ABSTRACT
The Serra do Urubu mountain range is considered a key biodiversity area. It is situated in the Pernambuco Endemism Center, one of the most threatened regions of the Brazilian Atlantic Forest. However, despite the high importance of this area little research on its herpetofauna has been performed. The present study presents an inventory of the herpetofauna of the region, through bibliographic review, searches in museum collections and field expeditions to the RPPNs Frei Caneca and Pedra D’Antas, in the municipalities of Jaqueira and Lagoa dos Gatos. The conservation status of the amphibians of the region is discussed. Five expeditions, between 2012 and 2013 were made. The methods employed were visual transect surveys, acoustic census and pitfall traps. We recorded a total of 46 amphibian species, belonging to nine families: Craugastoridae (3 spp.), Bufonidae (3 spp.), Ranidae (1 sp.), Hylidae (25 spp.), Leptodactylidae (8 spp.), Odontophrynidae (1 sp.), Hemiphractidae (2 spp.), Phyllomedusidae (2 spp.) and Microhylidae (1 sp.). We recorded 42 species of squamates: 16 species of lizards families Phyllodactylidae (1 sp.), Gekkonidae (1 sp.), Gymnophthalmidae (1 sp.), Polychrotidae (1 sp.), Leiosauridae (1 sp.), Tropiduridae (3 spp.), Dactyloidae (2 spp.), Diploglossidae (2 spp.), Teiidae (2 spp.), Scincidae (1 sp.), and Iguanidae (1 sp.); and 24 species of snakes: Boidae (3 spp.), Colubridae (2 spp.), Dipsadidae (13 spp.), Elapidae (2 spp.), Typhlopidae (1 sp.), and Viperidae (3 spp.). The occurrence of rare and/or threatened species such as the snakes Dipsas sazimai, Lachesis muta and Sibynomorphus sp. and the amphibians Hylomantis granulosa, Chiasmocleis alagoana, Boana freicanecae and Phyllodytes gyrinaethes reinforces the need for conservation measures at this highly threatened region of the Atlantic Forest.
KEY-WORDS:
Anura; Northeastern Brazil; Pernambuco; Squamata; RPPN Pedra D’Antas
RESUMO
O complexo da Serra do Urubu é considerado uma área chave para a conservação da biodiversidade, estando inserida no Centro de Endemismo de Pernambuco, uma das sub-regiões mais ameaçadas da Mata atlântica brasileira. Porém, apesar da alta importância dessa área, estudos relacionados com a herpetofauna local ainda são escassos. Para a elaboração da lista da herpetofauna da Serra do Urubu, foram feitas consultas na bibliografia, buscas em coleções herpetológicas e expedições de campo para a RPPN de Pedra D’Antas e RPPN Frei Caneca, localizadas nos municípios de Jaqueira e Lagoa dos Gatos. Uma discussão sobre o status de conservação dos anfíbios da região também foi realizada. Foram feitas cinco expedições entre os anos de 2012 e 2013, utilizando as metodologias de busca ativa em transectos lineares, censo auditivo e armadilhas de interceptação e queda. No total foram registrados 46 espécies de anfíbios anuros, pertencentes a nove famílias: Craugastoridae (3 spp.), Bufonidae (3 spp.), Ranidae (1 sp.), Hylidae (25 spp.), Leptodactylidae (8 spp.), Odontophrynidae (1 sp.), Hemiphractidae (2 spp.), Phyllomedusidae (2 spp.) e Microhylidae (1 sp.).Com relação aos répteis foram registradas 42 espécies, sendo 16 de lagartos: Phyllodactylidae (1 sp.), Gekkonidae (1 sp.), Gymnophthalmidae (1 sp.), Polychrotidae (1 sp.), Leiosauridae (1 sp.), Tropiduridae (3 spp.), Dactyloidae (2 spp.), Diploglossidae (2 spp.), Teiidae (2 spp.), Scincidae (1 sp.), Iguanidae (1 sp.); e 24 de serpentes: Boidae (3 spp.), Colubridae (2 spp.), Dipsadidae (13 spp.), Elapidae (2 spp.), Typhlopidae (1 sp.), Viperidae (3 spp.). Espécies consideradas raras e/ou ameaçadas de extinção como as serpentes Dipsas sazimai, Lachesis muta e Sibynomorphus sp. e os anfíbios Hylomantis granulosa, Chiasmocleis alagoana, Boana freicanecae e Phyllodytes gyrinaethes, reforçam a necessidade de conservação dessa região de Mata atlântica extremamente ameaçada.
PALAVRAS-CHAVE:
Anura; Nordeste do Brasil; Squamata; RPPN Pedra D’Antas
INTRODUCTION
The Atlantic Forest is a global hotspot of biodiversity and one of the most threatened biomes worldwide (Myers et al., 2000MYERS, N.; MITTERMEIER, R.A.; MITTERMEIER, C.G.; FONSECA, G.A.B. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403:853-858.), sheltering high levels of endemic species with restricted distribution (seeSilva & Castelleti, 2003SILVA, J.M.C. & CASTELLETI, C.H.M. 2003. Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal, C. & Câmara, I.G. (Eds.). The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington, CABS & Island Press. p. 31-42.; Haddad & Prado, 2005HADDAD, C.F.B. & PRADO, C.P.A. 2005. Reproductive modes in frogs and their unexpected diversity in the Atlantic Rain Forest of Brazil. Bioscience, 55(3):207-217.; Haddad et al., 2013HADDAD, C.F.B.; TOLEDO, L.F.; PRADO, C.P.A.; LOEBMANN, D.; GASPARINI, J.L. & SAZIMA, I. 2013. Guia dos anfíbios da Mata Atlântica: diversidade e biologia. São Paulo, Anolis Books.). The Atlantic Forest covers a very large area from the state of Rio Grande do Norte to the state of Rio Grande do Sul. It’s a very heterogeneous biome, with significant differences in species composition and origin along the various endemism centers occurring between the north and south Atlantic Forest (Silva & Castelleti, 2003SILVA, J.M.C. & CASTELLETI, C.H.M. 2003. Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal, C. & Câmara, I.G. (Eds.). The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington, CABS & Island Press. p. 31-42.; Silva et al., 2004SILVA, J.M.C.; SOUSA, M.C. & CASTELLETI, C.H.M. 2004. Areas of endemismo for passerine birds in the Atlantic Forest, South America. Global Ecology and Biogeography, 13:85-92.; Carnaval et al., 2014CARNAVAL, A.C.; WALTARI, E.; RODRIGUES, M.T.; ROSAUER, D.; VANDERWALL, J.; DAMASCENO, R.; PRATES, I.; STRANGAS, M.; SPANOS, Z.; RIVERA, D.; PIE, M.R.; FIRKOWSKI, C.R.; BORNSCHEIN, M.R.; RIBEIRO, L.F. & MORITZ, C. 2014. Prediction of phylogeographic endemismo of an environmentally complex biome. Proceedings of the Royal Society B, Biological Science, London, 281:1-8.).
