Abstracts
OBJECTIVES: To study the effect of passive cigarette smoking on plasma oxidative and antioxidative status in passive smoking preschool children and to compare them with controls. METHODS: Thirty-four passive smoking (five to 50 cigarettes per day) preschool children (study group) and 32 controls who had never been exposed to cigarette smoke were randomly chosen from children aged from 4 to 6 years. Urinary cotinine and plasma indicators of oxidative and antioxidative status, i.e., total oxidant status (TOS), total antioxidant capacity (TAC), and oxidative stress index (OSI), were determined. RESULTS: Mean environmental cigarette consumption was 22±13 cigarettes per day in passive smoking children. Mean urinary cotinine levels were 77.6±41.4 ng/mL and 11.9±2.3 ng/mL in the study and control groups, respectively (p < 0.001). Mean plasma TAC levels were 0.95±0.13 mmol Trolox equivalent/L and 1.01±0.09 mmol Trolox equivalent/L, respectively (p = 0.039). Mean plasma TOS levels were 28.6±7.9 µmol H2O2 equivalent/L and 18.5±6.3 µmol H2O2 equivalent/L, respectively (p < 0.001). Mean OSI levels were 3.08±0.98 arbitrary units and 1.84±0.64 arbitrary units, respectively (p < 0.001). A small amount of cigarette smoke (five to 10 cigarettes per day) causes considerable oxidative stress. There were significant correlations between number of cigarettes consumed and oxidant status and OSI levels. CONCLUSIONS: Passive smoke is a potent oxidant in preschool children. Its deleterious effects are not limited just to heavy passive smoking, but also occur with exposure to small amounts of smoke.
Antioxidants; cotinine; oxidants; passive smoking; preschool children
OBJETIVOS: Estudar o efeito do fumo passivo sobre o estado plasmático oxidativo e antioxidativo em pré-escolares fumantes passivos e compará-los com controles. MÉTODOS: Trinta e quatro pré-escolares fumantes passivos (cinco a 50 cigarros/dia) (grupo de estudo) e 32 controles que nunca estiveram expostos à fumaça de cigarro foram escolhidos aleatoriamente entre crianças de 4 a 6 anos. Foram determinados os níveis de cotinina urinária e de indicadores do estado oxidativo e antioxidativo, isto é, estado oxidante total (EOT), capacidade antioxidante total (CAT) e índice de estresse oxidativo (IEO). RESULTADOS: A média do consumo ambiental de cigarros foi de 22±13 cigarros por dia nas crianças fumantes passivas. Os níveis médios de cotinina urinária foram 77,6±41,4 ng/mL e 11,9±2,3 ng/mL nos grupos de estudo e controle, respectivamente (p < 0,001). Os níveis médios da CAT plasmática foram 0,95±0,13 mmol equivalente de Trolox/L e 1,01±0,09 mmol equivalente de Trolox/L, respectivamente (p = 0,039). Os níveis médios de EOT plasmático foram 28,6±7,9 µmol H2O2 equivalente/L e 18,5±6,3 µmol H2O2 equivalente/L, respectivamente (p < 0,001). Os níveis médios de IEO foram 3,08±0,98 unidade arbitrária e 1,84±0,64 unidade arbitrária, respectivamente (p < 0,001). Uma pequena quantidade de fumaça de cigarro (cinco a 10 cigarros/dia) causa estresse oxidativo considerável. Não houve correlações significativas entre o número de cigarros consumidos e os níveis de estado oxidante e de IEO. CONCLUSÕES: O tabagismo passivo é um potente oxidante em pré-escolares. Seus efeitos deletérios não se limitam apenas tabagismo passivo pesado, mas também ocorrem com a exposição a pequenas quantidades de fumaça.
