Belo Horizonte, MG, Brazil, Membro, Instituto de Previdência dos Servidores do Estado de Minas Gerais, Brazil, Professor associado, Departamento de Pediatria, Faculdade de Medicina, UFMG, Belo Horizonte, MG, Brazil
ABSTRACT
OBJECTIVE: To determine the impact of speech therapy on asthma and allergic rhinitis control in mouth breathing children and adolescents.
METHODS: This was a quasi-experimental randomized study of 24 mouth breathing patients with asthma and allergic rhinitis, aged from 6 to 15 years. All patients were taking beclomethasone diproprionate through oral inhalation at the start of the study. At enrollment on the study, oral inhalation was substituted with exclusively nasal inhalation and 1 month later half of the patients began speech therapy. They attended 16 speech therapy sessions in 8 weeks and continued taking beclomethasone dipropionate through exclusively nasal inhalation (BDT group). The comparison group received only beclomethasone diproprionate through exclusively nasal inhalation (BDI group). Both groups were assessed five times. Clinical scores were calculated for allergic rhinitis and asthma, an adapted version of the Marchesan orofacial myofunctional assessment protocol was applied, and parents/guardians' observations were recorded, in addition to spirometry measurements of peak inspiratory and peak expiratory flow.
RESULTS: There were significant improvements in the BDT group: clinical asthma score at T5 (p = 0.046); peak inspiratory flow at T4 (p = 0.030); peak expiratory flow at T3 (p = 0.008); breathing mode and lip position (p = 0.000) from T3 onwards; and parents/guardians' observations at T2, T4, and T5 (p = 0.010; p = 0.027; p = 0.030).
CONCLUSIONS: Speech therapy in combination with beclomethasone diproprionate through exclusively nasal inhalation resulted in earlier and longer-lasting clinical and functional control of asthma, allergic rhinitis, and mouth breathing than was achieved in the group that only took beclomethasone diproprionate.
Keywords: Myofunctional therapy, asthma, allergic rhinitis, mouth breathing.
Introduction
Interactions between asthma, allergic rhinitis and mouth breathing cause anatomic and functional changes that affect facial and somatic growth and which are most often diagnosed in children.1 The wide range of different symptoms of mouth breathing mean that patients must be treated by a multidisciplinary team.1-4
The efficacy of treating allergic rhinitis when it is associated with asthma has been widely documented.5 Recovery of upper airway function contributes to asthma control.5
For allergic patients and mouth breathers, the objective of speech therapy is to reestablish breathing through the nose and the respiratory function of the diaphragm while, at the same time, raising patient awareness about using their muscles. The treatment provides the patient with the necessary conditions to maintain nose breathing, which is unquestionably one of the basics of controlling respiratory diseases.6,7
The majority of clinical studies have studied the efficacy of speech therapy for improving the tonus of the orofacial muscles8-10 and for correcting orofacial functions.11 A literature review did not identify any investigations of the role of speech therapy for clinical and functional control of asthma and allergic rhinitis in mouth breathing children and adolescents.
The objective of this study is to investigate the impact of speech therapy on asthma and allergic rhinitis control in mouth breathing children and adolescents.
Methods
This was a randomized quasi-experimental study that selected 24 patients who met the inclusion criteria from among 169 asthmatics children/adolescents treated at the Brazilian national health service's Serviço de Pneumologia Pediátrica Padre Eustáquio in Belo Horizonte, Brazil.
The inclusion criteria were: positive skin test reaction to at least one of the allergens tested, with clinical presentation of allergic rhinitis and mouth breathing, on continuous treatment for persistent asthma with inhaled beclomethasone dipropionate oral spray. The diagnosis of mouth breathing at time of recruitment was based on the criteria of a permanently open mouth and parents' reports of daytime and nighttime breathing mode.11 Patients were excluded if they had moderate to severe adenoid hypertrophy, diagnosed during an ear nose and throat examination or on an X-ray of the cavum showing a reduction of more than 1/3 of the nasopharyngeal air column and nasal septum deviation, nasal polyps or upper or lower airway infections, which could interfere with assessment and treatment of asthma, allergic rhinitis and mouth breathing. Patients were also excluded if they had any type of comorbidity or if their forced expiratory volume in 1 second (FEV1) or FEV1/forced vital capacity (FVC) ratio was less than 40% of the normal figure.
