Abstract
Objective:
To characterize varicella zoster virus-related deaths and hospitalizations in Brazil before universal vaccination with the tetravalent (measles, mumps, rubella, and varicella) vaccine, attempting to collect baseline data on varicella morbidity and mortality in order to evaluate the impact of the varicella vaccination program.
Methods:
Varicella-associated mortality data were evaluated between 1996 and 2011 and varicella zoster virus-associated hospitalizations between 1998 and 2013. Data were gathered from the Informatics Department of the Unified Health System, considering the International Classification of Diseases, 10th Revision, code B01. All age groups were assessed. Varicella-specific mortality rates were calculated and seasonality of varicella-zoster virus-associated hospitalizations was described.
Results:
There were 2334 varicella deaths between 1996 and 2011, 19.3% in infants aged less than 1 year and 36% in children from 1 to 4 years. In infants under 1 year, varicella mortality rates reached 3.2/100,000/year. In children aged 1–4 years, varicella mortality rates reach 1.64/100,000/year. Average annual mortality rates for varicella in Brazil are 0.88/100,000 in infants under 1 year and 0.40/100,000 in children aged 1–4 years. The total number of hospitalizations associated with varicella zoster virus was 62,246 from 2008 to 2013. Varicella-associated hospitalizations have a seasonal distribution in children, peaking in November. In the elderly, monthly averages of herpes zoster-associated hospitalizations present no significant seasonal variation.
Conclusions:
Varicella is associated, in the pre-vaccine period, to significant morbidity and mortality in Brazil. The universal vaccination program is expected to decrease the disease burden from varicella.
Keywords
Varicella-zoster virus; Varicella; Tetraviral vaccine; Deaths; Hospitalizations
Resumo
Objetivo:
Caracterizar os óbitos e internações relacionados ao vírus varicela-zoster no Brasil antes da vacinação universal com a vacina tetravalente (sarampo, caxumba, rubéola e varicela), tentando coletar dados de referência sobre a morbidez e mortalidade por varicela, para avaliar o impacto do programa de vacinação contra a varicela.
Métodos:
Os dados de mortalidade associada à varicela foram avaliados entre 1996 e 2011 e as internações associadas ao vírus varicela-zoster, entre 1998 e 2013. Os dados foram coletados do Departamento de Informática do Sistema Unificado de Saúde, considerando a Classificação Internacional de Doenças, 10ª Revisão, código B01. Todas as faixas etárias foram avaliadas. Foram calculadas as taxas de mortalidade específicas por varicela e foi descrita a sazonalidade das internações associadas ao vírus varicela-zoster.
Resultados:
Houve 2.334 óbitos por varicela entre 1996 e 2011, 19,3% em neonatos com menos de 1 ano e 36% em crianças de 1 a 4 anos. Em neonatos com menos de 1 ano, as taxas de mortalidade por varicela atingiram 3,2/100.000/ano. Em crianças de 1–4 anos de idade, as taxas de mortalidade por varicela atingem 1,64/100.000/ano. As taxas de mortalidade anuais médias por varicela no Brasil são de 0,88/100.000 em neonatos com menos de 1 ano de idade e 0,40/100.000 em crianças de 1 a 4 anos de idade. O número total de internações associadas ao vírus varicela-zoster foi de 62.246 de 2008 a 2013. As internações relacionadas à varicela apresentaram distribuição sazonal em crianças, com pico em novembro. Em idosos, as médias mensais de internações associadas ao herpes zoster não apresentam variação sazonal significativa.
Conclusões:
A varicela está associada a morbidez e mortalidade significativas no período pré-vacinação no Brasil. O programa de vacinação universal deve diminuir a carga de doença da varicela.