Antioxidantes; cotinina; oxidantes; fumo passivo; pré-escolares
ORIGINAL ARTICLE
Increased oxidative stress in preschool children exposed to passive smoking
Faruk YıldırımI;Kabil SermetowII; Ali AycicekIII; Abdurrahim KocyigitIV; Ozcan ErelV
IMD. Pediatrician, Sanliurfa Children's Hospital, Pediatrics Clinic, Sanliurfa, Turkey
IIMD. Associate professor, Harran University, Medical Faculty, Pediatrics Department, Sanliurfa, Turkey
IIIMD. Associate professor, Harran University, Medical Faculty, Pediatric Hematology Department, Sanliurfa, Turkey
IVMD. Professor, Harran University, Medical Faculty, Clinical Biochemistry Department, Sanliurfa, Turkey
VMD. Professor, Ataturk Training and Research Hospital, Clinical Biochemistry Department, Ankara, Turkey
Correspondence Correspondence: Ali Aycicek, MD Harran University Medical Faculty Pediatric Hematology Department 63050 Sanliurfa - Turkey Tel.: +90 414 318 30 00-2156 Fax: +90 414 315 11 81 E-mail: ayciceka@hotmail.com
ABSTRACT
OBJECTIVES: To study the effect of passive cigarette smoking on plasma oxidative and antioxidative status in passive smoking preschool children and to compare them with controls.
METHODS: Thirty-four passive smoking (five to 50 cigarettes per day) preschool children (study group) and 32 controls who had never been exposed to cigarette smoke were randomly chosen from children aged from 4 to 6 years. Urinary cotinine and plasma indicators of oxidative and antioxidative status, i.e., total oxidant status (TOS), total antioxidant capacity (TAC), and oxidative stress index (OSI), were determined.
RESULTS: Mean environmental cigarette consumption was 22±13 cigarettes per day in passive smoking children. Mean urinary cotinine levels were 77.6±41.4 ng/mL and 11.9±2.3 ng/mL in the study and control groups, respectively (p < 0.001). Mean plasma TAC levels were 0.95±0.13 mmol Trolox equivalent/L and 1.01±0.09 mmol Trolox equivalent/L, respectively (p = 0.039). Mean plasma TOS levels were 28.6±7.9 µmol H2O2 equivalent/L and 18.5±6.3 µmol H2O2 equivalent/L, respectively (p < 0.001). Mean OSI levels were 3.08±0.98 arbitrary units and 1.84±0.64 arbitrary units, respectively (p < 0.001). A small amount of cigarette smoke (five to 10 cigarettes per day) causes considerable oxidative stress. There were significant correlations between number of cigarettes consumed and oxidant status and OSI levels.
CONCLUSIONS: Passive smoke is a potent oxidant in preschool children. Its deleterious effects are not limited just to heavy passive smoking, but also occur with exposure to small amounts of smoke.
Keywords: Antioxidants, cotinine, oxidants, passive smoking, preschool children.
Introduction
The main adverse effect of active or passive cigarette smoking is due to numerous compounds emitted in gases, many of which are oxidants and pro-oxidants; moreover, enhanced production of reactive oxygen species by smoke is related to increased free radical production, depleted serum antioxidants, and oxidative stress.1-4 Increased oxidant status can result in the oxidation of lipids, induction of DNA single-strand breakage, inactivation of certain proteins, and disruption of biological membranes,2,5,6 which are associated with numerous adverse health effects in fetuses, infants, children, and adults.1,2,7-12
Cotinine, which is the major metabolite of nicotine, is a commonly used indicator to reflect the level of smoking exposure, even though its half-life is less than 24 hours. Urinary cotinine is a good indicator, because it can be easily and accurately measured and is detectable in urine specimens at low concentrations.13
We previously reported that total antioxidant capacity (TAC), total oxidant status (TOS), and oxidative stress index (OSI) in fetal placental tissue, cord blood, and infants' and their mother's serum are altered by active or passive smoking.1,2,8,9 In the present study, we measured cotinine level to determine the level of passive cigarette exposure and evaluated the effect of passive smoking on the levels of total oxidant/antioxidant status in preschool children.