Patients were distributed into groups using the block randomization technique. The experimental group, hereafter the BDT group, had speech therapy and inhaled beclomethasone diproprionate through the nose, while the comparison group, hereafter the BDI group, only received the corticosteroid through exclusively nasal inhalation and did not have speech therapy.
By administering the inhaled corticosteroid through exclusively nasal inhalation, the same corticosteroid dose treats concomitantly asthma and allergic rhinitis in conjunction. The corticosteroid dosage was 500 mcg/day through exclusively nasal inhalation, using a pear-shaped valved spacer with a volume of 650 mL [Flumax®] fitted to a facemask. Patients took the inhaled dosage twice a day throughout the study, inhaling ten times for each dose.5,12 Compliance was monitored by systematically weighing the metered-dose inhalers on an analytic balance.13
The BDT group attended 16 speech therapy sessions covering three stages of treatment: awareness and proprioception of breathing mode and type using an atlas on respiratory physiology and anatomy, nose-blowing technique (Glatzel mirror), nasal lavage, olfactory experiences, massage and stretching of the orofacial, cervical, inspiratory and expiratory muscles; respiratory function training: muscle exercises isometric exercises to strengthen the lips, tongue, cheeks (the orbicularis oris, buccinator and lingual muscles) and, in parallel, correction of patients' habitual postures of body structures correction of lip position when sealed (using paper between the lips, antiallergic patches) and correction of habitual tongue position (chewing gum in the retroincisal region, sucking hard sweets against the palate), so that patients could practice nose breathing; respiratory exercises to stimulate nose breathing (rhythmic inspiration and expiration exercises and/or forced by occluding nostrils alternately) and breathing with ribs diaphragm and abdomen (using a party blower with a paper tube that rolls out when blown, strengthening respiratory muscles Bobath ball and rib cage expansion); correction of chewing and swallowing functions (using a range of food textures and densities). Patients were made aware of their body posture during all exercises. Sessions were individual and took place twice a week, lasting 40 minutes each. The speech therapy was provided by therapists blind to the results of clinical and functional assessments of asthma and allergic rhinitis.
All patients used saline solution in the form of a nasal spray for nasal lavage. No special allergen avoidance recommendations were made.
The study lasted 16 weeks and patients were assessed five times during that period. At the first session (time 1, T1), patients took beclomethasone dipropionate (BDP) through oral inhalation; at T2 patients had been taking BDP through exclusively nasal inhalation for 1 month and the BDT group began speech therapy; at T3 the BDT group had attended eight speech therapy sessions and both groups BDT and BDI had been taking BDP exclusively through nasal inhalation for 2 months; at T4, the BDT group had attended all 16 speech therapy sessions and patients in groups BDT and BDI had been taking BDP through exclusively nasal inhalation for 3 months; at T5 speech therapy had ceased 1 month previously and all patients had been taking BDP via exclusively nasal inhalation for 4 months.
The patients underwent the following clinical and functional assessments: A) asthma evaluation according to a clinical score14 made up of crises, ß2-agonist or systemic corticosteroid use, emergency service visits or hospitalizations, limitations to physical activities and nighttime symptoms - the final score is between 2 and 19 points and classifies asthma as mild (2 to 8), moderate (9 to 14) or severe (15 to 19); B) allergic rhinitis evaluation according to a clinical score,12,15 based on nasal itching, oropharyngeal itching, ocular itching, nasal obstruction, coryza and sneezing - each is scored from zero to three points depending on intensity and the sum of the scores for each item classifies rhinitis as mild (1 to 6), moderate (7 to 12) or severe (> 12 points); C) mouth breathing was assessed using a protocol for orofacial myofunctional assessment2 modified for this study respiratory mode, mouth breathing period and habitual lip position were reported by parents/guardians; respiratory mode and habitual lip position were confirmed by clinical orofacial myofunctional examination; the investigators instructed parents/guardians in order to standardize observations and records.
