Abstracts
Data on new specimens of the rare subterranean catfish Phreatobius cisternarum are reported. Specimens were collected from hand-dug wells in the region of the mouth of the Amazon river, Brazil. A revised diagnosis of Phreatobius is proposed, and P. cisternarum is redescribed on the basis of new and previously available material. Included are data on coloration, both in life and preserved, SEM observations of external morphology, morphometric and meristic data. One juvenile specimen, the first known of the species, is reported and illustrated. The new and previously available material reveal considerable intraspecific variation in several morphological traits of P. cisternarum. Despite its rarity in collections, P. cisternarum seems to be common in wells in the vicinity of the city of Belém and on Marajó Island, where many locals know of them. They occur in the superficial phreatic layer, and turn up in hand-dug wells usually from 4 to 13 meters deep. Individual fish hide in orifices of canga rocks at the bottom of wells. Some specimens were maintained in captivity for one-and-a-half year, during which some behavioral observations were recorded. In aquarium conditions, P. cisternarum is an oportunistic predator that feeds vigorously on earthworms and other live invertebrates.
Phreatobius cisternarum; siluriformes; subterranean fishes; fish ecology; feeding habits; behavior; taxonomy
São apresentados dados sobre novos exemplares do raro bagre subterrâneo Phreatobius cisternarum. Os espécimes foram coletados em poços na região da foz do Rio Amazonas, Brasil. Uma diagnose revisada é proposta para o gênero Phreatobius. P. cisternarum é redescrita com base em material novo e previamente disponível. São incluídos dados sobre coloração, tanto em vida quanto em preservação, observações de microscopia eletrônica de varredura da morfologia externa, dados morfométricos e merísticos. Um exemplar juvenil, o primeiro conhecido da espécie, é comparativamente descrito e ilustrado. O material novo e aquele previamente disponível revelam considerável variação intraespecífica em várias características morfológicas de P. cisternarum. A despeito de sua raridade em coleções, P. cisternarum parece ser bastante comum em poços na periferia da cidade de Belém e na Ilha de Marajó, onde muitos habitantes os conhecem. Os peixes ocorrem na camada freática superficial, e aparecem em poços manuais normalmente de 4 a 13 metros de profundidade. Os indivíduos da espécie se escondem em orifícios nas rochas de canga no fundo dos poços. Alguns exemplares foram mantidos por aproximadamente um ano e meio em cativeiro, ocasião em que foram registradas algumas observações comportamentais. Em condições de aquário, P. cisternarum é um predador oportunista que se alimenta vigorosamente de minhocas terrestres e outros invertebrados.
Phreatobius cisternarum; siluriformes; subterranean fishes; fish ecology; feeding habits; behavior; taxonomy
New data on cistern catfish, Phreatobius cisternarum, from subterranean waters at the mouth of the Amazon River (Siluriformes, Incertae Sedis)
Janice Muriel-CunhaI, II; Mário de PinnaI, III
IMuseu de Zoologia, Universidade de São Paulo, Caixa Postal 42494-970, 04218-970, São Paulo, SP, Brasil
IIGraduate Student. E-mail: jcunha@ib.usp.br
IIIProfessor and Director of Science. E-mail: pinna@ib.usp.br
ABSTRACT
Data on new specimens of the rare subterranean catfish Phreatobius cisternarum are reported. Specimens were collected from hand-dug wells in the region of the mouth of the Amazon river, Brazil. A revised diagnosis of Phreatobius is proposed, and P. cisternarum is redescribed on the basis of new and previously available material. Included are data on coloration, both in life and preserved, SEM observations of external morphology, morphometric and meristic data. One juvenile specimen, the first known of the species, is reported and illustrated. The new and previously available material reveal considerable intraspecific variation in several morphological traits of P. cisternarum. Despite its rarity in collections, P. cisternarum seems to be common in wells in the vicinity of the city of Belém and on Marajó Island, where many locals know of them. They occur in the superficial phreatic layer, and turn up in hand-dug wells usually from 4 to 13 meters deep. Individual fish hide in orifices of canga rocks at the bottom of wells. Some specimens were maintained in captivity for one-and-a-half year, during which some behavioral observations were recorded. In aquarium conditions, P. cisternarum is an oportunistic predator that feeds vigorously on earthworms and other live invertebrates.
Keywords:Phreatobius cisternarum, siluriformes, subterranean fishes, fish ecology, feeding habits, behavior, taxonomy.
RESUMO
São apresentados dados sobre novos exemplares do raro bagre subterrâneo Phreatobius cisternarum. Os espécimes foram coletados em poços na região da foz do Rio Amazonas, Brasil. Uma diagnose revisada é proposta para o gênero Phreatobius. P. cisternarum é redescrita com base em material novo e previamente disponível. São incluídos dados sobre coloração, tanto em vida quanto em preservação, observações de microscopia eletrônica de varredura da morfologia externa, dados morfométricos e merísticos. Um exemplar juvenil, o primeiro conhecido da espécie, é comparativamente descrito e ilustrado. O material novo e aquele previamente disponível revelam considerável variação intraespecífica em várias características morfológicas de P. cisternarum. A despeito de sua raridade em coleções, P. cisternarum parece ser bastante comum em poços na periferia da cidade de Belém e na Ilha de Marajó, onde muitos habitantes os conhecem. Os peixes ocorrem na camada freática superficial, e aparecem em poços manuais normalmente de 4 a 13 metros de profundidade. Os indivíduos da espécie se escondem em orifícios nas rochas de canga no fundo dos poços. Alguns exemplares foram mantidos por aproximadamente um ano e meio em cativeiro, ocasião em que foram registradas algumas observações comportamentais. Em condições de aquário, P. cisternarum é um predador oportunista que se alimenta vigorosamente de minhocas terrestres e outros invertebrados.