Palavras-chave
Vírus varicela-zoster; Varicela; Vacina tetravalente; Óbitos; Internações
Introduction
Varicella (chickenpox) is an acute, exanthematic and contagious infectious disease that occurs primarily in childhood.11 Bozzola E, Tozzi AE, Bozzola M, Krzysztofiak A, Valentini D, Grandin A, et al. Neurological complications of varicella in childhood: case series and a systematic review of the literature. Vaccine. 2012;30:5785-90. It is caused by varicella zoster virus (VZV), an alpha herpes virus belonging to the herpesviridae family.22 Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-76.–44 Ferreira RA, Pereira AC. Varicela. In: Coura JR, editor. Dinâmica das doenças infecciosas e parasitárias, v. 2, 2 ed. Rio de Janeiro: Guanabara Koogan; 2013. p. 1951-4. After resolution of chickenpox, VZV remains latent in dorsal root spinal ganglia and reactivation can arise at any stage of life, more frequently at an older age, causing herpes zoster.22 Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-76.,33 Arvin AM. Varicella-zoster virus. In: Long S, Pickering L, Prober C, editors. Principles and practice of pediatric infectious disease. Philadelphia: Elsevier Health Sciences; 2012. p. 1035-44.,55 Peña-Rey I, Martínez de Aragón MV, Villaverde Hueso A, Terres Arellano M, Alcalde Cabero E, Suárez Rodríguez B. Epidemiology of varicella in Spain pre- and post-vaccination periods. Rev Esp Salud Publica. 2009;83:711-24.–77 Breuer J, Fifer H. Chickenpox. BMJ Clin Evid. 2011;2011:0912.
Although it is generally considered a mild childhood disease, varicella can be severe in children, adults, and immunocompromised individuals88 Preblud SR. Age-specific risks of varicella complications. Pediatrics. 1981;68:14-7. due to risk of viral dissemination to internal organs, such as lungs, liver, brain, heart, and kidneys. The most frequent complications of varicella are secondary bacterial infections caused by Group A β-hemolyticus Streptococcus or Staphylococcus aureus, usually affecting the skin and soft tissues. Invasive bacterial infections such as pneumonia, arthritis, osteomyelitis, sepsis, and necrotizing fasciitis may be fatal.11 Bozzola E, Tozzi AE, Bozzola M, Krzysztofiak A, Valentini D, Grandin A, et al. Neurological complications of varicella in childhood: case series and a systematic review of the literature. Vaccine. 2012;30:5785-90. Neurological complications such as cerebellar ataxia, encephalitis, meningitis, and vasculitis can also occur.99 Science M, MacGregor D, Richardson SE, Mahant S, Tran D, Bitnun A. Central nervous system complications of varicella-zoster virus. J Pediatr. 2014;165:779-85.
The live attenuated vaccine against varicella was formulated in Japan in 1974.1010 Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288-90. Varicella vaccines, available worldwide, possess the single VZV or are combined with the measles, mumps, and rubella viruses, forming a tetravalent vaccine (MMRV).66 Ozaki T. Varicella vaccination in Japan: necessity of implementing a routine vaccination program. J Infect Chemother. 2013;19:188-95. According to Ozaki, the preventive effect of varicella vaccine was estimated at 75%. In the United States it ranged between 79% and 88% with the first dose, for all forms of the disease.66 Ozaki T. Varicella vaccination in Japan: necessity of implementing a routine vaccination program. J Infect Chemother. 2013;19:188-95.
In 2013, a product development partnership under the Brazilian Ministry of Health involving the pharmaceutical industry enabled the production of MMRV at the National Institute of Immunobiological Technology (Biomanguinhos) of the Oswaldo Cruz Foundation (Fiocruz, RJ, Brazil). Thus, universal vaccination against varicella began in September 2013, in Brazil, through the National Immunization Program (NIP). A single dose of MMRV is administered at the age of 15 months; for infants receiving the MMR (measles, mumps, and rubella), at age 12 months.
Universal vaccination is likely to change the epidemiology of varicella in Brazil, requiring baseline data to evaluate the impact of the varicella vaccination program. This study aims to describe the frequency and seasonal distribution of varicella-associated deaths, as well as the frequency of VZV-related hospitalizations in the pre-vaccination period in Brazil.
Materials and methods
Varicella-associated mortality data and VZV-associated hospitalizations were gathered from the Informatics Department of the Unified Health System (DataSUS), through the website http://www2.datasus.gov.br/DATASUS/index.php?area=02. On the Health Information page, the Mortality section of Vital Statistics was accessed in order to review varicella-related deaths. The Hospital Morbidity section in Epidemiological Information and Morbidity was accessed in order to assess VZV-related hospitalizations. Varicella-related deaths were verified according to the International Classification of Diseases, 10th Revision (ICD-10) code B01.