The functional assessments included measurements of peak expiratory flow (PEF) (Mini-Wright peak expiratory flow meter, Clement Clarke, Harlow, England), peak nasal inspiratory flow (PIF) (In-check-inspiratory flow meter, Clement Clarke, Harlow, England) and FEV1. Peak expiratory flow was measured three times with the patient standing and the highest value was taken for analysis.16 Before measuring PIF, the patient performed their habitual nasal lavage routine, with gentle nose blowing to eliminate nasal secretions. The mask was carefully fitted to the patient's face and the patient was asked to breath in hard through the nose, with the mouth closed and, starting from residual volume, keep breathing until total lung capacity was reached. At least three measurements were taken and the highest value was taken for analysis. Pulmonary function test guidelines15 were followed when measuring FEV1 with a spirometer (B100 Puritan Bennett-Renaissance).
The clinical and functional assessments of asthma and allergic rhinitis were performed at all five consultations, by observers blinded to which group patients were in.
For the statistical analysis, the Anderson-Darling and Shapiro-Wilk tests were used to check for normal distribution. For continuous variables with normal distribution, comparisons between groups (BDT and BDI) at T1 to T5 were performed using Student's t test. Intragroup comparisons at different times were made using Student's t test for paired samples where distribution was normal, and the Wilcoxon nonparametric test where it was not. Categorical variables were analyzed using Fisher's exact test. Results are described as maximum and minimum values plus means and standard deviations (SD). The significance level was set at 5% for all tests.
The research protocol and the free and informed consent form were approved by the Research Ethics Committees of the Belo Horizonte Municipal Health Department and the Universidade Federal de Minas Gerais (UFMG), also in Belo Horizonte.
Results
Twenty-four patients were initially enrolled on the study, however two patients were excluded from the BDT group, one because of incomplete compliance with the protocol and the other because of whooping cough. One patient in the BDT group received a concurrent prescription of systemic corticosteroid and anti-histamine at T3 and T4 and two patients in the BDI group were prescribed prednisone at T2, T3 and T4 because of acute asthma exacerbation.
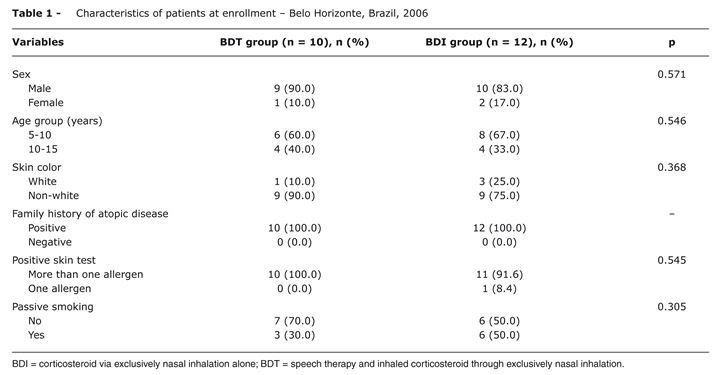
The patients' characteristics at the time of recruitment are given in Table 1.
There were no statistically significant differences between the two groups (p > 0.05). The mean age (mean ± SD) in the BDT group was 9.9±2.23 years and in the BDI group it was 9.41±2.27 years.
Table 2 lists asthma-related clinical and functional characteristics.
At the time of recruitment, there were no statistically significant differences between the groups in terms of the clinical scores for asthma or PEF and FEV1 measurements (p > 0.05). There was a statistical difference between groups at T5 for clinical asthma score (p = 0.046) and at T3 for PEF (p = 0.008). Peak expiratory flow > 80% was only observed in patients in the BDT group.
In the BDT group, the clinical score was reduced from 7.80 at T1 to 2.60 at T5, wile the BDI group's score dropped from 8.58 at T1 to 4.92 at T5 illustrating greater improvement in the BDT group.
Allergic rhinitis-related clinical and functional characteristics are shown in Table 3.
The BDT and BDI groups were comparable at the time of recruitment in terms of clinical score and PIF. A difference between the groups' PIF emerged at T4 (p = 0.030), indicating that the patients in the BDT group were improving faster than those in the BDI group. The mean clinical scores (Table 3) indicated that, at the time of enrollment on the study, all patients in both groups had persistent moderate allergic rhinitis which had already reduced to persistent mild allergic rhinitis by T2 and remained at this level until the end of the observation period. In both groups, the PIF figures increased from T1 to T5: more so in the BDT group (101.5 → 138.0) than in the BDI group (93.3 → 107.9). Of note was the T4 assessment, where mean PIF was significantly greater in BDT than in BDI (p = 0.003).
