Abstracts
The Neotropical genus Cyrtomyia Bigot has a distribution restricted to the Andean region of South America, with records only in Argentina and Chile. The genus is composed by two species, which are reviewed and redescribed herein: C. chilensis Paramonov, 1931 and C. pictipennis (Bigot, 1857). The main characters of the external morphology of adults are photographed. Illustrations of the male and female terminalia of C. chilensis are also included. An identification key to species is presented, and the species distribution is briefly discussed.
Cyrtomyia; Andean region; taxonomy; bee flies
O gênero Neotropical Cyrtomyia Bigot tem uma distribuição restrita à região Andina da América do Sul, com registros assinalados apenas para a Argentina e Chile. O gênero é composto por duas espécies, que são aqui revisadas e redescritas: C. chilensis Paramonov, 1931 e C. pictipennis (Bigot, 1857). Os principais caracteres da morfologia externa dos adultos estão fotografados. Ilustrações das terminálias de machos e de fêmeas de C. chilensis também são incluídas. Uma chave de identificação para as espécies é apresentada e a distribuição das espécies é brevemente discutida.
Cyrtomyia; região Andina; taxonomia; Bombyliidae
Review of Cyrtomyia Bigot (Diptera, BomByliidae, Ecliminae) with a key to the Neotropical genera of Ecliminae and Cyrtomyia species
Carlos José Einicker LamasI,II,* ** E-mails: einicker@usp.br; pfmotta@usp.br ; Paula Fernanda Motta RodriguesI,II,III,* ** E-mails: einicker@usp.br; pfmotta@usp.br
IMuseu de Zoologia, Universidade de São Paulo. Caixa Postal 42.494, 04218-970, São Paulo, SP, Brasil
IIConselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship
IIIPrograma de Pós-Graduação em Ciências Biológicas (Zoologia), Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brasil
ABSTRACT
The Neotropical genus Cyrtomyia Bigot has a distribution restricted to the Andean region of South America, with records only in Argentina and Chile. The genus is composed by two species, which are reviewed and redescribed herein: C. chilensis Paramonov, 1931 and C. pictipennis (Bigot, 1857). The main characters of the external morphology of adults are photographed. Illustrations of the male and female terminalia of C. chilensis are also included. An identification key to species is presented, and the species distribution is briefly discussed.
Key-words:Cyrtomyia; Andean region; taxonomy; bee flies.
RESUMO
O gênero Neotropical Cyrtomyia Bigot tem uma distribuição restrita à região Andina da América do Sul, com registros assinalados apenas para a Argentina e Chile. O gênero é composto por duas espécies, que são aqui revisadas e redescritas: C. chilensis Paramonov, 1931 e C. pictipennis (Bigot, 1857). Os principais caracteres da morfologia externa dos adultos estão fotografados. Ilustrações das terminálias de machos e de fêmeas de C. chilensis também são incluídas. Uma chave de identificação para as espécies é apresentada e a distribuição das espécies é brevemente discutida.
Palavras-chave:Cyrtomyia; região Andina; taxonomia; Bombyliidae.
INTRODUCTION
The bee fly genus Cyrtomyia Bigot, reviewed herein, is composed of medium-sized flies with dark ground color and wings. The genus includes only two species, Cyrtomyia chilensis Paramonov, 1931 and Cyrtomyia pictipennis (Bigot, 1857), both endemic to the Andean region. The genus has been placed in three different subfamilies (Cylleniinae, Toxophorinae and Ecliminae) by different authors.
Bigot (1857) erected the genus Cyrtophorus to include a single species from Chile: C. pictipennis. He considered the new genus, known at the time only from the female, as a neighbor genus of Lepidophora Westwood. Later, Bigot (1892) proposed Cyrtomyia as a new replacement name for Cyrtophorus, which was preoccupied by Le Conte, 1850 in Coleoptera (Cerambycidae).
Paramonov (1931) added a second species (C. chilensis) to the genus, based on a single male. The author suspected that this male might be the still unknown male of C. pictipennis, but decided to describe it as a new species.
Painter & Painter (1974) revised the type-series of C. chilensis housed in the BMNH collection, and designated a lectotype and paralectotypes. The authors presented a redescription of the external morphology of this species, and agreed with Bigot (1857), stating that "this genus apparently belongs near Lepidophora in the Toxophorinae".