The Pernambuco Endemism Center (PEC) (sensuPrance, 1982PRANCE, G.T. 1982. Forest refuges: evidences from woody angiosperms. In: Prance, G.T. (Ed.). Biological diversification in the tropics. New York, Columbia University Press. p. 137-158.; Silva & Castelleti, 2003SILVA, J.M.C. & CASTELLETI, C.H.M. 2003. Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal, C. & Câmara, I.G. (Eds.). The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington, CABS & Island Press. p. 31-42.) comprises the fragments of Atlantic Forest north of the São Francisco River; between the States of Rio Grande do Norte throughout Alagoas, in northeastern Brazil. The fauna and flora of this sub-biogegraphical region are more closely related to Amazonia than to the southern Atlantic Forest (Santos et al., 2007SANTOS, A.M.M.; CAVALCANTI, D.R.; SILVA, J.M.C. & TABARELLI, M. 2007. Biogeographical relantionships among tropical forests in north-eastern Brazil. Journal of Biogeography, 34:437-446.; Canedo & Haddad, 2012CANEDO, C. & HADDAD, C.F.B. 2012. Phylogenetic relationships within anuran clade Terrarana, with emphasis on the placement of Brazilian Atlantic rainforest frogs genus Ischnocnema (Anura: Brachycephalidae). Molecular Phylogenetics and Evolution, 65(2):610-620.; Fouquet et al., 2012FOUQUET, A.; LOEBMANN, D.; CASTROVIEJO-FISHER, S.; PADIAL, J.M.; ORRICO, V.G.D.; LYRA, M.L.; ROBERTO, I.J.; KOK, P.J.R.; HADDAD, C.F.B. & RODRIGUES, M.T. 2012. From Amazonia to the Atlantic Forest: molecular phylogeny of Phyzelaphryninae frogs reveals unexpected diversity and striking biogeographic pattern emphasizing conservation challenges. Molecular Phylogenetics and Evolution, 65(2):547-561.). This is the most fragmented and threatened region in the Atlantic Forest, with only 2% (360.455 hectares) of original forest cover left, of which only 3.371 are protected in Conservation Units (Brown & Brown, 1992BROWN, K.S.J.R. & BROWN, G.G. 1992. Habitat alteration and species loss in Brazilian forests. In: Whitmore, T.C. & Sayer, J.A. Tropical Deforestation and Species Extinction. London, Chapman & Hall. p. 119-142.; Ribeiro et al., 2009RIBEIRO, M.C.; METZGER, J.P.; MARTENSEN, A.C.; PONZONI, F.J. & HIROTA, M.M. 2009. The Brazilian Atlantic forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6):1141-1153.). The high level of deforestation since the colonial period, with accelerated rates of deforestation in the 1970’s and 1980’s, especially for sugar cane plantations and cattle grazing, lead to the actual disturbing level of deforestation in this region (Ranta et al., 1998RANTA, P.; BLOM, T.; NIEMELÃ, J.; JOENSUU, E. & SIITTONEN, M. 1998. The fragmented Atlantic forest of Brazil: size, shape, and distribution of forest fragments. Biodiversity and Conservation, 7:385-403; Tabarelli et al., 2005TABARELLI, M.; SIQUEIRA-FILHO, J.A. & SANTOS, A.M.M. 2005. A floresta atlântica ao norte do Rio São Francisco. In: Pôrto, K.C.; Almeida-Cortez, J.S. & Tabarelli, M. (Orgs.). Diversidade Biológica e Conservação da Floresta Atlântica ao norte do Rio São Francisco, Brasília, Ministerio do Meio Ambiente. p. 25-37 (Biodiversidade 14).; Pereira et al., 2014PEREIRA, G.A.; DANTAS, S.M.; SILVEIRA, L.F.; RODA, S.A.; ALBANO, C.; SONNTAG, F.A.; LEAL, S.; PERIQUITO, M.C.; MALACCO, G.B. & LEES, A.C. 2014. Status of the globally threatened forest birds of northeast Brazil. Papéis Avulsos de Zoologia, 54(14):177-194.). Most of the publications on the herpetofauna of the altitudinal seasonal tropical forest of the PEC, deal with species descriptions (e.g.,Carnaval & Peixoto, 2004aCARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004c. Hylomantis granulosa. In: The IUCN Red List of Threatened Species. Version 2014.3. Available at: Available at: www.iucnredlist.org
. Access in: 02/02/2015.
www.iucnredlist.org...