Palavras-chave:Phreatobius cisternarum, siluriformes, subterranean fishes, fish ecology, feeding habits, behavior, taxonomy.
INTRODUCTION
The subterranean catfish Phreatobius cisternarum is one of the most puzzling catfishes. Its unusual subterranean environment, bright red coloration, aberrant morphology, rarity in collections and uncertain phylogenetic relationships, all make the species an intriguing member of the Siluriformes. Described by Goeldi (1905), P. cisternarum remains the only species in its genus, although other, yet undescribed, species are currently known to exist. It inhabits exclusively the subterranean waters underlying the mouth of the Amazon. Specimens of the species have been collected on areas both north and south of the river mouth, as well as on the huge island in between (Marajó Island). So far, Carvalho (1967) is the only published reference to the biology of P. cisternarum, and is based mostly on observations on a single specimen kept in captivity. A detailed account of the anatomy of the species was published in Reichel (1927). Although it is a superb anatomical treatise, Reichel focused mostly on soft anatomy and had only a few specimens for study.
The phylogenetic position of P. cisternarum is still uncertain. Its aberrant external morphology, which includes several reductive features, has rendered traditional diagnostic characters for catfish families mostly uninformative for the taxonomic placement of Phreatobius. Since its discovery, P. cisternarum has been aligned with at least five different families, alone or in various combinations: Clariidae and Plotosidae (Fuhrmann, 1905, 1906), Trichomycteridae and Cetopsidae (Goeldi, 1905; Eigenmann, 1918; Myers, 1944), and Pimelodidae or Heptapteridae (Reichel, 1927; Myers & Weitzman, 1966; Buckup, 1988; Bockmann, 1998). Most of those proposals have not relied on osteological information, because of the scarcity of material for examination. Bockmann (1998) provided the most thorough investigation so far about the phylogenetic position of Phreatobius and proposed it as sister group to Gladioglanis, of the neotropical family Heptapteridae (formerly a subgroup of Pimelodidae).
The present paper reports on additional material obtained recently as a result of field efforts targeted specifically at P. cisternarum. During a series of collections made in a period of three months in 2003, we obtained a total of 13 specimens, which approximately equals the total number of specimens collected in the preceding 100 years. The new material available forms the basis for a complementary description of P. cisternarum, including a number of morphological and coloration traits not previously reported, information on intraspecific variation, and comparative data on a juvenile specimen, the first known of the species. We also report on the first direct field observations about the habitat of the species and on some behavioral observations done on specimens kept in captivity for approximately 19 months.
Our aim is to provide a source of basic comparative data on P. cisternarum which will help to characterize the species and to understand its peculiar biology. We also hope that the information will facilitate the diagnosis of a number of currently known, yet undescribed, forms assignable to Phreatobius.
MATERIAL AND METHODS
Morphometric data were taken point-to-point with digital calipers to the nearest 0.1 mm. Predorsal, preanal and prepelvic lengths were measured from the tip of mandibular symphysis (which is the anteriomost point of head in Phreatobius) to the base of the first ray of the respective fin. Head width was taken at the middle of head length. Body depth was measured immediately anterior to the origin of dorsal fin. Caudal peduncle length was the distance between the base of the first dorsal procurrent ray to the middle of caudal-fin base. Preorbital length was measured from the middle of upper jaw to the anterior margin of eye. Internarial width was the distance between mesial margins of posterior nares. Principal caudal-fin rays were counted as those directly attached to the hypural plate. Vertebral number included all unfused vertebrae (which in Phreatobius correspond to those bearing a neural spine) plus four anterior ones (three fused in the Weberian complex and a free first one). The compound caudal centrum (PU1 + U1) was counted as one. One specimen was cleared and counter-stained for bone and cartilage according to a slightly modified version of the method of Taylor & Van Dyke (1985). Internal-anatomical data on alcoholic specimens, including vertebral counts, were taken from x-rays made with a Faxitron MX20 digital microradiographic system. The specimen intended for Scanning Electron Microscope observation was the head of a formalin-fixed, alcohol preserved museum specimen. Its head was cut, cleaned of superficial debris in an ultrasound bath, dehydrated in alcoholic series, critical-point dried and gold-coated. Abbreviations are: FMNH (Field Museum, Chicago); MHNG (Muséum d'Histoire naturelle, Geneva); MNRJ (Museu Nacional do Rio de Janeiro, Rio de Janeiro); MPEG (Museu Paraense Emilio Goeldi, Belém); MZUSP (Museu de Zoologia da Universidade de São Paulo, São Paulo); SL (standard length); TL (total length); U (ural centrum); PU (preural centrum).