Hall (1976), in his study of the Chilean fauna of bee flies, presented a key to the species of Cyrtomyia and redescribed both, including the first descriptions of the male of C. pictipennis and the female of C. chilensis. Descriptions and illustrations of the male genitalia of both species were also presented. Hall pointed out the ambiguous placement of the genus between Toxophorinae and Cylleniinae by previous authors, but considered the former subfamily as being the correct choice.
This review of Cyrtomyia is being implemented as part of a further research which is being conducted on phylogenetic analysis of the Ecliminae subfamily. In this way, additional characters and illustrations are being added to the redescriptions, in order to complete them to the cladistics analysis.
MATERIAL AND METHODS
The studied material belongs to the following institutions: Museu de Zoologia da Universidade de São Paulo (MZUSP), São Paulo, SP, Brazil; the Natural History Museum (BMNH), London, UK; Deutsches Entomologisches Institut (DEI), Eberswalde, Germany.
Genitalia were macerated in 10% KOH at room temperature for one day to remove soft tissue, then rinsed in distilled water, dehydrated in an increasing ethanol series (30%, 50%, 70% and 95%) and dissected in glycerine. Genitalia preparations were placed in glycerine in microvials mounted on the pins beneath the specimen.
Terminology follows Cumming & Wood (2009), except for pleurae and terminalia, which follows Yeates (1994), wings, (Greathead & Evenhuis, 2001), and antennae, (Stuckenberg, 1999).
RESULTS
Taxonomy
Identification key to the Neotropical genera of Ecliminae and Cyrtomyia Bigot species:
Cyrtomyia Bigot, 1857
Cyrtophorus Bigot, 1857: 292. Type species: Cyrtophorus pictipennis Bigot, 1857, by monotypy. [Pre-occupied by Le Conte, 1850]. Loew, 1858: 347; Philippi, 1865: 652; Schiner, 1868: 113; Becker, 1913: 475; Painter et al., 1978: 22; Evenhuis, 1991: 33.
Cyrtomyia Bigot, 1892: 340 (new replacement for Cyrtophorus Bigot, 1857). Type species: Cyrtophorus pictipennis Bigot, 1857, automatic. Becker, 1913: 431, 437, 475, Edwards, 1930: 170; Paramonov, 1931: 40; Stuardo Ortiz, 1946: 91; Hall, 1969: 2, 3; Hull, 1973: 231, 232, 238; Painter & Painter, 1974: 94; Hall, 1976: 3, 10, 18, 119, 120, 124; Painter et al., 1978: 22; Greathead, 1988: 14, 16-20; Evenhuis, 1991: 33; Yeates, 1994: 21, 26, 64, 73, 74, 82, 113, 118, 125, 150, 163, 183, 190; Evenhuis & Greathead, 1999: 207.
Toxophorinarum Verrall, 1909: 479. Type species: Cyrtophorus pictipennis Bigot, 1857, by subsequent designation (Evenhuis, 1991: 75). [Unavailable; name proposed in synonymy with Cyrtophorus Bigot and not made available before 1961]. Evenhuis, 1991: 75; Evenhuis & Greathead, 1999: 207
Diagnosis
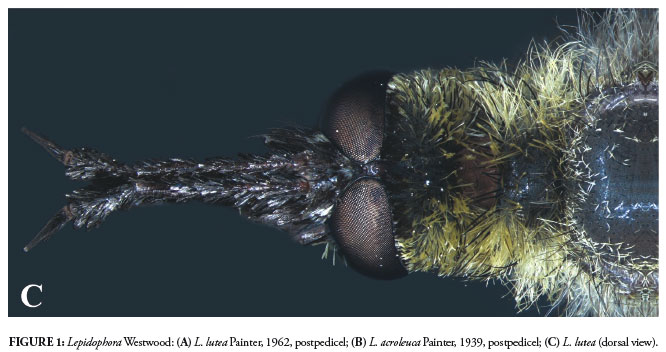
Among the genera of Ecliminae, Cyrtomyia shows similarities to Eclimus Loew and Palintonus François, as these three groups lack the strongly developed prothorax that is present in Lepidophora Westwood and Marmasoma White. However, the closest morphological similarities are observed between Cyrtomyia and Lepidophora. To separate these two genera, the bare postpedicel, weakly developed prothorax, male eyes set more closely together (Fig. 2), and lack of spatulate scales observed in Cyrtomyia are easy to observe.