; Rodrigues et al., 2005RODRIGUES, M.T.; FREIRE, E.M.X.; PELLEGRINO, K.C.M. & SITES JR. J.W. 2005. Phylogenetic relantionships of a new genus and species of microteiid lizard from the Atlantic forest of north-eastern Brazil (Squamata, Gymnophthalmidae). Zoological Journal of the Linnean Society, 144:543-557.; Freire et al., 2010FREIRE, E.M.X.; CARAMASCHI, U. & GONCALVES, U. 2010. A new species of Dendrophidion (Serpentes: Colubridae) from the Atlantic Rain Forest of Northeastern Brazil. Zootaxa, 2719:62-68.; Passos et al., 2010PASSOS, P.; FERNANDES, R.; BÉRNILS, R.S. & MOURA-LEITE, J.C. 2010. Taxonomic revision of the Brazilian Atlantic Forest Atractus (Reptilia: Serpentes: Dipsadidae). Zootaxa, 2364:1-63.; Gonçalves et al., 2012GONÇALVES, U.; TORQUATO, S.; SKUK, G. & SENA, G.A. 2012. A new species of Coleodactylus Parker, 1926 (Squamata: Sphaerodactylidae) from the Atlantic Forest of northeast Brazil. Zootaxa, 3204:20-30.); faunal inventories and natural history (e.g.,Santos & Carnaval, 2002SANTOS, A.M.M.; CAVALCANTI, D.R.; SILVA, J.M.C. & TABARELLI, M. 2007. Biogeographical relantionships among tropical forests in north-eastern Brazil. Journal of Biogeography, 34:437-446.; Silva et al., 2006SILVA, S.T.; SILVA, U.G.; SENA, G.A.B. & NASCIMENTO, F.A.C. 2006. A biodiversidade da Mata Atlântica alagoana: anfíbios e répteis. In: Moura, F.B.P.M. (Org.). A Mata Atlântica em Alagoas. Maceio, EDUFAL. p. 65-76.; Santana et al., 2008SANTANA, G.G.; VIEIRA, W.L.S.; PEREIRA-FILHO, G.A.; DELFIM, F.R.; LIMA, Y.C.C. & VIEIRA, K.S. 2008. Herpetofauna em um fragmento de Mata Atlântica no estado da Paraíba, região nordeste do Brasil. Biotemas, 21(1):75-84.; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.; Moura et al., 2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.; Roberto et al., 2015ROBERTO, I.J.; MELGAREJO, A. & ÁVILA, R.W. 2015. Répteis (Testudines, Squamata, Crocodylia) da Reserva Biológica de Pedra Talhada. In: Studer, A.; Nusbaumer, L. & Spichiger, N. (Eds.). Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas-Pernambuco, Brasil). Boissiera, 68:357-375.) or geographical distributional records (e.g.,Santos & Amorim, 2010SANTOS, E.M. & AMORIM, F.O. 2010. Geographic distribution: Chiasmocleis alagoana. Herpetological Review, 41(1):103.; Santos & Santos, 2010SANTOS, S.P.L. & SANTOS, E.M. 2010. Hypsiboas exastis. Geographic Distribution. Herpetological Review, 41:375-375.; Vilela et al., 2011VILELA, B.; LIMA, M.G.; GONÇALVES, U. & SKUK, G.O. 2011. Siphlophis compressus (Daudin, 1803) (Squamata: Dipsadidae): First records for the Atlantic forest north of the São Francisco river, northeastern Brazil. Cuadernos de Herpetologia, 25(1):23-24.; Rodrigues et al., 2013aRODRIGUES, K.C.; DELFIM, F.R.; CASTRO, C.S.S.; FRANÇA, F.G.R.; LEITE-FILHO, E.; MESQUITA, D.O.; OLIVEIRA, F.A.; SANTOS, A.C.A.; FERRARI, S.F. & VALENÇA-MONTENEGRO, M.M. 2013A. Strobilurus torquatus Wiegmann, 1834 (Squamata: Tropiduridae): New records from the Brazilian state of Paraíba and a geographic distribution map. Check List, 9(3):614-617.,bRODRIGUES, R.; ALBUQUERQUE, R.L.; SANTANA, D.J.; LARANJEIRAS, D.O.; PRÓTAZIO, A.S.; FRANÇA, F.G.R. & MESQUITA, D.O. 2013b. Record of the occurence of Lachesis muta (Serpentes, Viperidae) in an Atlantic Forest fragment in Paraíba, Brazil, with comments on the species’ preservation status. Biotemas, 26(2):283-286.).
Despite the increase of reptile and amphibian inventories in the PEC, still there are many large gaps of this biogeographical region that needs more studies regarding species composition, natural history, geographical distribution and conservation status of the species, especially to promote conservation action.
The PEC harbors at least five threatened species of snakes: Atractus caete (endangered), Bothrops muriciensis (endangered), Echinanthera cephalomaculata (vulnerable), Amerotyphlops amoipira (endangered), A. paucisquamus (vulnerable); and four species of anurans Hylomantis granulosa (vulnerable), Chiasmocleis alagoana (vulnerable), Phyllodytes gyrinaethes (critically endangered) and Physalaemus caete (endangered) (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.).
The present study aims to determine the composition, and the distribution of the herpetofauna of the the Serra do Urubu mountain range, a remnant of Atlantic Forest withinthe Pernambuco Endemism Center, and to discuss the conservation status of the herpetofauna in the area.
MATERIAL AND METHODS
Study area
The RPPN Pedra D’Antas (08°34’S, 35°37’W) has a total area of 330 hectares, located in the Serra do Urubu mountain range, in the municipalities of Lagoa dos Gatos and Jaqueira, State of Pernambuco, northeastern Brazil (Fig. 1). This Reserve lies adjacent to the RPPN Frei Caneca, (seefig. 1) and together these fragments cover approximately 1,200 hectares, being one of the largest remnants of Atlantic Forest in the region.
Map of RPPN Pedra D’Antas and RPPN Frei Caneca, at the Serra do Urubu mountain range municipalities of Jaqueira and Lagoa dos Gatos, Pernambuco State, Brazil, with the respectives study sites.
The Reserve is located in the Planalto of Borborema. The vegetation in the area is altitudinal seasonal tropical forest with 500-750 m of altitude (Veloso et al., 1991VELOSO, H.P.; RANGEL-FILHO, A.L.R. & LIMA, J.C.A. 1991. Classificação da vegetação brasileira adaptada a um sistema universal. Rio de Janeiro, IBGE.), with a mean annual rainfall of 1,000-1,500 mm. The regional climate is hot and humid (Nimer, 1972NIMER, E. 1972. Climatologia da região nordeste do Brasil. Revista Brasileira de Geografia, 34:3-51.), with four to five months of dry season between October to February and the wet season extending from March to September (IBGE, 1985INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA - IBGE. 1985. Atlas nacional do Brasil: região Nordeste. Rio de Janeiro, IBGE.).