Material examined
Phreatobius cisternarum, a total of 17 specimens, all from Brazil: MNRJ 11569, 1ex, c&s, 54.0 mm SL, State of Pará, Belém, hand-dug well 1015 m deep, col. R.P. Arlé, Nov/Dec 1962, and 2ex, 30.9 and 39.8 mm SL, State of Amapá, Macapá, hand-dug well, col. R.H.G. Damasceno, Dec 1965 (see notes below); MPEG 3325, 1 ex, 41.3 mm SL, State of Amapá, city of Amapá, Rio Amapá, upstream from Cachoeira Grande, flooded forest, col. M. Goulding, 01 Jan1984; MPEG 7649, 1 ex, 38.5 mm SL, State of Pará, Ananindeua, hand-dug well, col. A.F. Nascimento Filho, 01 Mar 1993; MZUSP 28309, 1 ex, 38.3 mm SL, State of Pará, Ananindeua, cistern, col. J. Batista Feb 1984; MZUSP 84568, 1 ex 44.5 mm SL, State of Pará, Benfica, hand-dug well 4.4 m deep, col. J.M. Cunha & Sr. Nei, 12 Dec 2003; MZUSP 85475, 1 ex 42.0 mm SL; same data as MZUSP 84568; MZUSP 85476, 1 ex, decapitated specimen, head critical-point dried and gold-coated for SEM observation, rest of specimen in alcohol, same data as MZUSP 84568; MZUSP 85469, 1 ex 45.2 mm SL, State of Pará, Marajó Island, Salvaterra, hand-dug well 4 m deep, col. J.M. Cunha & L. Gonçalves 18 Dec 2003; MZUSP 85470, 1 ex 21.8 mm SL; same data as MZUSP 85469; MZUSP 85471, 1 ex 34.0 mm SL; same data as MZUSP 85469; MZUSP 85472, 1 ex, 23.8 mm SL, State of Pará, Marajó Island, Salvaterra, hand-dug well 7.8 m deep, col. J.M. Cunha & S. Colares, 24 Dec 2003; MZUSP 85473 1 ex 44.2 mm SL; same data as MZUSP 85472; MZUSP 85474, 1 ex 44.6 mm SL; same data as MZUSP 85472; MZUSP 88395, 1 ex, 44.7 mm SL, State of Pará, Belém, hand-dug well, no further collection data; MZUSP 88396, 1 ex, 41.3 mm SL, State of Pará, Marajó Island, Salvaterra, hand-dug well, col. J.M. Cunha & J. da Conceição 11 Dec 2003.
Notes on MNRJ 11569
One of the three specimens of P. cisternarum reported by Carvalho (1967) comes from Belém, State of Pará, while two others are from Macapá, State of Amapá. Prof. Carvalho maintained the three preserved specimens in his possession for several years. Upon his passing, the material was transferred to MNRJ, but by that time the specimens were indistinguishably mixed in a single jar. Slightly different preservation characteristics in alcohol distinguished one of the specimens from the other two. That one specimen, now cleared and stained, is inferred to be the one from Belém. The other two, still in alcohol, are therefore inferred to be from Macapá. Such inference cannot be confirmed at present, and the three specimens are maintained in the same MNRJ catalogue number.
Notes on the type-specimens of P. cisternarum
Considerable confusion exists about the type material of P. cisternarum. The original description by Goeldi (1905) mentions two specimens, reportedly from a cistern near the city of Soure, Southeastern Marajó Island, collected in 1903 by Vicente Chermont de Miranda, a prominent figure in the cultural and political life of the State of Pará at the time (Chermont de Miranda, 1968). Four additional specimens from the same spot were subsequently obtained by Goeldi in 1905. Years later, the six specimens were sent to Europe, to the care of O. Furhmann. One of those six was sent to C. Eigenmann, deposited at the Carnegie Museum, and later transferred to the Field Museum (FMNH 58580), where it remains today. Two of the remaining five specimens kept by Fuhrmann were serially sectioned and mounted as a series of microscope slides. In 1923, Fuhrmann sent all the sections plus the three remaining whole specimens to M. Reichel, who used them as basis for his 1927 monograph. Apparently, no action has been taken to separate the original two syntypes from the subsequent four non-type specimens. The five specimens in Europe were eventually deposited at the Muséum d'Histoire naturelle, Geneva. One of the sectioned specimens (MHNG 2623.30, mounted in 12 slides, with number 7 missing) is definitely labeled as a type, but the other syntype can be any one of the remaining five specimens: MHNG 1213.97 (one specimen in alcohol), MHNG 1505.91 (two whole specimens and one sectioned, mounted in 53 slides), and FMNH specimen (Bockmann & Guazzelli, 2003).
Phreatobius Goeldi, 1905
Phreatobius Goeldi, 1905:549 [type-species: Phreatobius cisternarum Goeldi, 1905, by monotypy].
Heptapterus [in part].- Buckup (1988).
Diagnosis
A genus of siluriform diagnosed by the following combination of characters: 1- extended region of dorsal and ventral procurrent caudal-fin rays (4250 rays dorsally and 2226 ventrally), continuous with caudal fin, dorsally occupying position of adipose fin and ventrally continuous with anal fin; 2- caudal fin round; 3- all anal-fin rays unbranched (Bockmann, 1998); 4- mouth prognathous, and with jaws displaced dorsally on head; 5- brigh red color in life; 6- dorsal-fin spine and locking mechanism absent; 7- first pectoral-fin ray soft, not spinous; 8- adductor mandibulae muscle hypertrophied, covering most of skull and posteriorly inserting onto first neural spine (Reichel, 1927); 9- dorsal profile of skull concave in lateral view (Bockmann, 1998); 10- eyes tiny, orbital diameter 26% of HL; 11- eyes located anteriorly on head (preorbital length 1626% of HL); 12- two pleural ribs (Bockmann, 1998); 13- parapophysis of eighth vertebra elongated; 14- opercle narrow and curved dorsally (de Pinna, 1998); 15- single large cranial fontanel, occupying most of skull roof (Reichel, 1927). It is difficult at present to determine which of those characters are autapomorphies for Phreatobius, due to the uncertain familial relationships of the taxon. Most of the traits listed are rare or unusual across vast clades of siluriforms. On the basis of a possible heptapterid affinity and other reasonable possibilities suggested in the literature, characters 1, 4, 5, and 8-15 are likely autapomorphic.