Male
Head: Slightly narrower than thorax; dichoptic eyes almost touching below ocellar tubercle, separated by a distance much less than width of ocellar tubercle; ocellar tubercle strongly raised, dark brown with gray pruinescence; antenna dark brown with gray pruinescence; pedicel with ½ of length of postpedicel; postpedicel conical with longitudinal groove on inner surface, bare; style very small, located on a terminal depression; face polished light brown, long dark brown bristles bordering eyes on entire of face; proboscis velvety dark brown slightly larger than antenna; palpus formed by two palpomeres; occiput velvety dark brown with gray pruinescence, sparse short and thin dark brown bristles.
Thorax: Pronotum polished dark brown with long and thin dark brown bristles, anterior margin with long and thin dark brown scales and a row of long, strong and proclinate dark brown bristles, lateral with a row of long and strong dark brown bristles inserted on median portion, long and thin white scales on all surface; mesonotum polished dark brown with gray pruinescence, margins light brown; postpronotal lobe with sparse long dark brown bristles; pleurae light brown with gray pruinescence; anepisternum with long thin dark brown bristles; halter with knob with brown base and yellowish white apex with sparse short and thin dark brown scales on base; scutellum polished light brown with long dark brown bristles, posterior margin with dark brown bristles and strong dark brown bristles.
Wings: Circular whitish spot on apical ½ of discal cell and the center of the 3rd and 4th posterior cells; costal vein with two rows of modified bristles (toothed), with variable extent; calypter with long white hairs on anterior ½ of margin and short dark brown on posterior ½ of margin.
Legs: Polished light brown; tibiae with short dark brown bristles; tarsi with dark brown bristles, more concentrated ventrally; pulvilli light brown; claws light brown with dark brown apex. Leg I. Femur with light brown hairs, longer on ventral surface, a row of dark brown bristles on apical ⅓ of anteroventral surface. Leg II. Coxa with dark brown bristles on apical ⅓ of posterodorsal surface; trochanter with brown hairs; femur with light brown hairs on posterior and ventral surfaces, a row of dark brown bristles on anteroventral surface; pulvillus with ⅔ of length of claws. Leg III. Coxa with long brown hairs; trochanter with brown hairs; tarsi with rows of strong ventral dark brown bristles, strong apical dark brown bristles on ventral surface; pulvillus with ⅔ of length of claws.
Abdomen: Polished dark brown, long, cylindrical, with dark brown hairs and short dark brown scales; segment I with long brown hairs; segment IV with lateral long white scales.
Female
Similar to male, except: head as wide as thorax; dichoptic eyes, separated at ocellar tubercle level for 3.0 times tubercle width; ocellar tubercle not strongly raised; costal vein without toothed bristles characteristic of males.
Cyrtomyia chilensis Paramonov, 1931 (Figures 2, 3b, 4)
Cyrtomyia chilensis Paramonov, 1931: 41; Hull, 1973: 239; Hall, 1976: 18, 120, 122; Painter et al., 1978: 22; Theodor, 1983: 84; Yeates, 1994: 21, 125; Evenhuis & Greathead, 1999: 207.
Diagnosis
Brown spot on bifurcation of r4+5 large and running to anterior margin (Fig. 3B); abdominal segments I-IV with lateral long white scales; abdominal segment II without white tomentum; abdominal segments III and IV with short white scales and abdominal segments VI-VIII with tuft of long dark brown scales.
Male
Head: Ocellar tubercle with long dark brown bristles, anterior ones proclinate; frons velvety dark brown on upper ½ with short and thin white scales on upper ¼, lower ½ polished light brown with long and thin dark brown bristles and long and thin white scales; scape 2.8 times longer than pedicel; scape and pedicel with long and thin light brown scales, longer on ventral surface and long and thin dark brown bristles, shorter on pedicel; face with gray pruinescence on lower extremity, long dark brown bristles, more concentrated on upper ⅓, interspersed with long and thin white scales; proboscis with very small dark brown bristles, labellum with ¼ of length of proboscis; palpus brown with ½ of length of proboscis, with short dark brown bristles, palpomere I 2.0 times longer than palpomere II; occiput with short and thin dark brown scales concentrated on upper and lower thirds along margins of eyes, longer posteriorly, short and thin white scales more concentrated on median region along margins of eyes and longer and thinner around occipital foramen, interspersed with strong light brown bristles, strong dark brown bristles more concentrated around occipital foramen, interspersed with long and thin dark brown scales; lower region of head with long brown hairs.