The clinical and functional characteristics of mouth breathing at enrollment (T1) did not exhibit any statistically significant differences between the two groups, either when data was based on parents/guardians' observations or in terms of the results of the clinical examination.
Table 4 lists the clinical and functional characteristics of mouth breathing from T2 onwards.
There was a statistically significant difference between groups in terms of habitual lip position according to parents/guardians' observations at T2 (p = 0.010), in the BDT group 40% had closed lips, 50% partially open, 10% open, while in the BDI group, 100% had partially open lips; at T4 (p = 0.027), the BDT group had 70% closed lips and 30% partially open and the BDI group had 16.7% open lips and 83.3% partially open; and at T5 (p = 0.030), 80% of the BDT group had closed lips 80% and 20% had partially open lips and in the BDI group 25% had closed lips and 75% partially open.
Habitual lip position according to the clinical examination exhibited statistically significant difference between groups at T3 (p = 0.000), with 90% closed in the BDT group and 10% partially open, while in the BDI group 25% BDT alternated between open and closed, 58.3% had partially open lips and 16.7% open; at T4 (p = 0.000), 90% of the BDT group had closed lips, 10% alternated between open and closed while in the BDI group, 33.3% alternated between open and closed and 66.7% had partially open lips; and at T5 (p = 0.000), 100% of the BDT group had their lips closed and in the BDI group 41.7% alternated between open and closed, 50% had partially open lips and 8.3% still had fully open lips.
There were statistically significant differences in breathing mode according to the clinical examination at T3 (p = 0.002), in the BDT group 80% were nose breathing and 20% oronasally, in the BDI group 91.7% were breathing through both nose and mouth and 8.3% through the nose; at T4 (p = 0.000), 10% of the BDT group were oronasal and 90% nasal breathers and 100% of the BDI group were oronasal; and at T5 (p = 0.000), 10% of the BDT group were still breathing oronasally and 90% were breathing through the nose and the BDI group was still in oronasal breathing.
The mean weights of the inhalers, used to check compliance with BDP treatment, were similar between groups at all follow-up appointments, with no significant differences (p > 0.10).
Discussion
In this study we observed clinical and functional improvement in asthma, demonstrated as early as T2 by the reduction of the mean clinical score and the increase in PEF and FEV1 as percentages of normal values, indicating that nasal inhalation is superior.
Our literature review did not, however, identify any studies of the additional benefits of combining speech therapy with the nasal inhalation technique. The improvement in asthma hyperactivity control and reduction in asthma severity that comes with treating allergic rhinitis has been described in the literature. Some possible mechanisms have been suggested, such as the possibility that reducing nasal obstruction restores physiological breathing mechanisms, with resultant improvements in the quality of inspired air; that controlling nasal inflammation reduces secretion of inflammatory mediators; that the nasobronchial reflex is inhibited; or, less likely, that the dose has systemic effects.15
The efficacy of speech therapy for clinical and functional control of allergic rhinitis was demonstrated here, supporting Parolo & Bianchini,6 who have stated that speech therapy is effective for reducing allergic exacerbations and reducing their frequency.
Habitual lip position and nose breathing were only observed in the BDT group by the clinical examination and by parents' observations. The habitual lip position is lips permanently in contact when at rest, which aids nose breathing.8 One case report of a mouth breathing patient with allergic rhinitis found that the improvements in respiratory/allergic conditions after use of the medication did not alter oronasal breathing and that it was necessary to refer the patient for speech therapy in order to achieve nose breathing.9 in contrast, Henriksen & Wenzel17 observed a reduction in nasal obstruction and mouth breathing after treatment with budesonide in asthmatic patients.