Phreatobius cisternarum Goeldi, 1905
Phreatobius cisternarum Goeldi, 1905:549 [type-locality: Wasser einer Binnenlandzisterne tief im Innern der Mündung des Amazonestromes vorgelagertern Rieseninsel Marajó; 2 syntypes].- Fuhrmann, 1905, 1906 [taxonomic placement].- Eigenmann, 1918 [included in addendum to monograph on Pygidiidae (= Trichomycteridae)].- Reichel, 1927 [redescription, anatomy, relationships with Heptapterus, erection of subfamily Phreatobiinae].- Myers, 1944 [key, inclusion in Pygidiidae].- Myers & Weitzman, 1966 [exclusion from Trichomycteridae].- Carvalho, 1967 [biology; distribution, occurrence in State of Amapá].- de Pinna, 1998 [phylogenetic position].- Bockmann & Guazzelli, 2003 [check list; type status; distribution on Marajó Island].
Heptapterus cisternarum; Buckup, 1988 [relationships with Heptapteridae; comparison with H. sympterygium].
Description
Morphometric data for specimens examined are given in Table 1. Body nearly round in cross-section from head attachment to origin of anal fin, increasingly more compressed posteriorly, tapering to base of caudal fin. Depth of muscular part of body nearly constant, slightly less deep at posterior third of caudal region. Dorsal and ventral profiles of muscular part of body nearly straight. Caudal region expanded dorsally and ventrally by procurrent caudal-fin rays. In dorsal view, profiles of body mostly straight, gently converging to caudal fin. In dorsal view, head profile distinctly wider than trunk, making head not continuous with trunk.
Integument thick and opaque. Myotomes and skeletogenous septa not evident externally along most of body, except for posterior third of caudal peduncle in some specimens. Integument forming vertical folds regularly disposed along sides of trunk in most specimens.
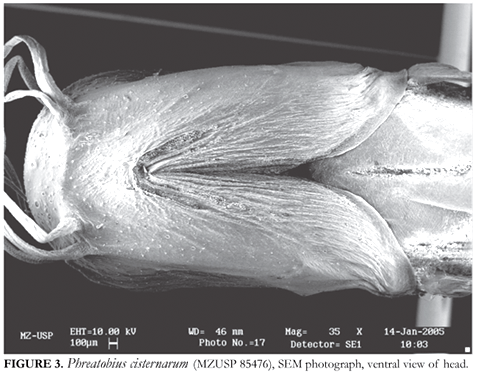
Lateral profiles of head convex in dorsal view. Snout blunt in dorsal and lateral views. Surface of head, especially on dorsal and upper lateral regions of posterior half, with network of superficial blood vessels visible by transparency and protruding slightly in profile on surface of skin (Fig. 1). Mouth prognathous, lower jaw extending further anteriorly than upper one (Fig. 1). In lateral view, cleft of mouth straight, located on dorsal fourth of head depth (Fig. 2). Lower jaw projected dorsally, its ventral surface markedly convex (Fig. 2). Upper jaw strongly depressed, its depth one-third or less that of lower one in lateral view. Corners of mouth well posterior to vertical through posterior margin of eyes. Mouth as wide as head, its lateral portions strongly curved posteriorly (Fig. 1). Upper lips narrow, well-defined laterally by fold of integument. Lower lip poorly defined, mostly continuous with ventral surface of head. Branchial membranes mostly free, narrowly attached to isthmus (Fig. 3). Posterior origin of membranes located immediately dorsal to origin of pectoral fin.
Eyes tiny, slightly larger than cephalic lateral-sensory pores, located on anterior 20% of HL, postero-laterally to posterior nares and posteriorly to base of maxillary barbel. Eyelens present, round in shape. Orbital margin not free.
Maxillary barbel long, when abducted reaching posterior limit of dorsal head musculature or to vertical through base of pectoral fin. Its base anterior to eye, close to anterolateral corner of upper jaw. Outer mental barbel longer than maxillary one, reaching pectoral-fin base or posterior third of pectoral fin. Inner mental barbel shortest, reaching maximally to slightly posterior to margin of branchial membrane when extended parallel to body. Its origin slightly anterior to that of lateral mental barbel. All barbels with fine round tips.
Relative position of nares shown in Fig. 1. Posterior naris close to base of maxillary barbel, but not continuous with its margin. Anterior nares close to upper lip. Distance between two posterior nares slightly larger than that between anterior ones. Anterior naris prolonged as tube of integument directed anterodorsally, about twice as long as wide. Posterior naris round and wide open, framed anteriorly by low rim of elevated integument.