Materials and methods
The study group included 45 preschool children (24 male, 21 female) aged from 4 to 6 years old (mean: 5.2±0.7 years) who had been exposed to at least five cigarettes per day at home (range: 5-45 cigarettes per day; mean: 22±13 cigarettes per day) for at least the past 2 months. The control group consisted of 44 preschool children (26 male, 18 female) aged from 4 to 6 years old (mean: 5.3±0.8) and of similar socioeconomic status. These subjects' mothers reported that the children had never been exposed to passive smoking. Participants were enrolled from Harran University pediatric outpatient clinic and Sanliurfa Children's Hospital pediatric outpatient clinic between February 2010 and April 2010. All subjects were healthy, and none had taken any antioxidant medications (vitamin C, vitamin E, selenium, etc.) or drunk fruit juice prior to or during the study. A brief history was taken and blood samples for complete blood count and renal and liver function tests were obtained from each individual in the study and control groups, and a complete physical examination was performed. Urine samples were collected into a sterile tube. Blood samples were withdrawn into heparinized tubes and plasma was separated from the cells by centrifugation at 3000 rpm for 10 minutes. The urine and plasma samples were stored at -80 °C until required for analysis. The plasma was analyzed for TAC and TOS. Subjects with any signs or symptoms of any acute or chronic illness or with abnormal biochemical test results were excluded from the study.
Eleven patients in the study group were excluded because their urinary cotinine levels were lower than 25 ng/mL (mean: 15.1 ng/mL; range: 10.2-24.9 ng/mL), although their mothers reported that children had been exposed to at least five cigarettes per day. It may be caused by the extent of their cigarette consumption, the rate of smoking, the type of cigarette (filter or non-filter, low tar, with different nicotine content, etc.), the proximity of the non-smoker, the duration of exposure, the magnitude of the space, the home or work ventilation system, the season of the year, and many other variables. Twelve subjects in the control group were excluded because their urinary cotinine levels were higher than 25 ng/mL (mean: 37.6 ng/mL; range: 25.1-59.4 ng/mL), although their mothers reported that the children had never been exposed to passive smoking. The mothers were fully informed about the aim of the investigation and gave consent for their children to be involved in the study. The study was approved by the Harran University Medical Faculty Ethics Committee and the Harran University Scientific Academic Council.
Analytical methods
Urinary cotinine levels were determined by chemiluminescence using a commercial kit (Immulite 2000 nicotine metabolite kit, Diagnostic Products Corporation, Los Angeles, CA, U.S.) with an automated hormone analyzer (Immulite 2000 immunoassay system, Siemens, Chicago, IL, U.S.). Urinary cotinine levels were expressed as ng/mL. The urinary cotinine cutoff level for passive smoking was 25 ng/mL.14 TAC and TOS levels were measured by Erel's methods (Rel Assay Diagnostics, Gaziantep, Turkey), which are automated and colorimetric.15-17 Erel's TOS method is based on the oxidation of ferrous ion to ferric ion in the presence of various oxidant species in acidic medium and on the measurement of the ferric ion by xylenol orange. The results were expressed in µmol H2O2 equivalent/L (H2O2 equiv./L).18 Erel's TAC method is based on the bleaching of the characteristic color of a more stable 2,2'-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) radical cation by antioxidants. The results were expressed in mmol Trolox equivalent/L.19 The percentage of TOS level to TAC level was regarded as the OSI.17 The plasma OSI value was calculated as follows: OSI = ([TOS, µmol H2O2 equiv./L][(TAC, µmol Trolox equivalent/L] × 100).7,17
Statistical analysis
Variables' homogeneity of variance was tested by Levene test. Differences in urinary cotinine and plasma parameters between study and control groups were analyzed by Student t test. Sex ratio was compared using a chi-square test. Bivariate associations between variables were assessed by Pearson's correlation test. The data were expressed as mean ± standard deviation (SD) and differences were considered statistically significant at p < 0.05. Statistical analyses were performed using SPSS for Windows Release 11.5 (SPSS Inc., Chicago, IL, U.S.).
