Abstracts
A new genus and species of parasitic copepod (Clausiidae), Spionicola mystaceus, associated with the polychaete Dipolydora armata (Spionidae) is described and figured. The new copepod has an elongate body, 5-segmented antennule, 2-segmented rami on legs 1 and 2, 2 spines representing leg 3, no leg 4, leg 5 well developed and reduced armature elements on feeding limbs. The host is a mollusk-shell borer, collected off São Sebastião Island, State of São Paulo, Brazil.
Crustacea; Copepod parasite; Polychaetes; Brazil; Clausiids
Novo gênero e nova espécie de Clausiidae (Crustacea, Copepoda) parasita de Dipolydora armata (Polychaeta, Spionidae) no Brasil: Descrição e ilustração de novo gênero e nova espécie de um copépodo parasita (Clausiidae), Spionicola mystaceus, associado ao poliqueto Dipolydora armata (Spionidae). O novo copépodo tem um corpo alongado, antenula penta articulada , ramos biarticulados nos pereiópodos um e dois, dois espinhos representando o pereiópodo 3, pereiópodo 4 ausente, pereiópodo 5 muito desenvolvido e estruturas dos apêndices bucais reduzidas. O hospedeiro é um perfurador de conchas de moluscos coletado ao largo da Ilha de São Sebastião, Estado de São Paulo, Brasil.
Crustacea; Copépodos parasitas; Poliquetas; Brasil; Clausiídeos
A new genus and a new species of Clausiidae (Crustacea, Copepoda) parasitic on Dipolydora armata (Polychaeta, Spionidae) in Brazil
Tagea K. S. BjörnbergI; Vasily I. RadashevskyI,II
ICentro de Biologia Marinha, Universidade de São Paulo, Caixa Postal 71, 11600-970, São Sebastião, SP, Brasil
IIInstitute of Marine Biology, Russian Academy of Sciences, Vladivostok 690041, Russia. E-mail: radashevsky@hotmail.com
ABSTRACT
A new genus and species of parasitic copepod (Clausiidae), Spionicola mystaceus, associated with the polychaete Dipolydora armata (Spionidae) is described and figured. The new copepod has an elongate body, 5-segmented antennule, 2-segmented rami on legs 1 and 2, 2 spines representing leg 3, no leg 4, leg 5 well developed and reduced armature elements on feeding limbs. The host is a mollusk-shell borer, collected off São Sebastião Island, State of São Paulo, Brazil.
Keywords: Crustacea; Copepod parasite; Polychaetes; Brazil; Clausiids.
RESUMO
Novo gênero e nova espécie de Clausiidae (Crustacea, Copepoda) parasita de Dipolydora armata (Polychaeta, Spionidae) no Brasil: Descrição e ilustração de novo gênero e nova espécie de um copépodo parasita (Clausiidae), Spionicola mystaceus, associado ao poliqueto Dipolydora armata (Spionidae). O novo copépodo tem um corpo alongado, antenula penta articulada , ramos biarticulados nos pereiópodos um e dois, dois espinhos representando o pereiópodo 3, pereiópodo 4 ausente, pereiópodo 5 muito desenvolvido e estruturas dos apêndices bucais reduzidas. O hospedeiro é um perfurador de conchas de moluscos coletado ao largo da Ilha de São Sebastião, Estado de São Paulo, Brasil.
Palavras-chave: Crustacea; Copépodos parasitas; Poliquetas; Brasil; Clausiídeos.
INTRODUCTION
A general survey of the literature on copepod parasites and associates of annelid polychaetes shows that off South America there are very few records of these taxa. Rocha (1986) found Clausia (Doviella) prima living in a mud flat in Juréia (São Paulo State) with characteristics of a parasite of polychaete. Selioides tardus Gravier, a parasite of a polynoid annelid, was described for the first time from the South American Atlantic (Gravier, 1912).
The species studied here was found on the spionid polychaete Dipolydora armata (Langerhans, 1880) boring in abandoned shells off São Sebastião, State of São Paulo, Brazil. Only five specimens were found after examining 200 polychaetes off the southeast and south of Brazil.