Pattern of cephalic latero-sensory canal pores visible in Fig. 1-3. Four pores present dorsally on anterior snout region between nares, corresponding to pores of the nasal latero-sensory canals (Fig. 1). Anterior pair of pores close to each other, separated by space slightly larger than a pore diameter, located mesial to base of anterior nares. Posterior pair widely apart, separated by space approximately six times a pore diameter, located posteromesially to posterior nares. Large pore directly posteriorly to base of maxillary barbel and slightly anterior to transverse line through corners of mouth in dorsal view, corresponding to terminal opening of infraorbital latero-sensory canal, which is incomplete. Two large latero-sensory pores visible on ventral half of head in lateral view (Fig. 2), one located slightly anterior to vertical through infraorbital pore and another, further ventrally, located at approximately middle of SL, corresponding to lateral part of mandibular latero-sensory canal. Anterior portion of mandibular canal with two pores on each ramus (only three pores visible in Fig. 3). Preopercular and epiphyseal branches of latero-sensory system, and associated pores, absent. Latero-sensory canal system absent along most of body. Lateral line reduced to short branch immediately posterior to head, with two pores. Numerous taste buds, superficial neuromasts and pit organs present in most regions of head. In dorsal view of SEM preparation, conspicuous pit line of four pores (corresponding to the medial supraorbital line of Arratia & Huaquin, 1995) extending obliquely (posterolaterally) from near posterior nasal latero-sensory pore (Fig. 1). Mandibular and hyoid pit lines also evident in lateral and ventral views (Figs. 2 and 3). Pectoral-fin rays 4 (n = 16), all soft, none, two or three posterior ones branched. Fin small, its length about 2025% of HL and its base very narrow. Pelvic fin with 2 to 4 unbranched rays with irregular lengths, all projecting beyond fin membrane. Pelvic fin reaching or covering anal opening, but not reaching anal fin. Pelvic fin morphology markedly variable. In some specimens, one or both pelvic-fins reduced to finger-like process, containing 2 rays, one normal and one vestigial: MZUSP 88395 and 85470, left pelvic fin process-like, right pelvic-fin with 3 rays; MZUSP 85476 and MNRJ 11569 (smallest specimen only), both pelvic fins process-like. Dorsal fin short, with 7 soft rays (n = 17), two to five posterior ones branched, its origin closer to tip of snout than to base of caudal fin, slightly anterior to vertical through origin of pelvic fins, its posterior insertion approximately at middle of SL. First dorsal-fin pterygiophore inserted posterior to neural spine of vertebra 17 (n = 1), 18 (n = 9) or 19 (n = 4). Anal fin long, with 19 (n = 1), 21 (n = 2), 22 (n = 4), 23 (n = 4), 24 (n = 2), or 25 (n = 1) soft rays, all unbranched. Origin of anal fin shortly posterior to anal opening. First anal-fin pterygiophore inserted posterior to haemal spine of vertebra 22 (n = 9), 23 (n = 4), or 24 (n = 1). Anal fin continuous posteriorly with ventral procurrent caudal-fin rays, but two regions distinguishable, in most specimens, by gentle indentation or concavity and by slightly more closely positioned procurrent rays, when compared to more widely spaced anal-fin rays (Fig. 4). Caudal fin round and continuous dorsally and ventrally with procurrent rays. Principal caudal-fin rays 8 (n = 1), 9 (n = 1), or 10 (n = 13), all soft, two to nine middle ones branched. Dorsal procurrent caudal-fin rays: 42 (n = 1), 46 (n = 4), 47 (n = 5), 48 (n = 2), or 50 (n = 2). Ventral procurrent caudal-fin rays 22 (n = 2), 23 (n = 4), 24 (n = 2), 25 (n = 3), or 26 (n = 3). Whole caudal region looks as framed by single extended fin, composed of dorsal procurrent caudal-fin rays, principal caudal fin rays, ventral procurrent caudal-fin rays and anal-fin rays. Vertebrae 59 (n = 1), 60 (n = 6), 61 (n = 3), 62 (n = 2), 63 (n = 1), or 64 (n = 2). Two pairs of pleural ribs, on vertebrae 6 and 7. Vertebra 8 with parapophyses elongated in rib-like shape, but lacking real pleural ribs.
Coloration in life
The color of live specimens of P. cisternarum is bright red to deep purple (Fig. 5). The color is a result of superficial blood seen by transparency, in combination with faint dark pigment on some parts of the body. Dark pigment is mostly inconspicuous against the red background and is described separately in the next section, except in a few instances where it perceptably interacts with the red color in the living fish. The red coloration covers more or less uniformly the whole surface of body and head. It does not extend onto fins, including region of accessory caudal-fin rays, which are hyaline. Barbels are whitish with a reflective core along most of their length, fading to a transparent tip. Under microscope, the rims of barbels are light pink. Dark pigment is most evident on dorsal and sometimes lateral part of head, forming a dark mesh that makes the red color look darker. Dark chromatophores concentrate around lips, which are outlined nearly in black, and dorsal snout region, particularly in area between bases of maxillary barbels, where two roughly round fields surround posterior nostrils and eyes. The tube-like extension of anterior nostril is transparent. The muscular separation between head and dorsum is delineated with a concentration of dark chromatophores. The whole dorsal region of body tends to be of a red darker and less uniform than that of sides and ventral region. This occurs because of the presence of dark pigment, which is irregularly and variably present on dorsum, while absent in abdomen. There is a small whitish area posterodorsally to pectoral-fin base, corresponding to a region lacking body wall musculature and where the lateral wall of the swimbladder contacts the integument (pseudotympanus). The red color on ventral side of body seems similar to that on dorsal side, but appears lighter because of total lack of integumentary dark pigment. A dark shade of some abdominal organs is visible by transparency on the abdomen.
Coloration in preservative
No trace of red coloration remains in preserved specimens. The overall color is a dull whitish with faint fields of dark pigment visible under close examination. Apparently the faint dark pigmentation also fades relatively quickly under preservation, because several specimens are nearly or entirely white, especially those with a long preservation history. Dark pigmentation is heaviest on dorsal surface of head, especially on lips and around eyes, with the dark covering extending onto lateral surface of head. Some specimens also have a concentration along the midline of head, forming a thin middorsal line, and on the separation between head and trunk. The pigmentation over most of the head is patterned with irregular thin white stripes forming anastomosing pattern over the dorsal part of head and cheeks, apparently outlining superficial blood vessels. The tubelike extension of anterior naris is white. All barbels are white, except for few streaks of dark pigment on base of maxillary and outer mental barbels. The body has a uniform covering of dark chromatophores extending along dorsum and upper third or half of sides, increasingly fader posteriorly. In a single specimen (MZUSP 84568), there is a longitudinal dark stripe along the lateral midline of body, not formed by chromatophores, but apparently by coagulated blood located below the integument. The ventral part of body and head lack dark pigment. The pectoral, pelvic and anal fins, as well as the region corresponding to the ventral procurrent caudal-fin rays lack dark pigment. The dorsal fin has irregular dark fields along its base, extending dorsally over individual rays. The caudal fin has elongated fields of dark chromatophores extending along the interradial membrane for the basal two-thirds of fin. The region corresponding to the dorsal procurrent caudal-fin rays has an irregular scattering of dark pigment over the basal two-thirds of its depth. The pigmentation of a juvenile specimen examined (MZUSP 85472, Fig. 4) follows the same general pattern as that of adults, but its dark pigmentation is heavier overall. That specimen differs from adults in that the side of the body has a well-defined separation between a darkly-pigmented dorsal half and a white ventral half, rather than a gradual ventral fading as observed in adults.