Thorax: Lateral of pronotum with long and thin brown scales; mesonotum with many short brown hairs on anterior ⅓ and short white scales on margins that form a characteristic draw: two longitudinal stripes on anterior ½, which is open to lateral and meet again on posterior margin; postpronotal lobe with long and thin white scales, long and strong light brown bristles on anterior ⅓, medium dark brown bristles on median ⅓ and short and thin on posterior ⅓; supra alar callus with long and strong light brown bristles, sparse dark brown bristles around and long and short white scales; anepisternum with long and thin brown scales and long and thin white scales on upper margin; katepisternum with long and thin brown bristles; meropleurite with long and thin dark brown hairs; proepimeron, proepisternum, anepimeron, metepisternum, metepimeron, notopleuron, laterotergite and metanotum bare; halter with stem yellowish brown with short and thin white scales; scutellum with short white scales, margins with long and thin white scales.
Wings (Fig. 3B): Dark brown with whitish area on apical ⅓ and all posterior cells; brown spot on apical white area that extends from bifurcation of R4 and R5, through R2+3 to anterior margin; circular brown point on apex of R4 and other on apex of R5 reaching the posterior margin of wing; circular brown point on apex of M1, reaching the margin of wing; axillary cell clearer on ½; costal vein with short white scales and toothed bristles from basal ¼ to apex of R4 vein; stem vein with long and thin white scales on base; anal cell open on wing margin by a distance equivalent to ½ length of r-m crossvein; calypter smoky with dark brown margin; basicosta with tuft of long and thin white scales; r-m crossvein positioned just beyond of ½ of discal cell; short white scales on both surfaces of wing, more concentrated on upper portion of basal ½.
Leg I. Coxa with long brown hairs on all surfaces and brown and light brown bristles on apical ⅓ of anterodorsal and anterior surfaces; trochanter with brown hairs; femur with dark brown scales, strong apical dark brown bristles on anterior (1) and posterodorsal (1) surfaces; tibia with short and thin dark brown scales, a row of short dark brown bristles on anterior, dorsal and posterodorsal, strong apical dark brown bristles on dorsal (1), ventral (2), anterior (2) and posterior (2); tarsomere I with strong apical dark brown bristles on dorsal (2), posterior (2) and ventral (2), other tarsomeres with strong apical dark brown bristles on ventral surface; pulvillus with ½ length of claws. Leg II. Coxa with long brown hairs on anterodorsal and posterodorsal surfaces; femur with light brown scales on apical ½ of dorsal and posterior surfaces, short and thin light brown scales on ventral and anterior surfaces and long and thin dark brown scales on apical ½ of ventral and anterior surfaces, strong dark brown bristles on apical ⅓ of posterior surface; tibia with short white scales on anterior, dorsal and ventral surfaces, a row of dark brown bristles on anterodorsal, posterodorsal, posterior, posteroventral and anteroventral, strong apical dark brown bristles on dorsal (2), ventral (3), posterior (2), anteroventral (1) and anterior (2); tarsi with ventral rows of short dark brown bristles, tarsomeres I and II with translucent scales like white with reflected light, tarsomere I with strong apical dark brown bristles on dorsal and ventral surfaces, other tarsomeres with strong apical dark brown bristles on ventral surface. Leg III. Coxa with strong light brown bristles on apical ⅓ of posterodorsal surface and strong dark brown bristles on apical ⅓ of anterodorsal surface; femur with short translucent scales like white with reflected light, longer on ventral surface, row of strong dark brown bristles on anteroventral surface and apical thirds of ventral and posteroventral surfaces; tibia with short translucent scales like white with reflected light, row of dark brown bristles on anterior, ventral, dorsal, posteroventral and posterodorsal surfaces, strong apical dark brown bristles on ventral (3), dorsal (1) and anterior (1) surfaces.