There are few records of copepod parasites on spionid polychaetes. Mesnilia cluthae (T. & A. Scott, 1896) was found on Dipolydora flava (Claparède, 1868) and Mesnilia martinensis Canu, 1898 found among polychaete tubes (Bocquet & Stock, 1959). Ho (1984) registered Spiophanicola spinulosus Ho, 1984 associated to three species of Spionidae: Spiophanes berkeleyorum Pettibone, 1962; S. kroyeri Grube, 1860, and S. missionensis Hartmam, 1941. Unidentified copepod parasites were also found associated with Polydorella dawydoffi Radashevsky, 1996 (described by one of us) and Polydorella stolonifera (Blake & Kudenov, 1978) (Williams, 2004).
MATERIAL AND METHODS
Field collections were made in Paranaguá Bay, Paraná (25º34'S, 48º21'W), and São Sebastião Channel, São Paulo (23º49'S, 45º25'W), southern Brazil, from November 2000 through June 2004. More than 40 species of spionid polychaetes living in temporary mucous tubes, in permanent silty tubes, boring in sponges and various calcareous substrata were collected intertidally and shallow subtidally using SCUBA. Samples were processed at Centro de Estudos do Mar, Universidade Federal do Paraná, and Centro de Biologia Marinha, Universidade de São Paulo. Mollusk shells were broken with a bench-vice and pliers. Living polychaetes were examined with light microscopy to obtain parasitic copepods. One copepod species was found on Dipolydora armata, a borer of shells of various mollusks off São Sebastião Island. Copepods were detached from the polychaetes, fixed in 10% formalin solution, rinsed in fresh water, and transferred to 70% ethanol. Drawings of entire copepods and dissected parts were made using a camera lucida mounted on a Nikon Labophot microscope. Fixed specimens are deposited at the Zoological Museum of the University of São Paulo, São Paulo (MZUSP), and at the Center of Marine Biology of the University of São Paulo (CEBIMar). Abbreviations used in the text: P1-P5 - legs. 1 to 5.
RESULTS
Copepoda Milne-Edwards, 1840
Clausiidae Giesbrecht, 1895
Genus Spionicola gen. nov.
Diagnosis: Body elongate, vermiform, in both sexes with limits of somites not always very clear, maintaining more or less the same width from first to seventh somite and narrowing very little towards the anal region. Antennule 5-segmented with pointed protuberances on first segment. Antenna 4-segmented. Labrum small. Mandible with strong claw and seta. Maxillule with 4 elements. Maxilla 2-segmented with claw-like spine and seta. Maxilliped very reduced in female and 4-segmented in male with long claw. Legs 1 and 2 biramous with 2-segmented rami tipped with 3 elements. Leg 3 reduced to spines. Leg 4 absent. Leg 5 well developed, composed of 2 segments and 3 long strong setae. Leg 6 in male reduced to 3 setae. Caudal ramus with 5 elements. Egg string uniseriate, very long.
Etymology: The generic name Spionicola is a composition of the host's family name Spionidae with the latin cola, inhabitant.
Type species: Spionicola mystaceus n. sp.
Spionicola mystaceus gen. nov. and sp. nov.
Material: The study is based on 2 females of which one was ovigerous and 3 males collected on 31 Jan.2004 and on 13 May 2004, at 1-5 m depth off Praia do Curral in São Sebastião Island, state of São Paulo, associated with the polychaete Dipolydora armata which inhabits shells of living gastropods or shells of the same gastropods occupied by hermit crabs. The holotype female (MZUSP 16319) is deposited in the Museum of Zoology of the University of São Paulo. The paratypes remained at the CEBIMar (1 male nº 011; 1 female nº 012). One male was lost during manipulation and the other dissected and retained in the authors collection.