Data on Habitat and Biology
The new specimens reported in this study were collected in private hand-dug wells in the vicinity of the city of Belém and on Marajó Island. Wells where P. cisternarum were secured varied in depth between 4 to 13 meters (measured to the bottom), and approximately one meter in diameter (Fig. 6). Some had masonry-finish walls while others were simply excavated in soil. All had concrete rims or fences around their tops and some also had lids. The substrate at the bottom of the wells was a mix of sand, fine chalk-like white silt and irregular rocks. The rocks correspond to "canga", which are iron-rich conglomerates formed by detrital mineral fragments cemented by a ferruginous matrix, usually with cavernous structure. The white silt is locally called "tabatinga" and is similar to a white clay. Wells visited where fish were collected had been open for one to eight years and all were being used regularly as water source for human use at time of collection. One local reported that a well in his property had been open for more than 30 years and still yielded P. cisternarum specimens regularly. Specimens were collected near the peak of dry season in the region (Nov-Dec), and water depth was usually less than 30 cm. Locals report that Phreatobius is far less usual in the rainy season, when water depth in wells rises to several meters. Maximum water depth usually corresponds to approximately two-thirds of the total well depth. Water in wells at time of collections was clear, warm (27-29ºC), and acidic (pH 5-6). Other water- chemical parameters were: ammonia 0.46 p.p.m.; nitrite 0.03-0.07 p.p.m.; iron 2.23 p.p.m.; phosphate 5.12 p.p.m.; hydrogenionic potential 5-6.
According to local well-drillers, Phreatobius cisternarum never surfaces from artesian wells, far deeper than hand-dug wells. If so, it does not inhabit artesian aquifers, but rather the superficial phreatic layer, which corresponds to the upper saturated stratum of soil and is the one normally reached by hand-dug wells. Water deposit in the superficial phreatic layer is directly fed by rains, so that the water level is strongly connected to immediate weather. Water level in hand-dug wells varies markedly between rainy and dry seasons, with fluctuations of up to several meters. Phreatobius specimens seem to be very common in hand-dug wells in the vicinity of the city of Belém and on Marajó Island, and many local residents with regular access to such wells have seen them.
Collection of specimens was done by removing rocks from the bottom of wells, with the help of hired local well-cleaning crews that descend into wells using ropes. The rocks have numerous irregular orifices and crevices where specimens of Phreatobius hide, with their bodies contorted along the path of tunnels and spaces. While some specimens obviously left the rock immediately upon disturbance, others stayed trapped in their hiding places while the rock was quickly moved from the well water to a collecting container.
Eight specimens intended for live observations were transported in plastic containers and placed into 50-liter aquaria. The aquaria were provided with substrate removed from the wells (sand, fine silt and rocks). Specimens were kept alive for 19 months and subsequently preserved. Artificial aeration was used initially, but soon terminated since air bubbles were obviously a source of permanent stress for the fish. The consequent lower level of dissolved oxygen does not seem to have negatively affected the specimens. Fish stayed still most of the time, hiding in rock orifices, and were active only when offered food. Tanks were kept dark most of the time. Sudden light clearly disturbed the fish, which became agitated and searched for deeper hiding places in the rocks. However, they were not as much disturbed by a gradual increase in illumination, and would readily search and eat food offered under lightened conditions. Their most preferred food item was live earthworms, which they pursued and ate eagerly. Sometimes they would swallow the worm whole and sometimes bite a piece off, especially when one end of the worm was anchored in the substrate. In those cases they would rotate the body until a piece was severed. The attack on prey was accompanied by vigorous contorting movements, with the body of the fish tangled with that of the worm. The jaws of P. cisternarum exert a firm grip on prey, something expected in view of its massive jaw muscles. Other food items were also accepted, such as live adult Artemia, and live tenebrionid larvae. Live ants, live Drosophila larvae and minced shrimp meat were not readily taken, although occasionally a specimen would eat them. One specimen was experimentally kept without food for six months, and some degree of emaciation was evident only towards the end of that period. No other health problems were evident, although the fish was noticeably less active than when normally-fed.
Specimens were mostly non-responsive to each other. They would often congregate in the same spot (Fig. 5), but that might be just a tendency to favor specific hiding sites, rather than actual gregarious behavior. They were never observed to bury in sand or to hide under vegetable debris. Under stress, usualy caused by disturbance, specimens would become hyperactive and then assume a vertical orientation, head up, with barbels stretched out at the water surface, spread in a circle. The distal third of the barbels joined the water film at the water-air interface, held by superficial tension. The fish hung down from the surface anchored by the set of barbels, either still or making gentle undulating movements. On that position, which lasted between one and two minutes, they would often expel a bubble of air through the mouth and apparently ingest some new air. This indicates that P. cisternarum has some form of aerial respiration, although the exact mechanism is still unknown. No indication of aerial respiration was observed under non-stress conditions.