Results
The cotinine-based study group consisted of 34 preschool children (17 male, 17 female) aged 4 to 6 years old (mean: 5.2±0.8 years). The control group consisted of 32 preschool children (19 male, 13 female) aged 4 to 6 years old (mean: 5.3±0.8 years). We found no significant differences between the groups with regard to mean age, body weight, height, body mass index, or male/female distribution (p > 0.05, Table 1).
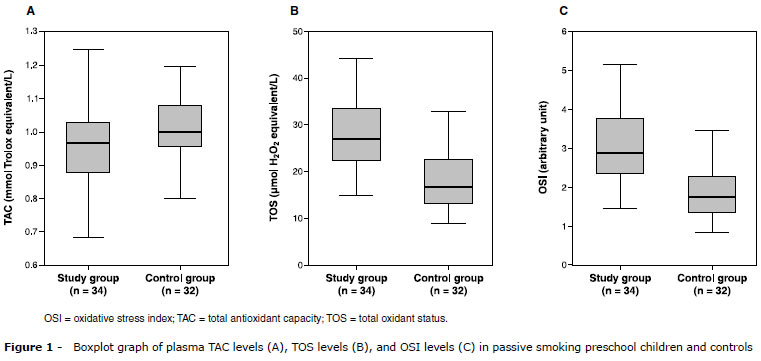
Mean environmental cigarette consumption was 22±13 cigarettes per day in the passive smoking children. Mean urinary cotinine levels were 77.6±41.4 ng/mL (range: 25.7-168 ng/mL) and 11.9±2.3 ng/mL (range: 10.1-22.8 ng/mL) in the study and control groups, respectively (p < 0.001). Plasma TAC, TOS, and OSI levels are given in Table 2. Plasma TAC levels were significantly lower in the study group than in the control group (Figure 1 A). Mean TAC levels were 0.95±0.13 mmol Trolox equiv./L and 1.01±0.09 mmol Trolox equiv./L, respectively (p = 0.039). Conversely, plasma TOS and OSI levels were significantly higher in the study group than in the control group (p < 0.001) (Figure 1 B, 1 C). Mean plasma TOS levels were 28.6±7.9 µmol H2O2 equiv./L and 18.5±6.3 µmol H2O2 equiv./L, respectively. Mean OSI levels were 3.08±0.98 arbitrary units (AU) and 1.84±0.64 AU, respectively. Either a small amount of cigarette smoke (five to 10 cigarettes/day) or a low level of urinary cotinine (25-50 ng/mL) causes important oxidative stress (p < 0.002). A significant positive correlation was found between the number of cigarettes to which children were exposed and TOS and OSI levels (p < 0.001) (Figure 2), but no correlation was found between urinary cotinine and oxidative parameters (p > 0.05).
Discussion
In this study, we found that the oxidative/antioxidative balance underwent a major shift towards the oxidative side in passive smoking preschool children. To the best of our knowledge, all the published studies related to the total oxidant/antioxidant effects of passive smoking are about placental tissue9 and fetal cord blood,8 infants and their mothers,1,2 and school children.10 This is the first report showing an association between increased plasma oxidative status and passive smoking in preschool children.
Exposure to passive smoking is very common and has been implicated as a significant health risk factor and as a habit that has adverse consequences for the establishment and progression of several diseases. Children are especially vulnerable to health risks from such exposure, including upper and lower acute respiratory tract infections, acute and chronic ear infections, exacerbation of asthma, changes in neurodevelopment, behavior problems, and decreased school performance.20,21 Although the underlying mechanisms involved in the pathologies associated with active or passive smoking are arguably still an area of active debate, free radical-induced oxidative damage has been alleged to play a major role in the pathogenesis of numerous smoking-related disorders.6,22,23 Free radicals are capable of directly and indirectly inducing oxidative stress in the body. Free radicals originating from cigarette smoke are considered an important cause of atherosclerosis and cancer.24 Cross et al. reported that tobacco smoke is a rich source of oxidants and reactive oxygen species.25 Our results showed that important changes occur in the TAC in passive smoking preschool children.