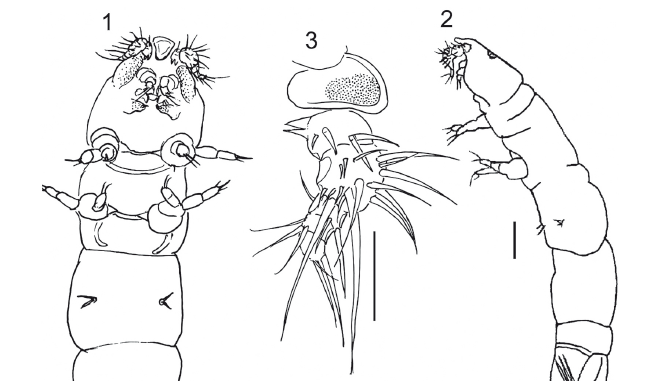
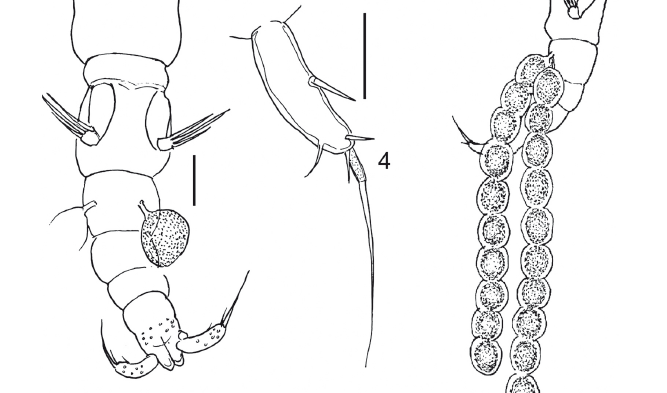
Description: Adult female 1.52 mm long, vermiform, poorly sclerotized, with two uniserial egg strings, each with 10 reddish-orange eggs (Figs. 1-2). Color: lightbrown-greenish. Rostrum pear-shaped, very protuberant, rounded anteriorly (Fig. 3). Cephalon not completely separated from first pedigerous somite. The ventrally expanded margins of the cephalothorax are densely ornamented with tiny spinules. Orange colored eye present dorsally. Pedigerous somites not distinctly separated from each other. Urosome with five somites. Caudal ramus about as long as anal somite, with 5 setae (Fig. 4). Caudal ramus 0.100 mm long and 0.025 mm wide. Paired egg strings 0.961 mm long (Fig. 2) attached to second urosomite.
Antennule five-segmented (Fig. 3); setal formula: 4, 15, 3, 3, 3. Segment 1 with two proximal ventral, pointed processes directed posteriorly. Antenna 4-segmented (Fig. 5); segment 3 with one seta; distal segment with a rounded projection and 5 setae, of which 2 longer.
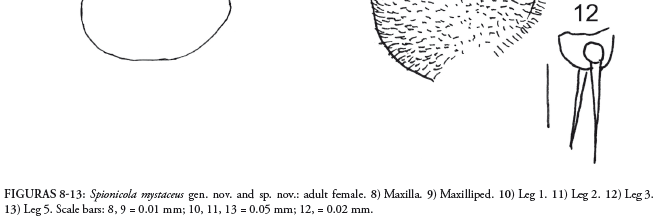
Mandible (Fig. 6) with distal pointed hook and 1 setule. Maxillule (Fig. 7): of velvety appearance, covered by microspines, with 2 digitiform internal protuberances and 3 spines. Maxilla covered with minute spines (Fig. 8); with 1 small, hook-like terminal spine, 1 cushion-like rounded projection and 2 small terminal spines. Maxilliped very small (Fig. 9), represented by small hook-like distal spine and 2 lateral minute spines on minute rectangular protuberance.
Legs (Figs. 10-13): with patches of minute setules giving them velvety appearance. Legs 1 and 2 with strong coxa, small basis with 1 outer spine, and 2-segmented rami. Exopod of leg 1: segment 1 with 1 outer spine; segment 2 with 1 outer and 3 terminal spines. Endopod of leg 1 with 3 terminal spines on segment 2 and 1 inner spine on segment 1. Exopod of leg 2: segment 1 with 1 outer spine and segment 2 with 3 terminal spines and 1 outer spine. Endopod of leg 2 with 3 terminal on segment 2 and 1 distal spine on segment 1. Leg 3 reduced to 2 spines. Fourth pair of legs absent. Fifth pair of legs 2-segmented; first segment long and wide with 1 spinule; segment 2 about half as long as first and half as wide, with 1 small spinule and 3 robust long setose spines.
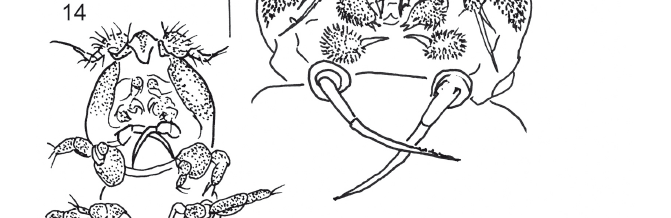
Males (Figs. 14-15): Two specimens, 0.94 mm and 1.03 mm long, vermiform, poorly sclerotized like females. Rostrum prominent, pear-shaped, tapering towards blunt posterior margin, more or less triangular in ventral view (Fig. 16). First pedigerous somite not clearly separated from cephalon. Prosome and urosome 5-segmented. First urosomite with bean-shaped spermatophores. Caudal rami about as long as anal somite, armed with 5 setae; mid-terminal seta, mounted on a pedestal, slightly longer than caudal ramus.