DISCUSSION
The material used in this study includes a relevant addition to the geographical distribution of P. cisternarum. The specimen MPEG 3325 comes from the city of Amapá, near the northern limit of the State of Amapá, far from the Amazon river. Previous records from that State (MNRJ 11569) were from the City of Macapá, on the southeastern part of the State and at the north shore of the Amazon river. The MPEG record expands approximately 300 km the distribution of the species, and almost doubles its previously known geographical range. Curiously, that specimen was collected in a flooded forest, rather than in a well. Whether it is part of a possibly epigean population of P. cisternarum, or a stray specimen accidentally washed out of its subterranean environment, cannot currently be known. There have been anecdotal reports of Phreatobius specimens showing up in domestic water reservoirs in the outskirts of the city of Belém, and even in a swimming pool, having entered it through an underwater crack in pavement. So, their accidental presence in epigean environments is possible.
The new data obtained from recent collections of P. cisternarum allowed a detailed assessment of the intraspecific morphological variation in the species. That variation, however, does not show any clear geographical correlation. Specimens from south and north of the mouth of the Amazon and from Marajó Island show no taxonomically-consistent differentiation. However, the vastness of the area concerned, along with the presumably limited vagility of P. cisternarum in its underground habitat, makes it unlikely that there is no isolation. Unless there is a vast system of interconnected underground canals spanning the mouth of the Amazon river and adjoining areas (a possibility considered by Carvalho, 1967), some degree of populational segregation should be expected. In this study, the only possible variation indicative of differentiation was in the number of haemal spines associated with PU2. In all specimens reported from the State of Amapá, there are two such spines, while specimens from other localities have a single PU2 haemal spine. The significance of such observations, however, are restricted by the limited number of specimens from the northern range (three specimens known from State of Amapá, two of which with some locality uncertainties; see Notes on MNRJ 11569, above). Further investigations on that issue must await additional material from north of the mouth of the Amazon.
There are few other siluriforms with a blood-red live coloration similar to that in P. cisternarum. One of them is the silurid Silurichthys sanguineus, described by Roberts (1989) from the Kapuas drainage in Western Borneo and known from a single specimen. No ecological information is currently available on the species. The clariid Horaglanis krishnai, from the Indian State of Kerala, is another catfish which is bright red when alive (cf. Babu, 2002). Among non-siluriform freshwater fishes, an impressive case of similarity in red color and general body shape is Bihunichthys monopteroides, of the Southeast Asian family Chaudhuriidae (Kottelat & Lim, 1994; figs. 7, 8). Those cases of apparently independent similarity invite an investigation into possible environmental factors that may be associated with a rich integumentary blood supply. Low dissolved oxygen concentrations is a factor to be considered, since cutaneous respiration exists in many fishes (Graham, 1997), potentially with a particularly important role in very small species. Underground water may have lowered dissolved oxygen levels (Trajano, 2001), because of their limited aerial contact and lack of green plants. The same possibly occurs in epigean fossorial environments such as leaf litter, the habitat of Bihunichthys. At least one undescribed species of Phreatobius, with the same red color as P. cisternarum, also occurs in leaf litter (Henderson & Walker, 1990). The red skin color of Phreatobius and other fossorial or subterranean fishes (Trajano, 2001) is probably related to cutaneous respiration.
The feeding habits of P. cisternarum in aquarium conditions conform to those of an opportunistic predator of macro invertebrates. The vigorous attacks observed on earthworms and beetle larvae, shown by all specimens kept alive, leave little doubt that the species is well adapted to secure and ingest relatively large live prey. Our observations differ markedly from those reported by Carvalho (1967), where a specimen kept in aquarium for a year never showed any interest in tubificid worms and daphnia offered. Carvalho speculated that the fish might be feeding on algae or microorganisms. That possibility seems to us unlikely in view of the mouth and head morphology of the species, which seems fit for taking large prey. We notice furthermore that his reported Phreatobius specimen would spend much of its time in a vertical position at one corner of the aquarium. As seen above, our studied specimens only took such position under conditions of stress. Thus, we suspect that the discrepancy in feeding behavior observed is due to the specimen reported by Carvalho being stressed much of the time and therefore unwilling to feed.
Little can be said at present about the specific prey items of P. cisternarum in its natural habitat. No invertebrates were found during our collection of fish, and no information is available on the faunal composition of the superficial phreatic layer in the region.
As most cave fishes, P. cisternarum displays low levels of activity. It is likely that in its natural habitat the fish stays motionless for large periods of time, only moving when there is some sign of prey. Specimens of P. cisternarum kept in captivity were capable of ingesting very large amounts of food in a single taking. Some of the earthworms eaten were almost as long as the fish itself, yet were ingested whole with little trouble. Given the depth and nature of the soils where the fish is found, availability of earthworms, and possibly other prey, in natural conditions is possible but probably unusual. Such availability must be spotty and irregular, and the fish must be capable of eating large prey at long intervals. One of our studied specimens was left with no food for six months, and showed no evident health problems (see Data on Habitat and Biology, above). Also, the specimen reported by Carvalho (1967) survived in good condition for a whole year without food. Obviously, P. cisternarum can sustain long periods of fasting, which agrees with the inferred spotty availability of food items in its habitat. Low metabolic levels and discontinuous food supplies have been reported for troglobitic fishes (Trajano, 2001) and P. cisternarum seems to fit that pattern. It is possible that the richer offer of food near artificial wells makes P. cisternarum concentrate at those spots. The relatively mild photophobic reactions observed in aquarium mean that sunlight at wells might not be a strong repulsive factor for the fish. There is anecdotal indication from locals that old wells yield more Phreatobius than recently opened ones, but this remains to be investigated.