We selected six study sites for the data collection, described as follow:
-
Site 1: (08°41’35.1”S, 35°51’27.8”W, 584 m). Secondary growth forest, with a predominance of arbustive-arboreal vegetation. Banana plantations, with a permanent stream. Presence of rocky outcrops, with accumulation of leaf litter.
-
Site 2: (08°41’47.4”S, 35°51’17.3”W, 586 m). Secondary growth forest, with a predominance of arbustive-arboreal vegetation. Presence of rocky outcrops, with, accumulation of leaf litter. Absence of bodies of water.
-
Site 3: (08°41’48.8”S, 35°51’25.9”W, 592 m). Secondary forest, at the forest edge, 500 m away from the pond next to the center house in the RPPN. During the raining season a marsh is formed connecting with this pond.
-
Site 4: (08°42’20.7”S, 35°51’10.4”W, 717 m). Primary forest, with arboreal vegetation 20-30 meters high, abundance of terrestrial and arboreal bromeliads. Without bodies of water.
-
Site 5: (08°42’36.2”S, 35°51’23.5”W, 687 m). Primary forest, with arboreal vegetation 20-30 meters high, abundance of terrestrial and arboreal bromeliads. Located at the margins of the Açude das Moças pond. The pond is approximately 30 meters wide, with 3 km of length and a maximum depth of 10 meters.
-
Site 6: (08°41’36.6”S, 35°51’20.5”W, 548 m). Permanent pond located next to the center house in the RPPN, with a length of 1.5 km, 800 meters wide, and a maximum depth of 5 meters, arbustive-arboreal vegetation in the margins.
Information regarding the herpetofauna of RPPN Frei Caneca was gathered using bibliographic references (Santos & Carnaval, 2002SANTOS, E.M. & CARNAVAL, A.C.O.Q. 2002. Anfíbios anuros do Estado de Pernambuco. In: Tabarelli, M. & Silva, J.M.C. (Orgs.). Diagnóstico da Biodiversidade de Pernambuco. Recife, Editora Massangana. p. 529-535.; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.; Moura et al., 2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.), and additional occasional records made by our team.
Surveys
Five expeditions were done at RPPN Pedra D’Antas, of four to seven days duration, in August and December of 2012, March, June and September of 2013. Totalizing 24 field days of effort.
For the herpetofauna inventory we selected four methodologies: visual and acoustic transect survey, pitfall traps and opportunistic encounter methodology. The visual and acoustic transect survey (sensuCrump & Scott Jr., 1994CRUMP, M.L. & SCOTT JR., N.J. 1994. Visual encounter surveys. In: Heyer, W.R.; Donnelly, M.A.; McDiarmid, R.W.; Hayek, L.A.C. & Foster, M.S. Measuring and monitoring biological diversity. Standard methods for amphibians. Washington & London, Smithsonian Institution Press. p. 17-39.; Rödel & Ernst, 2004RÖDEL, M.O. & ERNST, R. 2004. Measuring and monitoring amphibian diversity in tropical forests. I. An evaluation of methods with recommendations for standardizations. Ecotropica, 10:1-14.), consisted of five transects 250 × 2 meters, in Sites 1-5. Each transect was surveyed by two researchers for one hour of effort, during both daily and nightly periods. Totaling 35 transects surveyed, seven transects in each site (four nocturnal and three diurnal), with a total effort of 14 hours/man per transect of visual and acoustic surveys respectively.
At the same area of the transects we installed five stations of pitfall traps with drift fence (Cechin & Martins, 2000CECHIN, S.Z. & MARTINS, M. 2000. Eficiência de armadilhas de queda (pitfall traps) em amostragens de anfíbios e répteis. Revista Brasileira de Zoologia, 17:729-740.), each station was composed of eight 60 liter buckets in a linear arrangement, separated by a 1 meter high plastic fence, and each bucket installed 10 meters apart. Each station was separated by at least 1 km of distance. The traps remained opened for 18 nonconsecutive days, totaling 720 buckets/day.
At Site 6 only acoustic surveys for amphibians were performed, totalizing 48 man/hours. For reptiles we also used the opportunistic encounter methodology to record species in the RPPN, outside our study sites, or recorded by others.
Voucher specimens were collected (under licence SISBIO 34734-1), euthanized with xilocaine anesthetic, fixed in formol 10% and conserved in alcohol 70%. The specimens were deposited at the Coleção de Herpetologia da Universidade Regional do Cariri (URCA-H), Crato, Ceará.
Data analysis
The effectiveness of the sampling effort was estimated based on rarefaction curves, through 10,000 randomizations of a matrix in which each row represents a species and its number of captures while each column represents one day of sampling effort (pitfall traps, visual transect survey, acoustic survey and opportunistic encounter). This analysis was performed using Past 3.1 (Hammer et al., 2001HAMMER, O.; HARPER, D.A.T. & RYAN, P.D. 2001. Past: Paleontological statistics software package for education and data analysis. Available at: Available at: http://palaeo-electronica.org/2001_1/past/issue1_01.htm
. Access in: 02/02/2015.
http://palaeo-electronica.org/2001_1/pas...
). We use the program Estimates (Colwell, 2006COLWELL, R.K. 2006. Estimates: Statistical Estimation of Species Richness and Shared Species from Samples. Version 7.5. User’s Guide and Application. Available at: Available at: http://viceroy.eeb.uconn.edu/estimates
. Access in: 02/02/2015.
http://viceroy.eeb.uconn.edu/estimates...
) to estimate species richness through the indicators Jacknife I and ICE.