The quantities of smoke-derived chemicals (e.g., oxide of nitrogen, nicotine, carbon monoxide, and various carcinogens) in the environment depend on a host of factors, such as the number of smokers, the extent of their cigarette consumption, the rate of smoking, the type of cigarette (filter or non-filter, low tar, with different nicotine content, etc.), the proximity of the passive smoker, the duration of exposure, the magnitude of the space, the home or work ventilation system, the season of the year, and many other variables.26 All these factors, combined with the results of our studies, led us to conclude that further studies are needed to more clearly identify the effects of passive smoking on the oxidant/antioxidant system.
An indicator of cigarette smoke exposure should be measurable and should represent the magnitude, duration, and frequency of exposure.20 Urinary cotinine is a good indicator, because it varies with the strength of the source of exposure, can be easily and accurately measured at an affordable cost, and is detectable in urine specimens at low concentrations.13 In this study, we measured cotinine to determine the level of passive cigarette exposure in preschool children. Parents smoked on average 22 cigarettes per day and children's urinary cotinine levels were on average 77 ng/mL, yet no correlation was found between oxidant status and cotinine level. However, a positive correlation was observed between number of cigarettes smoked and TOS. It seemed to be more pronounced in the number of cigarettes consumed than in the cotinine level. Although maternal report may be less accurate than measured levels of cotinine, no correlation was seen between reported amount of smoking and measurable levels of cotinine. Moreover, in our previous study, no correlation was found between cotinine and other parameters.1
The limitation of the study was that creatinine and specific gravity (relative density) were not measured; both allow differences in urine concentration to be taken into account when determining urinary cotinine concentrations. This study showed that assessing urinary cotinine levels without measuring urinary creatinine or specific gravity levels is not more reliable than assessing parental reports of cigarette exposure levels. Measurements of the biochemical products cotinine and creatinine in urine are often adjusted to account for the influence of hydration. Such adjustments have traditionally relied on creatinine or specific gravity measurements in the sample being assayed, the final values being expressed as a ratio of the biochemical product to creatinine or specific gravity.27 Specific gravity can be measured rapidly, reliably, and inexpensively - characteristics particularly attractive for routine clinical use.27
The values of lipid peroxidation products can be used as an index of oxygen free radical generation. The measurement of TOS provides a sensitive index of lipid peroxidation and oxidative stress.17,19 It has been argued that the increased production of reactive oxygen species associated with smoking may exceed the capacity of the oxidant defense system, resulting in oxidative damage to selected proteins, lipids and DNA.23,28 In infants exposed to passive smoking, several components of the antioxidant defense system have been reported to be impaired as compared with those in non exposed infants.2,9,19,29 Similar to our previous studies, this study showed that TOS levels were significantly higher in passive smokers. In addition to this oxidative marker, OSI, which is a significant marker of both oxidant and antioxidant power, was significantly increased in the passive smoking preschool children.
In conclusion, passive parental smoking is associated with important alterations in plasma oxidant/antioxidant balance and causes potent oxidative stress.
Acknowledgements
We are most grateful to the technical staff of the laboratory and of the outpatient clinic at Sanliurfa Children's Hospital and Harran University Medical Faculty, Clinical Biochemistry Department for their assistance in conducting this study.
References
1. Aycicek A, Erel O, Kocyigit A. Decreased total antioxidant capacity and increased oxidative stress in passive smoker infants and their mothers. Pediatr Int. 2005;47:635-9.
2. Aycicek A, Erel O, Kocyigit A. Increased oxidative stress in infants exposed to passive smoking. Eur J Pediatr. 2005;164:775-8.
3. Fayol L, Gulian JM, Dalmasso C, Calaf R, Simeoni U, Millet V. Antioxidant status of neonates exposed in utero to tobacco smoke. Biol Neonate. 2005;87:121-6.
4. Polidori MC, Mecocci P, Stahl W, Sies H. Cigarette smoking cessation increases plasma levels of several antioxidant micronutrients and improves resistance towards oxidative challenge. Br J Nutr. 2003;90:147-50.