Abdomen: Sternites light brown polished, short white scales on segments III and IV; segment I-IV with sparse lateral long white scales; segments VI-VIII with lateral tuft of long dark brown scales. Epandrium short, crescent shaped, posterior margin rounded with long dark brown bristles, short and spatulate dark brown bristles on posterior ½; gonocoxite elongated with rounded apex in lateral view (Fig. 4B), 2.4 times longer than wide; gonocoxal apodeme rounded and dilated on distal ½ in lateral view (Fig. 4B), with rounded apex in dorsal view (Fig. 4A); hypandrium not fused to gonocoxite, semicircular, anterior margin slightly concave (Fig. 4C); basiphallus wide, rounded in lateral view (Fig. 4B); epiphallus long, dilated dorsally, with rounded apex that exceeds the apex of distiphallus in lateral view (Fig. 4B); distiphallus short and thin; epiphallus and distiphallus exceed the posterior margin of the gonocoxite in lateral view (Fig. 4B); lateral aedeagal apodeme no exceeds the lateral margin of gonocoxite in dorsal view (Fig. 4A); ejaculatory apodeme rounded in lateral view (Fig. 4B), exceeds the anterior margin of gonocoxite in dorsal view (Fig. 4A); gonostylus robust, apex dilated with pointed apical process (Fig. 4A).
Female
Similar to male, except: frons polished dark brown with long and thin white scales on lower ⅔; palpus slightly shorter than proboscis; costa with very small white scales; anal cell open on margin of wing by a distance equivalent to ¼ of length of r-m crossvein; r-m crossvein positioned well beyond ½ of discal cell. Female terminalia (Figs. 4D,E): Spermathecae short; bulbs rhomboidal, as long as wide, longitudinal axis slightly longer than sperm pump, with numerous tubules on basal ½; sperm pump short, surrounded by small glands; spermathecal duct 5.2 times longer than sperm pump, connected directly to furca; distal portion of spermathecal duct with numerous tubules and dilated on base with claw shaped thorns well developed; proximal sclerotized collar well developed, with ornamentation, delimiting the base of sperm pump; furca formed by two bars, each with basal extremity forked.
Examined Material
Holotype male: Chile. Valparaíso, Limache, I-1918, P. Herbst col. (DEI). Chile. 1 male (B.M. 1881-56), T. Edmonds col. (BMNH); 1 male (B.M. 1937-27) and 2 females (B.M. 1934-265 and B.M. 1937-27), 1932, E.P. Reed col. (BMNH); Malleco, Termas Tolhuaca, 1 female (J.C. Hall Collection), 15-20.I.1959, L.E. Peña col. (BMNH); Pichinahuel, Cordillera de Nahuelbuta, 1 female, (J.C. Hall Collection), 10-20.I.1959, L.E. Peña col. (BMNH); Curicó, El Coigo, 1 male, 15.XII.1967 (MZUSP).
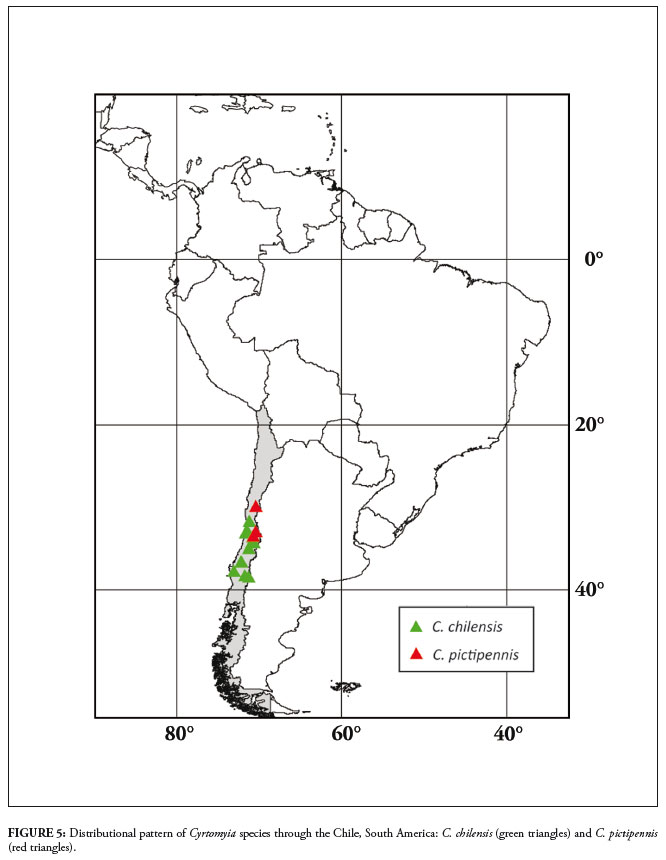
Geographic records
Chile (Valparaíso, Malleco, Arauco, Coquimbo, Curicó, Ñuble, Santiago, O'Higgins).