The taxonomy adopted follows (Frost, 2016FROST, D.R. 2016. Amphibian species of the world: an online Reference. version 5.6. Available at: Available at: http://research.amnh.org/vz/herpetology/amphibia
. Access in: 02/12/2016.
http://research.amnh.org/vz/herpetology/...
for amphibians, (Costa & Bérnils, 2014COSTA, H.C. & BÉRNILS, R.S. 2014. Répteis brasileiros: Lista de espécies. Herpetologia Brasileira, 3(3):74-84.) for reptiles, with the exception of the lizard family Scincidae, where we follow (Pyron et al., 2013PYRON, R.A.; BURBRINK, F.T. & WIENS, J.J. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionay Biology, 13:1-93.). The conservation status of the species was classified according to (IUCN, 2016INTERNATIONAL UNION FOR CONSERVATION OF NATURE - IUCN. 2016. The IUCN Red List of Threatened Species. version 2016.2. Available at: Available at: www.iucnredlist.org
. Access in: 01/12/2016.
www.iucnredlist.org...
) and (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.).
RESULTS
Amphibians
We recorded 38 amphibian species at RPPN Pedra D’Antas, all anurans, distributed in nine famlilies: Hylidae was the most representative (n = 23 spp.), followed by Leptodactylidae (n = 8 spp.), Phyllomedusidae, Bufonidae and Craugastoridae (n = 2 spp.), Microhylidae, Ranidae, Odontophrynidae has only one species (Table 1; Figs. 2-7). The visual transect survey methodology was able to detect 24 species, the acoustic survey detected 26 species, while the pitfall traps only captured seven species, but Chiasmocleis alagoana was only recorded through this method (Table 1). The rarefaction curve for amphibians tends close to reach an asymptote (Fig. 8), however the non-parametric richness estimators Jacknife I and ICE recovered 48 spp.
Amphibians species recorded at the Serra do Urubu mountain range. (A) Rhinella crucifer, (B) Rhinella granulosa (Photo by C.O. Gussoni), (C) Rhinella jimi (Photo by C.O. Gussoni), (D) Gastrotheca fissipes, (E) Gastrotheca pulchra (Photo by B. Lisboa), (F) Hylomantis granulosa, (G) Dendropsophus branneri, (H) Dendropsophus elegans.
Amphibians species recorded at the Serra do Urubu mountain range. (A) Dendropsophus haddadi, (B) Dendropsophus minutus, (C) Dendropsophus oliveirai, (D) Dendropsophus soaresi, (E) Boana albomarginata, (F) Boana atlantica, (G) Boana crepitans, (H) Boana exastis.
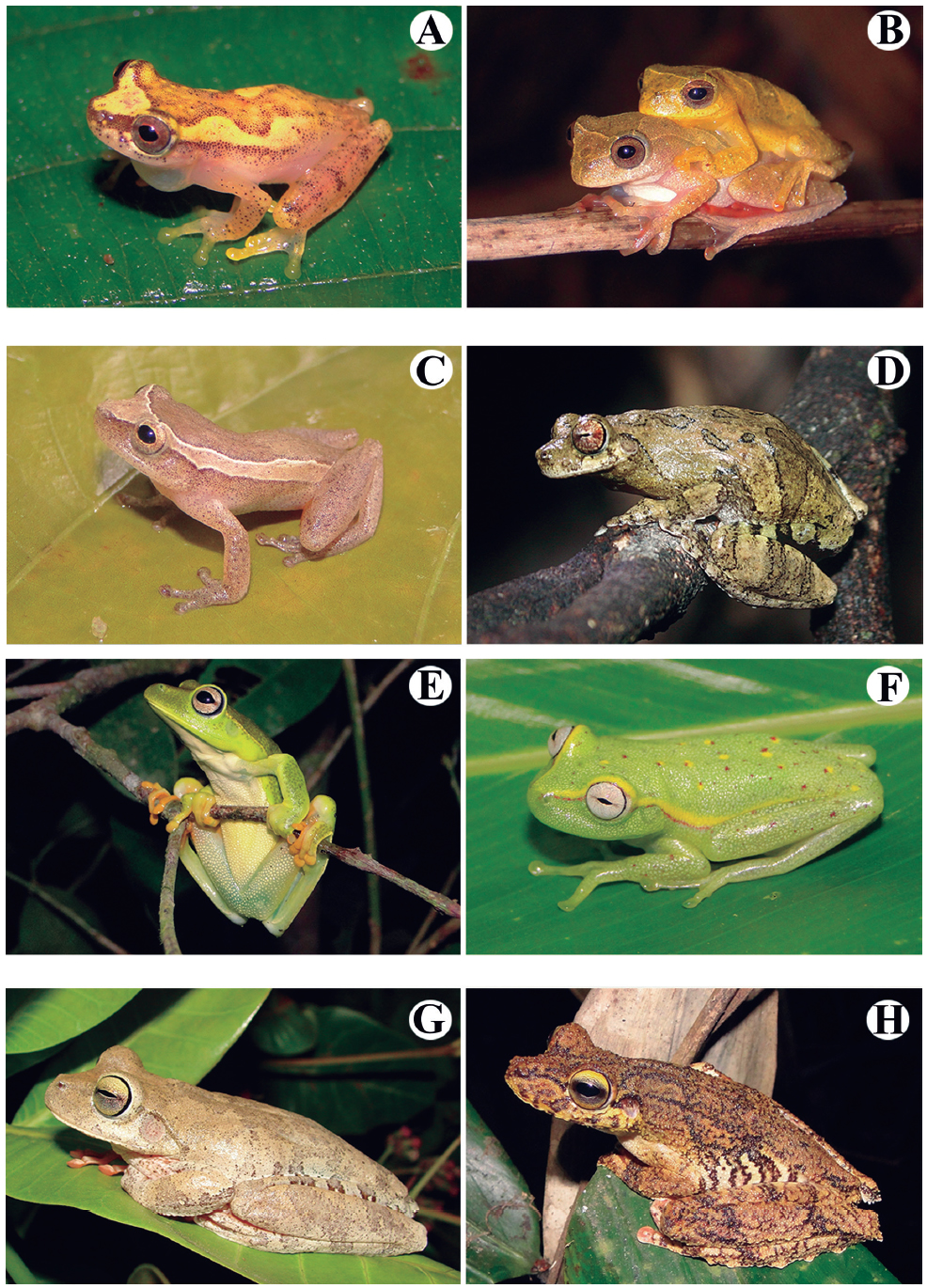
Amphibians species recorded at the Serra do Urubu mountain range. (A) Boana faber, (B) Boana freicanecae, (C) Boana raniceps (Photo by C.O. Gussoni), (D) Boana semilineata, (E) Phyllodytes edelmoi, (F) Phyllodytes gyrinaethes, (G) Pithecopus nordestinus, (H) Scinax auratus.