5. Durak I, Elgün S, Kemal Bingöl N, Burak Cimen MY, Kaçmaz M, Büyükkoçak S, et al. Effects of cigarette smoking with different tar content on erythrocyte oxidant/antioxidant status. Addict Biol. 2002;7:255-8.
6. Liu X, Lu J, Liu S. Synergistic induction of hydroxyl radical-induced DNA single-strand breaks by chromium (VI) compound and cigarette smoke solution. Mutat Res. 1999;440:109-17.
7. Arab K, Steghens JP. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Ana Biochem. 2004;325:158-63.
8. Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81-5.
9. Aycicek A, Varma M, Ahmet K, Abdurrahim K, Erel O. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur J Pediatr. 2011;170:645-51.
10. Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol. 2005;100:61-4.
11. Weiss ST, Tager IB, Schenker M, Speizer FE. The health effects of involuntary smoking. Am Rev Respir Dis. 1983;128:933-42.
12. Valenzuela PM, Matus MS, Araya GI, Paris E. Environmental pediatrics: an emerging issue. J Pediatr (Rio J). 2011;87:89-99.
13. Wong GC, Berman BA, Hoang T, Bernaards C, Jones C, Bernert JT. Children's exposure to environmental tobacco smoke in the home: comparison of urine cotinine and parental reports. Arch Environ Health. 2002;57:584-90.
14. Wang X, Tager IB, Van Vunakis H, Speizer FE, Hanrahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26:978-88.
15. Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309-15.
16. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2000;37:277-85.
17. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103-11.
18. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112-9.
19. Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52:7286-92.
20. Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31:14-30.
21. Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003-2006. Pediatrics. 2009;124:1299-305.
22. Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, et al. Enhanced protein glutathion and oxidative stress in cigarette smoking. Free Radic Biol Med. 2004;36:464-70.
23. Rahman I, MacNee W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax. 1996;51:348-50.
24. Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med. 1999;27:1173-81.
25. Cross CE, O'Neill CA, Reznick AZ, Hu ML, Marcocci L, Packer L, et al. Cigarette smoke oxidation of human plasma constituents. Ann NY Acad Sci. 1993;686:72-89.
26. Bartal M. Health effects of tobacco use and exposure. Monaldi Arch Chest Dis. 2001;56:545-54.
27. Haddow JE, Knight GJ, Palomaki GE, Neveux LM, Chilmonczyk BA. Replacing creatinine measurements with specific gravity values to adjust urine cotinine concentrations. Clin Chem. 1994;40:562-4.
28. Yoshie Y, Ohshima H. Synergistic induction of DNA strand breakage by cigarette tar and nitric oxide. Carcinogenesis. 1997;18:1359-63.
29. Becker AB, Manfreda J, Ferfuson AC, Dimich-Ward H, Watson WT, Chan-Yeung M. Breast-feeding and environmental tobacco smoke exposure. Arch Pediatr Adolesc Med. 1999;153:689-91.
Manuscript submitted Jun 09 2011, accepted for publication Aug 28 2011
No conflicts of interest declared concerning the publication of this article.
Suggested citation: Yıldırım F, Sermetow K, Aycicek A, Kocyigit A, Erel O. Increased oxidative stress in preschool children exposed to passive smoking. J Pediatr (Rio J). 2011;87(6):523-8.
- 1. Aycicek A, Erel O, Kocyigit A. Decreased total antioxidant capacity and increased oxidative stress in passive smoker infants and their mothers. Pediatr Int. 2005;47:635-9.
- 2. Aycicek A, Erel O, Kocyigit A. Increased oxidative stress in infants exposed to passive smoking. Eur J Pediatr. 2005;164:775-8.
- 3. Fayol L, Gulian JM, Dalmasso C, Calaf R, Simeoni U, Millet V. Antioxidant status of neonates exposed in utero to tobacco smoke. Biol Neonate. 2005;87:121-6.