Cyrtomyia pictipennis (Bigot, 1857) (Fig. 3A)
Cyrtophorus pictipennis Bigot, 1857: 292; Becker, 1913: 475; Edwards, 1930: 170; Hull, 1973: 239; Painter & Painter, 1974: 94; Hall, 1976: 18, 120, 123, 124; Painter et al., 1978: 22; Evenhuis & Greathead, 1999: 207.
Diagnosis
Isolated brown spot on bifurcation of r4+5 not connected on any way with color on anterior margin (Fig. 3A); abdominal segments IV-VII with lateral long white scales; abdominal segment II with white tomentum; abdominal segments II-IV with short white scales and abdominal segments VI-VIII without tuft of long dark brown scales.
Female
Head: Dark brown with gray pruinescence and long dark brown bristles, anterior ones proclinate; front dark brown with gray pruinescence, long and thin white scales and long dark brown bristles, stronger laterally on upper ½; tuft of short white scales, very dense on center of upper ½, right in front of ocellar tubercle; scape 3.0 times longer than pedicel; scape with yellowish white scales and long dark brown bristles; pedicel with short dark brown scales and short and strong dark brown bristles, larger on ventral surface; face with gray pruinescence on adjacent area to eyes, long and strong dark brown bristles, more concentrated on upper ⅓, interspersed with long and thin white scales at time of antennal base and gena; proboscis with very small light brown bristles, labellum with ½ of length of proboscis; palpus light brown, with ⅔ of length of proboscis, with short dark brown bristles, palpomere I 1.5 times greater than palpomere II; occiput with long and thin yellowish white scales, pedunculate white scales more concentrated on median region along the margins of eyes and dark brown near vertex, strong dark brown bristles more concentrated around occipital foramen; lower region of head with long dark brown hairs.
Thorax: Mesonotum with many short brown hairs and short yellowish white scales; postpronotal lobe with long and thin white yellowish scales, sparse long dark brown bristles, long and strong light brown bristles on anterior ⅓, medium on median ⅓ and short and thin on posterior ⅓; supra alar callus with long and strong dark brown bristles, sparse dark brown bristles around and long and short yellowish white scales; postalar callus with long strong dark brown bristles and long and thin yellowish white scales; anepisternum with long and thin white yellowish scales on upper margin; katepisternum with long and thin white yellowish scales on upper ⅓; metepisternum with long and thin brown hairs on posterior ½; metepimeron with long and thin white scales on upper ½; meropleurite, proepimeron, proepisternum, anepimeron, notopleuron, laterotergite and metanotum bare; halter with stem dark brown with short white scales; scutellum with short and long yellowish white scales, margins with long and thin yellowish white scales.
Wings (Fig. 3A): Light brown with whitish area on apical ⅓ and all posterior cells; brown spot on apical whitish on bifurcation of R4 and R5; axillary cell slightly clearer; costal vein with short yellowish white scales; stem vein with long and thin yellowish white scales on base; anal cell open on margin of wing by a distance equivalent to ¼ of length of r-m crossvein; calypter light brown, dark brown margin with long white hairs on anterior ½ and dark brown on posterior ½; basicosta with tuft of long and thin yellowish white scales; r-m crossvein positioned just beyond the ½ of discal cell; short yellowish white scales on both surfaces of wing, more concentrated on upper region of basal ½.
Leg I. Coxa with long and thin yellowish white scales and long dark brown hairs, dark brown bristles on apical ⅓ of anterodorsal and anterior surfaces; trochanter with dark brown hairs; femur with short yellowish white scales on anterior, ventral and posterior surfaces and dark brown scales on dorsal surface, a strong apical dark brown bristle on anterior surface; tibia with yellowish white scales, except on ventral surface, a row of short dark brown bristles on anterodorsal, dorsal and posterodorsal surfaces, strong apical dark brown bristles on dorsal (2), ventral (2), anterior (3) and posterior (3) surfaces; tarsomere I with strong apical dark brown bristles on dorsal (1), posterior (2) and ventral (2) surfaces, other tarsomeres with strong apical dark brown bristles on ventral surface; pulvillus with ⅓ of length of claws. Leg II. Coxa with long and thin yellowish white scales and long dark brown hairs on anterodorsal and posterodorsal surfaces; femur with yellowish white scales on posterior and ventral surfaces and light brown scales on apical ½ of anterior and dorsal surfaces; tibia with short white scales on anterior, dorsal and posterior surfaces, a row of dark brown bristles on anterodorsal, dorsal, posterodorsal, posterior, posteroventral and anteroventral surfaces, strong apical dark brown bristles on dorsal (2), ventral (2), posterior (2), anteroventral (1) and anterior (2) surfaces; tarsomere I with strong apical dark brown bristles on dorsal (1), anteroventral (1) and posterior (1), other tarsomeres with strong apical dark brown bristles on ventral surface. Leg III. Coxa with long and thin yellowish white scales, strong dark brown bristles on apical ⅓ of posterodorsal surface and strong brown bristles on apical ⅓ of anterodorsal surface; femur with short white scales, except on surface posterodorsal, a row of strong dark brown bristles on anteroventral and ventral surfaces, a row of shorter bristles on posteroventral surface; tibia with short white scales, except on ventral surface, a row of dark brown bristles on anteroventral, anterodorsal, posterodorsal and ventral surfaces, strong apical dark brown bristles on ventral (1), dorsal (2) and anterior (3) surfaces.