Amphibians species recorded at the Serra do Urubu mountain range. (A) Scinax eurydice, (B) Scinax fuscomarginatus, (C) Scinax x-signatus pattern 1, (D) Scinax clade ruber, (E) Scinax nebulosus, (F) Scinax pachycrus, (G) Scinax x-signatus pattern 2, (H) Adenomera cf. hylaedactyla.
Amphibians species recorded at Serra do Urubu mountain range. (A) Leptodactylus fuscus, (B) Leptodactylus cf. latrans, (C) Leptodactylus natalensis, (D) Leptodactylus troglodytes, (E) Leptodactylus vastus, (F) Physalaemus cuvieri, (G) Pseudopaludicola mystacalis, (H) Chiasmocleis alagoana.
Amphibians species recorded at the Serra do Urubu mountain range. (A) Proceratophrys renalis, (B) Lithobates palmipes, (C) Pristimantis ramagii, (D) Pristimantis sp.
Amphibian species list, Serra do Urubu mountain range, recorded at RPPN Frei Caneca (Santos & Carnaval, 2002SANTOS, E.M. & CARNAVAL, A.C.O.Q. 2002. Anfíbios anuros do Estado de Pernambuco. In: Tabarelli, M. & Silva, J.M.C. (Orgs.). Diagnóstico da Biodiversidade de Pernambuco. Recife, Editora Massangana. p. 529-535.; Santos & Santos, 2009SANTOS, S.P.L. & SANTOS, E.M. 2009. Gastrotheca pulchra. Geographic distribution. Herpetological Review, 40:445-445.) and RPPN Pedra D’Antas (present study), discriminating the study sites, methodology (VTS = visual transect survey; AS = acoustic survey, PF = pitfall traps; VS = visual survey; *= opportunist encounter, outside the study sites), conservation status, according to MMA and IUCN (VU = vulnerable; EN = endangered; CR = critically endangered; NE = not evaluated; DD = data deficient); and Pernambuco Endemism Center endemic.
Chiasmocleis alagoana, Dendropsophus soaresi and Scinax fuscomarginatus were recorded for the first time in the Serra do Urubu Mountain range. We found three endangered species in RPPN Pedra D’Antas: Hylomantis granulosa which is classified as vulnerable by (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.), Chiasmocleis alagoana (endangered), and Phyllodytes gyrinaethes classified as critically endangered. Dendropsophus soaresi and Pithecopus nordestinus were only found in the vicinities of the Reserve, in open deforested areas including provisory ponds. Seven species were recorded only in Site 6: Dendropsophus haddadi and Scinax fuscomarginatus vocalizing in Cyperaceae inside the pond; Leptodactylus fuscus, L. troglodytes and L. vastus at the margins of the pond; Boana faber in the arboreal vegetation at the forest edge, 15 meters away from the pond; and Physalaemus cuvieri inside the pond, at the water surface between the aquatic vegetation. This site has a total of 26 species recorded, followed by the Site 5 (n = 22 spp.), Site 3 (n = 16 spp.), 4 (n = 9), 1 and 2 (n = 6). Three species were recorded along all the study sites: Pristimantis ramagii, Pristimantis sp. and Rhinella crucifer. Phyllodytes gyrinaethes despite having been recorded at Site 2, was more abundant at Sites 4 and 5, while P. edelmoi was frequently recorded at Sites 3 and 4. Proceratophrys renalis was only found at Site 4, with low encounter rates. The hylids Dendropsophus branneri, D. elegans, D. minutus, Scinax auratus, S. eurydice, S. fuscovarius and S. nebulosus were associated with the large ponds in Sites 5 and 6. Hylomantis granulosa was found associated with permanent and provisory rocky streams in Sites 1 and 2.
Reptiles
We recorded 31 species of squamate reptiles at the RPPN Pedra D’Antas: two species of amphisbaenids (Amphisbaenidae), 12 species of lizards: Dactyloidae (n = 2 spp.), Diploglossidae (n = 1 sp.) Gekkonidae (n = 1 sp.), Gymnophthalmidae (n = 1 sp.), Leiosauridae (n = 1 sp.), Phyllodactylidae (n = 1 sp.), Polychrotidae (n = 1 sp.), Scincidae (n = 1 sp.), Teiidae (n = 1 sp.) and Tropiduridae (n = 2 spp.); and 19 species of snakes: Boidae (n = 2 spp.), Colubridae (n = 1 sp.), Dipsadidae (n = 11 spp.), Elapidae (n = 2 spp.), Typhlopidae (n = 1 sp.), and Viperidae (n = 1 sp.) (Table 2; Figs. 9-14).
Reptile species recorded at the Serra do Urubu mountain range. (A) Amphisbaena alba, (B) Amphisbaena pretrei, (C) Norops fuscoauratus, (D) Dactyloa punctata, (E) Diploglossus lessonae (juvenile), (F) Diploglossus lessonae (adult), (G) Ophiodes sp. (H) Hemidactylus mabouia (Photo by C.O. Gussoni).
Reptile species recorded at the Serra do Urubu mountain range. (A) Dryadosaura nordestina, (B) Iguana iguana, (C) Enyalius aff. catenatus (fêmea), (D) Enyalius aff. catenatus (macho), (E) Gymnodactylus darwinii, (F) Polychrus marmoratus, (G) Mabuya nigropunctata, (H) Ameiva ameiva (Photo by C.O. Gussoni).