Antennule 5-segmented (Fig. 17); setal formula 3, 13, 4, 3, 5. Antenna, mandible, maxillule and maxilla as in female. Maxilliped 4-segmented (Fig. 18), with 1 strong, very long serrate claw on the distal serrate segment.
Leg 1 covered with setules with separate coxa and basis (Fig. 19); exopod 2-segmented with outer spine on segment 1 and segment two, with 3 terminal setae; endopod 2-segmented, 2 setulate spines and a thin spine on last segment. Leg 2 (Fig. 20) covered with setules, with 1 seta on coxobasis and endopod 2-segmented with 1 inner seta on segment 2, and 3 distal spines on distal segment; exopod 2-segmented, with 1 outer spine on first segment, and 3 terminal setulate spines on distal segment. Leg 3: 2 spines on pedestal. Leg 4 absent. Leg 5 as in female; Leg 6 (Fig. 21) represented by 3 small spines on each side of first urosomite.
Young female: (copepodid V?) 1.18 mm long (Fig. 22). Cephalon not separate from first pedigerous somite. Pedigerous somites 2, 3, 4 and 5 well delimited. Rostrum, antennules, antennae, mandibles, maxillules, maxillipeds and legs as in adult female. Urosome 5-segmented. Caudal rami as in adult female.
Habitat: The host polychaete, Dipolydora armata is a borer of coralline algae and shells of various mollusks (Radashevsky & Nogueira, 2003). The largest polychaetes with 35 segments, reach 5.5 mm long and 0.3 mm wide. In São Sebastião Channel, D. armata was found in the shallow subtidal in shells of a variety of live gastropods Astraea olfersii (Philippi, 1846), Morula nodulosa (CB Adams, 1845), Pisania auritula (Link, 1807), Pisania pusio (Linnaeus, 1758), Stramonita haemastoma (Linnaeus, 1757) and shells of the same gastropods occupied by hermit crabs Paguristes tortugae Schmitt, 1933 and Pagurus brevidactylus (Stimpson, 1859). The polychaetes resided in burrows within the shells. Tens of polychaetes were found in a single shell, and up to 15/cm2 were found on the shell surface. Of 220 shells of the gastropod Stramonita haemastoma (22-35 mm high), 79.1% (174 shells, including 10 live mollusks and 164 shells occupied by the hermit crab P. tortugae) were free from D. armata; 20.9% (46 shells, including 2 live mollusks and 44 shells occupied by hermit crabs P. tortugae) were infested by polychaetes. Hundreds of them were examined and only 5 copepods were found. Copepods were firmly attached to the dorsal side of the middle segments of the polychaete and probably sucked from the host's body. We can therefore conclude that Spionicola mystaceus is not a common parasite in the region where it was found.
DISCUSSION
Wilson & Illg (1955) modernized the concept of the family Clausiidae. O'Reilly (1995) discussed the data obtained about the family and refined its diagnosis..
The specimens studied belong apparently to the family Clausiidae because they have an elongate body, 5-segmented antennule, simple mandible with one subdistal seta, 1+3 setae on the maxillule, reduced number of leg segments, and reduction or absence of the prosomal posterior legs. O'Reilly (1995) considers four or five elements on the maxillule as characteristic for clausiids. The maxilla is 2-segmented and armed with a cutting edge, and a hook-like spine as in Clausia antiqua Kim (2001). The maxilliped in the female is strongly reduced as in Pseudoclausia with a distal and a proximal element. According to O'Reilly (1995, p. 59) "the leg morphology exhibits a trend towards reduction" This is true for the new species which has a strongly reduced third pair of legs (present in the form of 2 spines) and lacks the fourth pair of legs. The fifth leg of the new species is well developed as in Megaclausia mirabilis O'Reilly, 1995, but is distinguished from the latter and other clausiid species by having 3 large and 1 minute setae in the female.