Abdomen: Short white scales concentrated on segments III and IV; segment I with long and thin yellowish white scales; segment II with white tomentum; segments I-IV with long lateral white scales; segments VI-VIII with lateral tuft of long dark brown scales.
Male
Similar to female, except: ocellar tubercle with long proclinate dark brown bristles; frons with long and thin dark brown bristles, all with same thickness; postpedicel with very small dark brown bristles on basal ⅓ of outer surface; palpus with dark brown bristles, longer on outer surface; costal vein with toothed bristles that extends from apical ½ of wing to r4; r-m crossvein positioned beyond ½ of discal cell; abdomen with short white scales concentrated on segments II-IV; segments IV-VII with long lateral white scales more concentrated on segment IV; segments VI-VIII without lateral tufts of long dark brown scales.
Remarks
It was not possible to describe the male and female terminalia of the C. pictipennis by the unavailability of specimens for dissection. Complete description of male terminalia was presented by Hall (1976).
Material Examined
Lectotype female: Chile. (B.M. 1960-539) (BMNH (E) 240557); Paralectotypes: 1 female (BMNH (E) 240556); and 1 male (B.M. 1960-539) (ex. Bigot Collection).
Geographic records
Chile (Coquimbo, Santiago, Valparaíso).
DISCUSSION
Cyrtomyia and Lepidophora are the genera of Ecliminae with the most morphological similarities.
Both genera share a well-developed pronotum ornamented with spines, dichoptic males, and the presence of scales on the wings. The phenetic analysis implemented by Greathead (1988) corroborated this hypothesis of proximity, and a cladistic analysis of the Ecliminae is being prepared in order to test it through this approach.
Cyrtomyia has a Trans-Andean distribution (Fig. 5) in an area of low rainfall (Montgomery et al., 2001). Indeed, the entire central-Andes region (15º to 33ºS) is arid, with drier conditions than the other portions of the mountain range (Montgomery et al., 2001). Considering only ecological conditions, it seems likely that the distribution of Cyrtomyia might extend northwards, but, on the other hand, Cyrtomyia is presumed not to occur in South America east of the Andes, nor in Central and North America, based on the examination of extensive material from the New World. If the sister-group relationship of the two genera is corroborated by the cladistic analysis, it will strongly suggest that the Andean uplift was the ancient vicariant event separating Cyrtomyia and Lepidophora on opposite sides of the mountain range.
ACKNOWLEDGMENTS
We thank Dr. Erica McAlister for the kind assistance during the visit of CJEL to the Natural History Museum, London. To Dr. Ramon Mello (UNESP) for taking photos and checking the redescription of C. pictipennis with the holotype of this species housed in DEI, during his visit to this institution, and also to Dr. Joachim Ziegler (DEI) for the assistance and facilities made available to Dr. Mello. Thanks also to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the grant to CJEL (Proc. No. 481024/2008-5) and the grant to PFMR (Proc. No. 143050/2011-7).
Aceito em: 28/02/2013
Publicado em: 31/03/2013
- Becker, T. 1913. Genera Bombyliidarum. Ezhegodnik Zoologicheskago Huzeya Imperatorskoi Akademiia Nauk., 17:421-502.
- Bigot, J.M.F. 1857. Diptéres nouveaux provenant du Chili. Annales de la Société de Entomologique de France, (3)5:277-308.