Nothing is known about the reproductive habits of P. cisternarum. The collection of juvenile specimens in the same spots as adults suggests that there is not much ecological change along the late part of the ontogeny of the species. However, larvae and very small juvenile specimens are still unknown.
ACKNOWLEDGMENTS
Research funding is provided by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). Horacio Higuchi, Maria Ivaneide Assumpção and Wolmar Wosiacki (all from MPEG) helped with information on Phreatobius and/or loans of specimens. Lara Guimarães operated the SEM facilities at MZUSP and Ulf Mehlig helped with the photograph of the juvenile specimen. We thank Priscila Carmona, Cesar Braga, Alexandre Cunha, Renan Bernardi, Iracilda Sampaio, Horácio Schneider, Sra. Neide, Lino do Santos, Silvio Colares and Sr. Nei for help in field work and/or access to wells in private land. For conversations on subterranean fishes and other relevant subjects, we thank Augusto Auler, Heraldo Britski and Eleonora Trajano. For editorial help, we are grateful to Airton Cruz. The manuscript benefitted from reviews by Alberto Akama, Fábio Di Dario and José Luis Birindelli.
Babu, S. 2002. No title. Available at: http://www.planetcatfish.com/images/full/clariidae/horaglanis/krishnai/1.jpg
Recebido em: 25.08.2005
Aceito em: 11.11.2005
- Arratia, G. & Huaquin, L. 1995. Morphology of the lateral line system and of the skin of diplomystid and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonner Zoologische Monographien, 36:1-110.
- Bockmann, F.A. 1998. Análise filogenética da família Heptapteridae (Teleostei, Ostariophysi, Siluriformes) e redefinição de seus gêneros. Tese (Doutorado), Universidade de São Paulo, São Paulo.
- Bockmann, F.A. & Guazzelli, G.M. 2003. Family Heptapteridae. In: Reis, R.; Kullander, S.O.; Ferraris Jr, C.J. (Org.), Check list of the freshwater fishes of South and Central America. Edipucrs, Porto Alegre, p.406-431.
- Buckup, P.A. 1988. The genus Heptapterus (Teleostei, Pimelodidae) in southern Brazil and Uruguay, with the description of a new species. Copeia, (3):641-653.
- Carvalho, A.L. 1967. Novos dados para o conhecimento de "Phreatobius cisternarum" Goeldi (Pisces, Pygidiidae, Phreatobiinae). In: Atas do simpósio sôbre a Biota Amazônica. Conselho Nacional de Pesquisas, Rio de Janeiro, p.8388, v.3 (Limnologia).
- Chermont de Miranda, V. 1968. Glossário Paraense (coleção de vocábulos peculiares à Amazônia e especialmente à Ilha do Marajó). Reprint of 1905 edition. Universidade Federal do Pará, Belém.
- Eigenmann, C.H. 1918. The Pygidiidae, a family of South American catfishes. Memoirs of the Carnegie Museum, 7:259-398.
- Fuhrmann, O. 1905. Zoologie: Phreatobius cisternarum. Compte Rendu des Travaux, quatre-vingt-huitième de la Société Helvétique des Sciences Naturelles, Lucerne. Archives des Sciences Physiques et Naturelles, (1905): 68-69.
- Fuhrmann, O. 1906. Scleropages formosum und über Phreatobius cisternarum. Verhandlungen der Schweizerischen Naturforschenden Gesellschaft, 88:50-51.
- Goeldi, E. 1905. Nova zoologica aus der Amazonas-Region: Neue Wirbeltiere. In: Congrès International de Zoologie, 6. Compte-Rendu. W. Kundig & Fils, Genève, p.542-549.
- Graham, J.B. 1997. Air-Breathing Fishes. Academic Press, San Diego.
- Henderson, P.A. & Walker, I. 1990. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. Journal of Fish Biology, 37:401-411.
- Kottelat, M. & Lim, K.K.P. 1994. Diagnoses of two new genera and three new species of earthworm eels from the Malay Peninsula and Borneo (Teleostei: Chaudhuriidae). Ichthyological Exploration of Freshwaters, 5:181-190.
- Myers, G.S. 1944. Two extraordinary new blind nematognath fishes from the Rio Negro, representing a new subfamily of Pygidiidae, with a rearrangement of the genera of the family and illustrations of some previously described genera and species from Venezuela and Brazil. Proceedings of California Academy of Science, 23:591-602.
- Myers, G.S. & Weitzman, S.H. 1966. Two remarkable new trichomycterid catfishes from the Amazon basin in Brazil and Colombia. Journal of Zoology, 149:277-287.
- de Pinna, M.C.C. 1998. Phylogenetic relationships of neotropical Siluriformes (Teleostei: Ostariophysi): historical overview and synthesis of hypotheses. In: Malabarba, L.R.; Reis, R.E.; Vari, R.P.; Lucena, Z.M. & Lucena, C.A.S. (Eds.), Phylogeny and classification of Neotropical fishes. EDIPUCRS, Porto Alegre, p.279-330.
- Reichel, M. 1927. Etude anatomique du Phreatobius cisternarum Goeldi, silure aveugle du Brésil. Revue Suisse Zoologie, 34:285-403.
- Roberts, T. R. 1989. The freshwater fishes of Western Borneo (Kalimantan Barat, Indonesia). Memoirs of the California Academy of Science, 14. California Academy of Sciences, San Francisco.
- Taylor, W.R. & Van Dyke, G.C. 1985. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage. Cybium, 9:107-119.
- Trajano, E. 2001. Ecology of subterranean fishes: an overview. Environmental Biology of Fishes, 62:133160.
Publication Dates
-
Publication in this collection
16 Dec 2005 -
Date of issue
2005
History
-
Received
25 Aug 2005 -
Accepted
11 Nov 2005