Reptile species recorded at the Serra do Urubu mountain range. (A) Salvator merianae, (B) Strobilurus torquatus (Photo by C.O. Gussoni), (C) Tropidurus hispidus, (D) Tropidurus semitaeniatus (Photo by C.O. Gussoni), (E) Boa constrictor, (F) Corallus hortulanus, (G) Epicrates assisi, (H) Spilotes pullatus.
Reptile species recorded at the Serra do Urubu mountain range. (A) Tantilla melanocephala, (B) Atractus potschi, (C) Dipsas sazimai, (D) Erythrolamprus aesculapii (Photo by C.O. Gussoni), (E) Imantodes cenchoa, (F) Leptodeira annulata, (G) Oxyrhopus petolarius (Photo by C.O. Gussoni), (H) Oxyrhopus trigeminus.
Reptile species recorded at the Serra do Urubu mountain range. (A) Philodryas olfersii (Photo by C.O. Gussoni), (B) Pseudoboa nigra, (C) Sibynomorphus sp. (D) Taeniophallus affinis, (E) Xenodon sp. (Photo by C.O. Gussoni), (F) Xenopholis scalaris, (G) Micrurus sp. (H) Micrurus lemniscatus carvalhoi.
Reptile species recorded at the Serra do Urubu mountain range. (A) Amerotyphlops arenensis, (B) Crotalus durissus, (C) Lachesis muta.
Reptile species list, Serra do Urubu mountain range, recorded at RPPN Pedra D’Antas (present study), discriminating the study sites, methodology (VTS = visual transect survey; PF = pitfall traps; * = opportunist encounter, outside the study sites; ** = photographic record by the researcher Carlos Otávio Gussoni).
The visual transect survey detected 34% of the species (n = 11 spp.), with 19 individuals recorded. The lizard Norops fuscoauratus, and the snakes Corallus hortulanus and Lachesis muta were exclusively found by this methodology.
Despite the low capture rate 0,02 individuals/trap (n = 13), the pitfall traps recorded eight species. The lizard Dryadosaura nordestina, and the snakes Taeniophallus affinis and Amerotyphlops arenensis were exclusively recorded by this method. The opportunistic encounter survey recorded the majority of reptiles species (18 spp.), including the amphisbaenids Amphisbaena alba and A. pretrei, the lizards Hemidactylus mabouia, and Tropidurus semitaeniatus; and the snakes Atractus potschi, Boa constrictor, Dipsas sazimai, Imantodes cenchoa, Leptodeira annulata, Oxyrhopus petolarius, Pseudoboa nigra, Tantilla melanocephala. The lizard Ophiodes sp. and the snake Erythrolamprus aesculapii were recorded by photographs taken by an ornithologist researcher who worked at the Serra do Urubu mountain range (Carlos Otávio Gussoni archives, pers. com.).
Regarding the lizard and amphisbaenids species richness, the rarefaction curve was close to reach an asymptote (Fig. 15). The estimators Jacknife I and ICE indicated the possible occurrence of 12 or 13 species in the area respectively, indicating that our sample effort was capable to detect at least 77% of the species (n = 10).
Sample rarefaction curve for lizards and amphisbaenids in RPPN Pedra D’Antas, after 24 days of effort.
During the study period we recorded a total of 41 lizard specimens, (Table 3). Dactyloa punctata and Enyalius aff. catenatus were the most commonly captured species. The capture rates of snakes totalizes 22 captured species. The most captured species were Xenopholis scalaris (n = 4), Amerotyphlops arenensis (n = 4) and Lachesis muta with three individuals.
Distribution of the recorded reptile species along the study sites at RPPN Pedra D’Antas, municipality of Lagoa dos Gatos, Pernambuco, Brazil.
DISCUSSION
Amphibians
Previous research in the Serra do Urubu mountain range by (Santos & Carnaval, 2002SANTOS, E.M. & CARNAVAL, A.C.O.Q. 2002. Anfíbios anuros do Estado de Pernambuco. In: Tabarelli, M. & Silva, J.M.C. (Orgs.). Diagnóstico da Biodiversidade de Pernambuco. Recife, Editora Massangana. p. 529-535.) pointed to the occurrence of 22 anurans species, later (Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.) recorded 42 species in the RPPN Frei Caneca, municipality of Jaqueira. Comparing with our results in the RPPN Pedra D’Antas (n = 38 spp.), we can assume that RPPN Frei Caneca besides having a higher species richness, shelters unique species in the region such as Gastrotheca fissipes, G. pulchra, Boana freicanecae, and Scinax pachycrus, by the presence of specific habitats. The “Complexo do Cruzeiro” for example is a rocky outcrop area with high abundance of terrestrial bromeliads, suitable for Gastrotheca spp. and Scinax pachycrus (seeIzecksohn et al., 2009IZECKSOHN, E.; CARVALHO-E-SILVA, S.P. & PEIXOTO, O.L. 2009. Sobre Gastrotheca fissipes (boulenger, 1888), com a descrição de uma nova espécie (Amphibia, Anura, Amphignathodontidae). Arquivos do Museu Nacional, Rio de Janeiro, 67(2):81-91.; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.; Teixeira Jr. et al., 2012TEIXEIRA JR., M.; DAL VECHIO, F.; RECODER, R.S.; CARNAVAL, A.C.; STRANGAS, M.; DAMASCENO, R.P.; SENA, M.A. & RODRIGUES, M.T. 2012. Two new species of marsupial tree-frogs genus Gastrotheca Fitzinger, 1843 (Anura, Hemiphractidae) from the Brazilian Atlantic Forest. Zootaxa, 3437:1-23.; Haddad et al., 2013HADDAD, C.F.B.; TOLEDO, L.F.; PRADO, C.P.A.; LOEBMANN, D.; GASPARINI, J.L. & SAZIMA, I. 2013. Guia dos anfíbios da Mata Atlântica: diversidade e biologia. São Paulo, Anolis Books.). Boana freicanecae until now has only been found in the “Mata do Quengo” region, the most pristine area in the Serra do Urubu mountain range, in rocky streams inside de forest (Carnaval & Peixoto, 2004aCARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004a. A new species of Hyla from Northeastern Brazil (Amphibia, Anura, Hylidae). Herpetologica, 60:387-395.; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.).