VZV-associated hospitalizations were verified according to an uncoded morbidity list (ICD-10). Varicella deaths comprised the period 1996–2011, and hospitalizations for varicella and herpes zoster from 2008 to 2013 were studied. When data collection was performed, the latest period available for death records was prior 2011, and for hospitalizations, before 2013. Moreover, in the period prior to 1996, the age-related data were uneven. The authors considered both varicella and herpes zoster for hospitalizations because DataSUS does not discriminate hospitalization records between varicella and herpes zoster. Varicella-related deaths were evaluated because the disease is more frequent in children.
Varicella-specific mortality in age groups was calculated as the number of varicella deaths in infants aged less than 1 year × 100,000/population less than 1 year, and in children aged 1–4 years × 100,000/population aged 1–4 years in five Brazilian macro-regions (North, Northeast, South, Southeast, and Midwest). Population size used in the denominators was obtained from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística [IBGE]) based on the 1991, 2000, and 2010 censuses and respective annual intercensal projections. Seasonality of VZV-associated hospitalizations was evaluated through the averages and standard deviations of the monthly number of VZV-associated hospitalizations from 2008 to 2013 in the age groups. It was assumed that VZV-associated hospitalizations among infants aged under 1 year and 1–4 years are predominantly related to varicella, while hospitalizations among adults older than 65 years old are mainly caused by herpes zoster. The study was submitted to the Ethics Committee of Oswaldo Cruz Institute and approved with reference number CAAE 27704214.3.0000.5248.
Results
There were 2334 varicella-associated deaths between 1996 and 2011 in Brazil. From these, 19.3% (n = 450) were in infants aged less than one year, 36% (n = 840) were in children aged 1–4 years, 11.7% (n = 273) in children aged 5–9 years, and 33% (n = 771) in patients more than 9 years old. Table 1 shows the average mortality rates for varicella in age groups in distinct Brazilian regions, from 1996 to 2011. In infants under 1 year of age, varicella mortality rates vary in distinct Brazilian regions, from 0 to 3.2/100,000/year. In children aged 1–4 years, varicella mortality rates vary from 0 to 1.64/100,000/year. Average annual mortality rates for varicella in Brazil from 1996 to 2011 were 0.88/100,000 in infants under 1 year and 0.40/100,000 in children aged 1–4 years.
Average year mortality rates for varicella per 100,000 by age group in Brazilian regions, 1996–2011.
The number of VZV-associated hospitalizations by age group in the period before MMRV vaccine introduction in Brazil from 2008 to 2013 is represented in Fig. 1. The majority of hospitalizations emerged in children under 9 years of age, with peaks in the age group 1–4 years and in the elderly after 80 years of age. The present study demonstrates that the frequency of hospitalizations for herpes zoster ranges from 4378, 5084, and 5151 cases per month in Brazil in the age groups 60–69, 70–79, and over 80 years old, respectively. The total number of hospitalizations associated with VZV was 62,052, and varicella-associated hospitalizations had a seasonal distribution in children, peaking in November.
Varicella-zoster virus hospitalizations by age groups before tetraviral vaccine introduction in Brazil, 2008–2013.