Three studies mentioned the efficacy of speech therapy for orofacial muscles, assessed in terms of the functional changes. One study investigated the effects of speech therapy for correcting the morphology and function of the mentual and orbicular muscles when at rest, with lips closed, in mouth breathing patients.10 Another study observed that speech therapy in associated with the removal of sucking habits presented a better and faster improvement of the swallowing pattern and the tongue rest position.8 In the third study, speech therapy reestablished nose breathing and corrected orofacial musculature in mouth breathing children without organic cause (mouth breathing by habit).11
These studies used a greater number of weekly 30-minute sessions. This differs from our study, in which significant clinical and functional improvements in allergic rhinitis, asthma and mouth breathing were observed in relation to the comparison group after eight individual 40-minute sessions, twice a week.
Junqueira et al. followed a different methodology with respect to the referral of patients for speech therapy, which was only done after 6 months' exclusive use of the medication. In our study, the speech therapy was initiated just 1 month after starting to administer the beclomethasone dipropionate exclusively through nasal inhalation. The speech therapy should be given in parallel with the treatment prescribed by the treating physician.18
This study investigated a diseases with high prevalence rates, especially in developing countries,15,16,19,20 and the very high prevalence of allergic rhinitis and asthma comorbidity,20-22 in combination with the fact that allergic rhinitis has been identified as the main cause of mouth breathing23-26 is a powerful argument for recommending a unified treatment strategy. Patients benefit from first being made aware of nose breathing and then making it automatic, meaning that their respiratory capacity is increased through speech therapy, which is a drug-free technique. The impact can be assessed in terms of the extent to which correct functional use of the airways becomes automatic and, possibly, in terms of the degree of control of asthma and allergic rhinitis.
Both groups had similar compliance with the medication regime. Notwithstanding, the speech therapy contributed to correcting the experimental group's respiratory pattern and led to earlier and longer lasting control of the allergic rhinitis and mouth breathing, having a favorable impact on clinical and functional asthma and allergic rhinitis control in the mouth breathers studied here.
Therefore, speech therapy, combined with beclomethasone dipropionate exclusively through nasal inhalation using a facemask, can be considered as a treatment option for patients with persistent asthma, allergic rhinitis and mouth breathing.
This is a quasi-experimental study because of its limited sample size. Barriers to conducting ideal study designs are inherent to countless research scenarios; this does not however erase the possibility of bias. Studies should be undertaken with larger patient samples and with observers also blinded to the progression of the mouth breathing condition. It is nevertheless worth pointing out that the majority of articles located both in Brazilian and international literature that aimed to detect the efficacy of speech therapy for mouth breathing patients with oral myofunctional disorders had sample sizes that were smaller than or equal to the sample studied here.
Acknowledgements
The authors are grateful to the help received from the pediatric respiratory medicine department at the Hospital das Clínicas, UFMG, and the Unidade de Referência Secundária Padre Eustáquio, particularly from professors Irmgard de Assis and Cássio da Cunha Ibiapina, doctors Geralda Magela Costa Calazans, Alessandra Gazire Alves Affonso, Corina Toscano Sad, physiotherapist Simone Nascimento Santos Ribeiro, and medical students Alexandre Martins da Costa El-Aouar, Joana Mendes Bretas, Érica de Brito D'Andréa and Flávia Albuquerque de Rezende Dutra. The authors would also like to thank professors Dr. Irene Queiroz Marchesan and Dr. Ricardo Godinho for their important comments during the viva for the dissertation that led to this article.
References
- 1. Campanha SM, Freire LM, Fontes MJ. O impacto da asma, da rinite alérgica e da respiração oral na qualidade de vida de crianças e adolescentes. Rev CEFAC. 2008;10:513-9.
- 2. Marchesan IQ. A equipe de trabalho no respirador oral. In: Krakauer HL, Francesco R, Marchesan IQ, editores. Respiração oral: abordagem interdisciplinar. São José dos Campos: Pulso; 2003. p.163-167.
- 3. Shapiro PA. Effects of nasal obstruction on facial development. J Allergy Clin Immunol. 1988;81:967-71.
- 4. Trask GM, Shapiro GG, Shapiro PA. The effects of perennial allergic rhinitis on dental and skeletal development: a comparison of sibling pairs. Am J Orthod Dentofacial Orthop. 1987;92:286-93.
- 5. Camargos PA, Rodrigues ME, Lasmar LM. Simultaneous treatment of asthma and allergic rhinitis. Pediatr Pulmonol. 2004;38:186-92.