Ho & Kim (2003) analysed cladistically the 21 representatives of the family Clausiidae, based on characters and character states of the legs 1 to 4 of each species. Our specimens are excluded from Ho & Kim's first 4 groups because they have 2-segmented rami on their legs, and these 4 groups exhibit 3-segmented rami on all or some of the legs. Ho & Kim's Group VI contains two species Pseudoclausia giesbrechti and P. longiseta with 3-segmented exopods on legs 1 and 2. Group V has no endopod on legs 3 and 4 and bears 2-segmented rami on legs 1 and 2. Group V is therefore the group to which our species shows the greatest affinity, but not all the characters of the Group V species agree with those of Spionicola: Clausia lubocki and Indoclausia bacescui have leg 3 with a 1-segmented ramus (in our species, leg 3 is represented by 2 spines); Megaclausia mirabilis, Rhodinicola rugosum and R. thomassini have 2-segmented exopods on legs 3 and 4 (our species bears 2 spines instead of leg 3 and no legs 4). These apomorphies characterize our species combined with the minute female maxilliped, which is reduced to a protuberance bearing minute spines and the very prominent rostrum. Therefore it cannot be included in any of the genera or species just mentioned. The specific name refers to the moustache-like aspect of the antennules when viewed ventrally (moustacia, Latin for moustache).
ACKNOWLEDGEMENTS
This work was supported by the State of São Paulo Research Foundation (FAPESP) within the BIOTA/FAPESP - The Biodiversity Virtual Institute Program (www.biota.org.br) (proc. 1998/07090-3; 2003/08688-0). Financial support was also provided to VIR by the Ministério da Educação do Brasil through the Universidade Federal do Paraná (contract 125/2000).
We thank the Center of Marine Biology (CEBIMar) of the University of São Paulo, Brazil, for providing research space and equipment, also for the help from the staff.
Recebido em: 10.03.2009
Aceito em: 07.07.2009
Impresso em: 30.09.2009
- Bocquet, C. & Stock, J.H. 1959. Copépodes parasites d'invertebres des Côtes de la Manche. V. Redescription de Mesnilia cluthae (Th. et A. Scott) (Copépode Cyclopoide, Famille des Clausiidae). Travaux de la Station Biologique de Roscoff, 47:1-18.
- Gravier, C. 1912. Sur un Crustacé parasite d'un Polynoidien de l'Atlantique sudaméricaine (Selioides tardus nov. sp.). Museum d'Histoire Naturelle de Paris Bulletin, 18:63-67.
- Ho, J-S. 1984. New family of poecilostomatoid copepods (Spiophanicolidae) parasitic on polychaetes from Southern California, with a phylogenetic analysis of nereicoliform families. Journal of Crustacean Biology, 4:134-146.
- Ho, J-S. & Kim, I-H. 2003. New clausiid copepods (Poecilostomatoida) associated with polychaetes of Korea, with cladisric analysis of the family Clausiidae. Journal of Crustacean Biology, 23:568-581.
- Kim, I-H. 2001. A new species of Clausia (Copepoda, Poecilostomatoida, Clausiidae) associated with a polychaete in Korea. Hydrobiologia, 452:217-223.
- O'Reilly, M. 1995. A new genus of Copepod (Copepoda: Poecilostomatoida) commensal with the maldanid polychaete Rhodine gracilior, with a review of the family Clausiidae. Journal of Natural History, 29:47-64.
- Radashevsky, V.I. 1996. Morphology, ecology and asexual reproduction of a new Polydorella species (Polychaeta: Spionidae) from the South China Sea. Bulletin of Marine Science, 58:684-693.
- Radashevsky, V.I.; Nogueira, J.M.M. 2003. Life history, morphology and distribution of Dipolydora armata (Polychaeta: Spionidae). Journal of the Marine Biological Association of the United Kingdon, 83:375-384.
- Rocha, C.E.F. 1986. Copepoda of the Juréia ecological reserve, State of São Paulo, Brazil. I. Doviella prima, new genus, new species (Poecilostomatoidea: Clausidiidae). Boletim de Zoologia, Universidade de São Paulo, 10:173-187.
- Wilson, M.S. & Illg, P.L. 1955. The family Clausiidae (Copepoda, Cyclopoida). Proceedings of the Biological Society of Washington, 68:129-142.
Publication Dates
-
Publication in this collection
01 Oct 2009 -
Date of issue
2009
History
-
Reviewed
07 July 2009 -
Received
10 Mar 2009 -
Accepted
30 Sept 2009