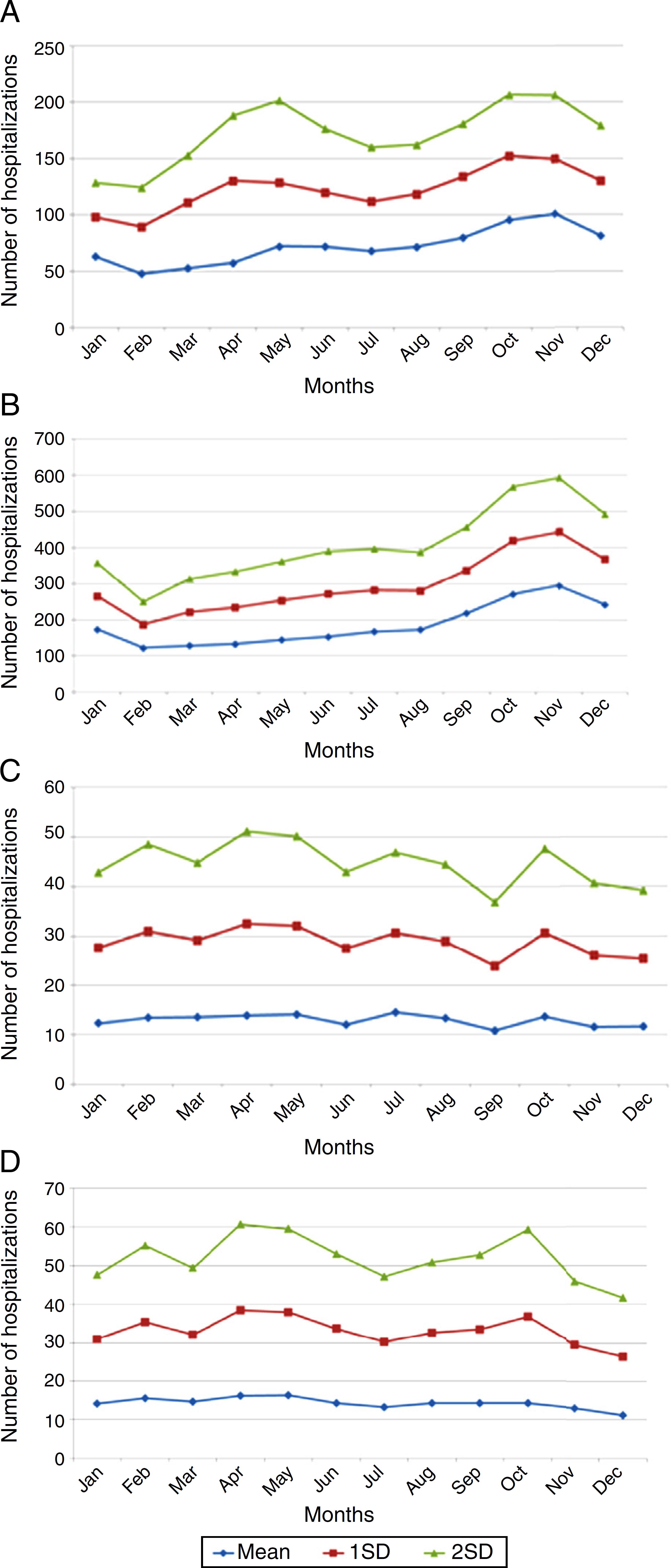
In adults older than 65 years, monthly averages of herpes-zoster-associated hospitalizations presented no variation. Averages and standard deviations of monthly numbers of hospitalizations from 2008 to 2013 are depicted in Fig. 2A–D.
Monthly averages of hospitalizations for varicella in different age categories, showing a seasonal distribution in children (A, B) and no variation in adults (C, D). (A) Infants aged under 1 year. (B) Children aged 1–4 years. (C) Adults aged 65–69 years. (D) Adults aged 75–79 years.
Discussion
This study demonstrated significant morbidity and mortality from varicella in Brazil prior to the introduction of MMRV into the NIP. Data suggest that varicella should not be considered as a benign disease, as it presents frequent complications. Although the majority of deaths were in children aged 1–4 years, the mortality rate is higher in infants aged less than 1 year. Data suggest that the risk of a child dying from varicella is twice as high for infants under 1 year than children aged 1–4 years. However, the MMRV vaccine in the Brazilian NIP is given to children aged 15 months. Thus, varicella would not be prevented in younger infants. In some countries practicing universal varicella vaccination, the vaccine is administered earlier, at 12 months of age, as in the United States, Canada, Japan, China, and Uruguay.66 Ozaki T. Varicella vaccination in Japan: necessity of implementing a routine vaccination program. J Infect Chemother. 2013;19:188-95.,1111 Quian J, Rüttimann R, Romero C, Dall’Orso P, Cerisola A, Breuer T, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch Dis Child. 2008;93:845-50.–1414 Russell ML, Dover DC, Simmonds KA, Svenson LW. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-24. Importantly, the cohort of immunized children will increase progressively over the next years, putatively leading to reduced VZV circulation among immunized children, which will benefit children less than 1 year old by herd immunity, as described by Streng et al.1515 Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and the effects on varicella epidemiology – results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis. 2013;13:303. However, the potential reactivation of VZV in adults represents a continuous repository of wild strains. In this case, children under 15 months old will be susceptible to acquiring the infection.