- 4. Polidori MC, Mecocci P, Stahl W, Sies H. Cigarette smoking cessation increases plasma levels of several antioxidant micronutrients and improves resistance towards oxidative challenge. Br J Nutr. 2003;90:147-50.
- 5. Durak I, Elgün S, Kemal Bingöl N, Burak Cimen MY, Kaçmaz M, Büyükkoçak S, et al. Effects of cigarette smoking with different tar content on erythrocyte oxidant/antioxidant status. Addict Biol. 2002;7:255-8.
- 6. Liu X, Lu J, Liu S. Synergistic induction of hydroxyl radical-induced DNA single-strand breaks by chromium (VI) compound and cigarette smoke solution. Mutat Res. 1999;440:109-17.
- 7. Arab K, Steghens JP. Plasma lipid hydroperoxides measurement by an automated xylenol orange method. Ana Biochem. 2004;325:158-63.
- 8. Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81-5.
- 9. Aycicek A, Varma M, Ahmet K, Abdurrahim K, Erel O. Maternal active or passive smoking causes oxidative stress in placental tissue. Eur J Pediatr. 2011;170:645-51.
- 10. Kosecik M, Erel O, Sevinc E, Selek S. Increased oxidative stress in children exposed to passive smoking. Int J Cardiol. 2005;100:61-4.
- 11. Weiss ST, Tager IB, Schenker M, Speizer FE. The health effects of involuntary smoking. Am Rev Respir Dis. 1983;128:933-42.
- 12. Valenzuela PM, Matus MS, Araya GI, Paris E. Environmental pediatrics: an emerging issue. J Pediatr (Rio J). 2011;87:89-99.
- 13. Wong GC, Berman BA, Hoang T, Bernaards C, Jones C, Bernert JT. Children's exposure to environmental tobacco smoke in the home: comparison of urine cotinine and parental reports. Arch Environ Health. 2002;57:584-90.
- 14. Wang X, Tager IB, Van Vunakis H, Speizer FE, Hanrahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26:978-88.
- 15. Cao G, Prior RL. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem. 1998;44:1309-15.
- 16. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2000;37:277-85.
- 17. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103-11.
- 18. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112-9.
- 19. Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52:7286-92.
- 20. Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31:14-30.
- 21. Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003-2006. Pediatrics. 2009;124:1299-305.
- 22. Muscat JE, Kleinman W, Colosimo S, Muir A, Lazarus P, Park J, et al. Enhanced protein glutathion and oxidative stress in cigarette smoking. Free Radic Biol Med. 2004;36:464-70.
- 23. Rahman I, MacNee W. Oxidant/antioxidant imbalance in smokers and chronic obstructive pulmonary disease. Thorax. 1996;51:348-50.
- 24. Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med. 1999;27:1173-81.
- 25. Cross CE, O'Neill CA, Reznick AZ, Hu ML, Marcocci L, Packer L, et al. Cigarette smoke oxidation of human plasma constituents. Ann NY Acad Sci. 1993;686:72-89.
- 26. Bartal M. Health effects of tobacco use and exposure. Monaldi Arch Chest Dis. 2001;56:545-54.
- 27. Haddow JE, Knight GJ, Palomaki GE, Neveux LM, Chilmonczyk BA. Replacing creatinine measurements with specific gravity values to adjust urine cotinine concentrations. Clin Chem. 1994;40:562-4.
- 28. Yoshie Y, Ohshima H. Synergistic induction of DNA strand breakage by cigarette tar and nitric oxide. Carcinogenesis. 1997;18:1359-63.
- 29. Becker AB, Manfreda J, Ferfuson AC, Dimich-Ward H, Watson WT, Chan-Yeung M. Breast-feeding and environmental tobacco smoke exposure. Arch Pediatr Adolesc Med. 1999;153:689-91.
Correspondence:
Publication Dates
-
Publication in this collection
01 Feb 2012 -
Date of issue
Dec 2011
History
-
Accepted
28 Aug 2011 -
Received
09 June 2011