- 6. Parolo AM, Bianchini EM. Pacientes portadores de respiração bucal: uma abordagem fonoaudiológica. Rev Dental Press Ortodon Ortop Facial. 2000;5:76-81.
- 7. Rahal A, Krakauer LH. Avaliação e terapia fonoaudiológica com respiradores bucais. Rev Dental Press Ortodon Ortop Facial. 2001;6:83-6.
- 8. Degan VV, Puppin-Rontani RM. Terapia miofuncional e hábitos orais infantis. Rev CEFAC. 2004;6:396-404.
- 9. Junqueira P, Parro FM, Toledo MR, Araújo RL, Di Francesco RD, Rizzo MC. Conduta fonoaudiológica para pacientes com diagnóstico de rinite alérgica: relato de caso. Rev CEFAC. 2005;7:336-9.
- 10. Schievano D, Rontani RM, Bérzin F. Influence of myofunctional therapy on the perioral muscles: clinical and electromyographic evaluations. J Oral Rehabil. 1999;26:564-9.
- 11. Korbmacher HM, Schwan M, Berndsen S, Bull J, Kahl-Nieke B. Evaluation of a new concept of myofunctional therapy in children. Int J Orofacial Myology. 2004;30:39-52.
- 12. Camargos P, Ibiapina C, Lasmar L, Cruz AA. Obtaining concomitant control of allergic rhinitis and asthma with a nasally inhaled corticosteroid. Allergy. 2007;62:310-6.
- 13. Rubin BK, Durotoye L. How do patients determine that their metered-dose inhaler is empty? Chest. 2004;126:1134-7.
- 14. Rosier MJ, Bishop J, Nolan T, Robertson CF, Carlin JB, Phelan PD. Measurement of functional severity of asthma in children. Am J Respir Crit Care Med. 1994;149:1434-41.
- 15. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31:616-24.
-
16Global Iniciative for Asthma - GINA. Global strategy for asthma management and prevention. Washington: National Institutes of Health; 2006.
- 17. Henriksen JM, Wenzel A. Effect of an intranasally administered corticosteroid (budesonide) on nasal obstruction, mouth breathing, and asthma. Am Rev Respir Dis. 1984;130:1014-8.
- 18. Campiotto AR, Reis DM, Gaeta RT. Princípios da intervenção fonoaudiológica nos distúrbios miofuncionais orofaciais. In: Filho OL, organizador. Tratado de Fonoaudiologia. São Paulo: Tecmedd; 2004. p.735-40.
- 19. The International Study of Asthma and Allergies in Childhood - ISAAC. Prevalence of asthma symptoms. Eur Respir J. 1998;12:315-35.
-
20ARIA. Manejo da rinite alérgica e seu impacto na asma: guia de bolso. ARIA no Brasil; 2002. 23p.
- 21. Berger WE. Overview of allergic rhinitis. Ann Allergy Asthma Immunol. 2003;90:S7-S12.
- 22. Fiore RW, Comparsi AB, Reck CL, Oliveira JK, Pampanelli KB, Fritscher CC. Variação na prevalência de asma e atopia em um grupo de escolares de Porto Alegre. J Pneumol. 2001;27:237-42.
- 23. Barros JR, Becker HM, Pinto JA. Evaluation of atopy among mouth-breathing pediatric patients referred for treatment to a tertiary care center. J Pediatr (Rio J). 2006;82:458-64.
- 24. Cintra CF, Castro FF, Cintra PP. As alterações orofaciais apresentadas em pacientes respiradores bucais. Rev Bras Alergia Imunopatol. 2000;23:78-83.
- 25. Sennes LU, Sanches TG. Doenças associadas e complicações da rinite alérgica. In: Castro FFM. Rinite alérgica. São Paulo: Lemos; 1998. p.196-7.
- 26. Abreu RR, Rocha RL, Lamounier JA, Guerra AF. Etiology, clinical manifestations and concurrent findings in mouth-breathing children. J Pediatr (Rio J). 2008;84:529-35.
Publication Dates
-
Publication in this collection
08 July 2010 -
Date of issue
June 2010
History
-
Received
29 July 2009 -
Accepted
27 Jan 2010