- Bigot, J.M.F. 1892. Diptéres nouveaux ou peu connus. 37e partie. XLVI Bombylidi (mihi) 1re partie. Annales de la Société de Entomologique de France, 61:321-76.
- Cumming, J.M. & Wood, D.M. 2009. Morphology and terminology. In: Brown, B.V.; Borkent, A.; Cumming, J.M.; Wood, D.M.; Woodley, N.E. & Zumbado, M., Manual of Central American Diptera. NRC Research Press, Ottawa. v.1, p. 9-50.
- Edwards, F.W. 1930. Bombyliidae, Nemestrinidae and Cyrtidae. In: Diptera of Patagonia and south Chile based mainly on material in the British Museum (Natural History). British Museum (Natural History), London. Part 5, fasc. 2, p. 62-197.
- Evenhuis, N.L. 1991. World catalog of genus-group names of bee flies (Diptera: Bombyliidae). Bishop Museum Bulletin in Entomology 5. Bishop Museum Press, Honolulu.
- Evenhuis, N.L. & Greathead, D.J. 1999. World Catalog of Bee Flies (Diptera: Bombyliidae). Backhuys Publishers, Leiden.
- Greathead, D.J. 1988. The relationships of Tillyardomyia Tonnoir with a redefinition of the subfamily Ecliminae (Diptera: Bombyliidae). New Zealand Entomologist, 11:14-21.
- Greathead, D.J. & Evenhuis, N.L. 2001. Annotated keys to the genera of African Bombylioidea (Diptera: Bombyliidae; Mythicomyiidae). African Invertebrates, 42:105-224.
- Hall, J.C. 1969. A review of the Cylleniinae with a world revision of the genus Thevenemyia Bigot (Eclimus auct.) (Diptera: Bombyliidae). University of California Publications in Entomology, 56:1-85.
- Hall, J.C. 1976. The Bombyliidae of Chile. University of California Publications in Entomology, 76[1975]:1-278.
- Hull, F.M. 1973. The Bee Flies of the World. The genera of the family Bombyliidae. Bulletin of the United States National Museum, 286:3-687.
- Loew, H. 1858. Bericht über die neueren Erscheinungen auf dem Gebiete der Dipterologie. Berliner Entomologisch Zeitschrift, 2:325-349.
- Montgomery, D.R.; Balco, G. & Willett, S.D. 2001. Climate, tectonics, and the morphology of the Andes. Geology, Boulder, 29(7):579-582.
- Ortiz, C.S. 1946. Catalogo de los Dípteros de Chile. Ministerio de Agricultura, Santiago.
- Painter, R.H. & Painter, E.M. 1974. Notes on, and redescription of types of South American Bombyliidae (Diptera) in European and United States Museums. Kansas States University, Agricultural Experimental Station, Research Publications, 168:1-322.
- Painter, R.H.; Painter, E.M. & Hall, J. 1978. Family Bombyliidae. In: A Catalogue of the Diptera of the Americas South of the United States, 38:1-92.
- Paramonov, S.J. 1931. Beiträge zur Monographie der Bombyliiden Gattungen Amictus, Lyophlaeba etc. (Diptera). Zbirnik Prats' Zoologichnogo Muzeyu, 11:1-218.
- Philippi, R.A. 1865. Aufzählung der chilenischen Dipteren. Verhandlungen der Zoologisch-Botanischen Gesellschaft in Wien, 15:595-782.
- Schiner, I.R. 1868. Diptera. In: Reise der Österreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859, unter den Befehlen des Commodore B. von Wüllerstorf-Urbair. Zoologischer Theil, Wien.v. 2, pt. 1, Section B.
- Stuckenberg, B.R. 1999. Antennal evolution in the Brachycera (Diptera), with a reassessment of terminology relating to the flagellum. Studia Dipterologica, 6:33-48.
- Theodor, O. 1983. The genitalia of Bombyliidae (Diptera). Israel Academy of Sciences and Humanities, Jerusalem.
- Yeates, D.K. 1994. The cladistics and classification of the Bombyliidae (Diptera: Asiloidea). Bulletin of the American Museum of Natural History, 219:1-191.
- Verrall, G.H. 1909. British flies. Vol. V. Stratiomyidae and succeeding families of the Diptera Brachycera of Great Britain. Gurney & Jackson, London.
Publication Dates
-
Publication in this collection
26 Mar 2013 -
Date of issue
2013
History
-
Received
28 Feb 2013 -
Accepted
31 Mar 2013