Although there was a significant reduction in varicella-associated hospitalizations in countries that have adopted universal vaccination, like the United States, outbreaks have still been reported, even among populations with high single-dose coverage. In the United States, the Advisory Committee on Immunization Practices passed the recommendation for routine two-dose varicella vaccination in 2006, which was 10 years after the one-dose varicella vaccination program started in the United States. Moreover, the second dose was recommended not only for outbreaks, but also to increase protection against varicella.1616 Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S, et al. Impact of vaccination on the epidemiology of varicella: 1995–2009. Pediatrics. 2014;134:24-30. Consequences of vaccine failure are potentially serious due to the continuous transmission of wild VZV and the accumulation of susceptible young adults.1717 Gershon AA, Katz SL. Perspective on live varicella vaccine. J Infect Dis. 2008;197:S242-5. Consequently, the need for a second dose of varicella vaccine in some countries has been considered.1818 Prymula R, Bergsaker MR, Esposito S, Gothefors L, Man S, Snegova N, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind, randomised, controlled trial. Lancet. 2014;383:1313-24. Nevertheless, the high morbidity and mortality in children aged 1–4 years in Brazil demonstrates the potential positive impacts of a single MMRV dose given at the age of 15 months.
According to Goldman and King,1919 Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-94. there has been a significant increase in the incidence of herpes zoster over the years following implementation of universal vaccination against VZV in the United States, concomitantly with the decline in varicella incidence. Therefore, it has been argued that the potential increase in the incidence of herpes zoster could nullify the benefits of vaccination.1919 Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-94.,2020 Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis. 2008;197:S224-7. This increase would be related to the low circulation of wild VZV in a vaccinated population, which represents a vaccination booster, increasing the immune response to VZV and preventing viral reactivation in the nervous system.1919 Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-94.,2121 Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500-7. Data from the present study provide a baseline for comparing the post-vaccination period.
Despite the existence of vaccination programs in several developed and developing countries, a question remains: whether or not to vaccinate children against varicella?
Varicella in Brazil cannot be considered a benign disease, since it is responsible for an average of 155 deaths per year, representing almost one death every two days by the disease, generating an average of 34 hospitalizations per day. In the population of children aged 1–4 years, an average of nine hospitalizations per day by the disease has been reported. This scenario leads the authors to believe that varicella in developing countries – in conjunction with factors such as poverty, malnutrition, and lack of access to health care – frequently requires hospitalization, possibly resulting in death. In addition, the indirect costs of the disease must be considered, such as parental work leave and caregiver expense. The estimated average cost of workday loss for a mother with a child under 15 years old with varicella was US$ 5.90, in 2004.2222 Valentim J, Sartori AM, de Soárez PC, Amaku M, Azevedo RS, Novaes HM. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine. 2008;26:6281-91.
In must be emphasized that during 14 years, varicella vaccination was available in private vaccination clinics widely used by the population of the middle and upper classes in Brazil. However, there was circulation of wild VZV in this period, which exposed the vaccinated population to the boosters that prevented weaning of immunity after vaccination.
Varicella had always been associated in the pre-vaccine period to a significant number of deaths and hospitalizations. The introduction of MMRV into the Brazilian NIP generated expectations of a positive impact, especially for children.
Varicella-specific mortality rates vary in different years. These rates in infants under one year are twice as high as those observed in children aged 1–4 years, although the absolute number is greater in the range of 1–4 years. This indicates that a child under 1 year has double the risk of dying from chickenpox when compared to children aged 1–4 years. Varicella-specific mortality rates are higher in the Brazilian Midwest and Southeast. VZV-related hospitalizations have a bimodal distribution, peaking in children under 9 years old and persons older than 80. There is a seasonal pattern in hospitalizations for varicella in children, with a higher monthly average from September to November; this is not observed in adults, which may be related to non-seasonal VZV-reactivation associated with herpes zoster.
This survey has some limitations, because it is a retrospective study using secondary data. Furthermore, the diagnosis of varicella and herpes zoster are eminently clinical and varicella was not a reportable disease in all Brazilian states until September 2013. Therefore, it is possible that the disease is underdiagnosed, especially in country's regions where access to health care programs is still precarious.
Because varicella in Brazil is a disease that leads to almost one death every two days and because it has a worse prognosis in developing countries, the authors conclude that universal varicella vaccination is fully justified in the country. An epidemiologic follow-up of breakthrough varicella and herpes zoster cases that will possibly occur more often in the coming years is essential, and it is vital to discuss the inclusion of a second dose of varicella vaccine in the Brazilian NIP.
-
☆
Please cite this article as: Martino Mota A, Carvalho-Costa FA. Varicella zoster virus related deaths and hospitalizations before the introduction of universal vaccination with the tetraviral vaccine. J Pediatr (Rio J). 2016;92:361–6.
-
FundingThis study was supported with funds from Fiocruz.
References
-
1Bozzola E, Tozzi AE, Bozzola M, Krzysztofiak A, Valentini D, Grandin A, et al. Neurological complications of varicella in childhood: case series and a systematic review of the literature. Vaccine. 2012;30:5785-90.
-
2Heininger U, Seward JF. Varicella. Lancet. 2006;368:1365-76.
-
3Arvin AM. Varicella-zoster virus. In: Long S, Pickering L, Prober C, editors. Principles and practice of pediatric infectious disease. Philadelphia: Elsevier Health Sciences; 2012. p. 1035-44.
-
4Ferreira RA, Pereira AC. Varicela. In: Coura JR, editor. Dinâmica das doenças infecciosas e parasitárias, v. 2, 2 ed. Rio de Janeiro: Guanabara Koogan; 2013. p. 1951-4.
-
5Peña-Rey I, Martínez de Aragón MV, Villaverde Hueso A, Terres Arellano M, Alcalde Cabero E, Suárez Rodríguez B. Epidemiology of varicella in Spain pre- and post-vaccination periods. Rev Esp Salud Publica. 2009;83:711-24.
-
6Ozaki T. Varicella vaccination in Japan: necessity of implementing a routine vaccination program. J Infect Chemother. 2013;19:188-95.
-
7Breuer J, Fifer H. Chickenpox. BMJ Clin Evid. 2011;2011:0912.
-
8Preblud SR. Age-specific risks of varicella complications. Pediatrics. 1981;68:14-7.
-
9Science M, MacGregor D, Richardson SE, Mahant S, Tran D, Bitnun A. Central nervous system complications of varicella-zoster virus. J Pediatr. 2014;165:779-85.
-
10Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288-90.
-
11Quian J, Rüttimann R, Romero C, Dall’Orso P, Cerisola A, Breuer T, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch Dis Child. 2008;93:845-50.
-
12Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-12.
-
13Lu L, Wang C, Suo L, Li J, Liu W, Pang X, et al. Varicella disease in Beijing in the era of voluntary vaccination, 2007 to 2010. Pediatr Infect Dis J. 2013;32:e314-8.
-
14Russell ML, Dover DC, Simmonds KA, Svenson LW. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319-24.
-
15Streng A, Grote V, Carr D, Hagemann C, Liese JG. Varicella routine vaccination and the effects on varicella epidemiology – results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006–2011. BMC Infect Dis. 2013;13:303.
-
16Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S, et al. Impact of vaccination on the epidemiology of varicella: 1995–2009. Pediatrics. 2014;134:24-30.
-
17Gershon AA, Katz SL. Perspective on live varicella vaccine. J Infect Dis. 2008;197:S242-5.
-
18Prymula R, Bergsaker MR, Esposito S, Gothefors L, Man S, Snegova N, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine versus one dose of monovalent varicella vaccine: a multicentre, observer-blind, randomised, controlled trial. Lancet. 2014;383:1313-24.
-
19Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-94.
-
20Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis. 2008;197:S224-7.
-
21Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500-7.
-
22Valentim J, Sartori AM, de Soárez PC, Amaku M, Azevedo RS, Novaes HM. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine. 2008;26:6281-91.
Publication Dates
-
Publication in this collection
Jul-Aug 2016
History
-
Received
16 June 2015 -
Accepted
21 Oct 2015