Some species recorded by (Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.) at the RPPN Frei Caneca, were misindentified (Dendropsophus cf. oliveirai, Phyllodytes luteolus, Phyllodytes sp. and Pseudopaludicola sp.), and represent in fact D. haddadi, Phyllodytes edelmoi, P. gyrinaethes, and Pseudopaludicola mystacalis (Santos, E.M. pers. com.), all of them recorded at RPPN Pedra D’Antas.
Scinax x-signatus and S. fuscovarius were also recorded by (Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.) from the RPPN Frei Caneca., these two species were unassigned to any species group of Scinax and belong to the Scinax ruber clade (sensuFaivovich, 2002FAIVOVICH, J. 2002. A cladistic analysis of Scinax (Anura: Hylidae). Cladistics, 18:367-393.; Faivovich et al., 2005FAIVOVICH, J.; HADDAD, C.F.B.; GARCIA, P.C.; FROST, D.R.; CAMPBELL, J.A. & WHEELER, W.C. 2005. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History, 294:1-240.). At the RPPN Pedra D’Antas we collected two species with a different color pattern Scinax x-signatus and another slender species. This species we mostly found vocalizing in bromeliads, we called it Scinax sp. (clade ruber); it is also found at RPPN Frei Caneca.
Another taxonomically unresolved group is Pristimantis of northeastern Brazil. At the Serra do Urubu, (Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.) attest the occurrence of three species, one of them P. ramagii, and the other two Pristimantis sp.1 and Pristimantis sp.2. The species of this group present highly polymorphic variation (Napoli et al., 2009NAPOLI, M.F.; ANANIAS, F.; FONSECA, P.M. & SILVA, A.P.Z. 2009. Morphological and karyotypic contributions for a better taxonomic definition of the frog Ischnocnema ramagii (Boulenger, 1888) (Anura, Brachycephalidae). South American Journal of Herpetology, São Paulo, 4(2):164-172.). Taxonomic studies are needed to determine the range of all the Pristimantis species occurring both north and south of the São Francisco River. We found Pristimantis ramagii and Pristimantis spp., at RPPN Pedra D’Antas mainly differing in acoustic analysis.
Adenomera marmorata is also mentioned to occur at Serra do Urubu and in the Atlantic Forest of Pernambuco state (Moura et al., 2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.). However (Fouquet et al., 2013FOUQUET, A.; CASSINI, C.S.; HADDAD, C.F.B.; PECH, N. & RODRIGUES, M.T. 2013. Species delimitation, patterns of diversification and historical biogeography of the Neotropical frog genus Adenomera (Anura, Leptodactylidae). Journal of Biogeography, 12250:1-16.), performed phylogenetic and biogeographic analyses of Adenomera, and delimited the populations of the Atlantic Forest of Pernambuco and Alagoas in the Adenomera hylaedactyla clade, closely related to the Maranhão and Pará populations. The clade A. hylaedactyla has the wider distribution in the genus, occurring east from French Guyana throughout the Brazilian Amazon region, the center of Brazil and the Atlantic Forest of northeastern Brazil, with a highly genetic structure among the different populations, possibly being a species complex (Fouquet et al., 2013FOUQUET, A.; CASSINI, C.S.; HADDAD, C.F.B.; PECH, N. & RODRIGUES, M.T. 2013. Species delimitation, patterns of diversification and historical biogeography of the Neotropical frog genus Adenomera (Anura, Leptodactylidae). Journal of Biogeography, 12250:1-16.). So, until more elaborate taxonomic studies of the Adenomera hylaedactyla clade are made, we prefer to call the species that occurs at Serra do Urubu and in the Pernambuco State Adenomera cf. hylaedactyla.
Another species that occurs at the Serra do Urubu mountain range with unresolved taxonomy is a species of the Leptodactylus latrans complex (sensude Sá et al., 2014DE SÁ, R.O.; GRANT, T.; CAMARGO, A.; HEYER, W.R.; PONSSA, M.L. & STANLEY, E. 2014. Systematics of the neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): Phylogeny, the relevance of non-molecular evidence, and species accounts. South American Journal of Herpetology, São Paulo, 9(S1):S1-S128.). De Sá et al. (2014) mentioned that the taxonomy of the populations of L. latrans outside the type locality, state of Rio de Janeiro, is still unresolved and needs to be investigated. The individuals of Serra do Urubu do not have auxiliary dorsal folds, which differentiates them from L. chaquensis (de Sá et al., 2014DE SÁ, R.O.; GRANT, T.; CAMARGO, A.; HEYER, W.R.; PONSSA, M.L. & STANLEY, E. 2014. Systematics of the neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): Phylogeny, the relevance of non-molecular evidence, and species accounts. South American Journal of Herpetology, São Paulo, 9(S1):S1-S128.), and can be included in the Leptodactylus latrans complex.
Considering the inventory in the RPPN Frei caneca (Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.) and RPPN Pedra D’Antas we can affirm that the Serra do Urubu mountain range is the most diverse area of amphibians species in the Pernambuco state (n = 46 spp.), with 59% of the species that occur in the State (seeSantos & Carnaval, 2002SANTOS, E.M. & CARNAVAL, A.C.O.Q. 2002. Anfíbios anuros do Estado de Pernambuco. In: Tabarelli, M. & Silva, J.M.C. (Orgs.). Diagnóstico da Biodiversidade de Pernambuco. Recife, Editora Massangana. p. 529-535.; Moura et al., 2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.). The Serra do Urubu mountain range has one of the richest amphibian faunas of the Brazilian Atlantic Forest (seeDias et al., 2014DIAS, I.R.; MEDEIRO, T.T.; VILA NOVA, M.T. & SOLÉ, M. 2014. Amphibians of Serra Bonita, southern Bahia: a new hotpoint within Brazil’s Atlantic Forest hotspot. Zookeys, 449:105-130. for a review), and is the fourth richest area in the northeastern Atlantic Forest, only the Reserva Ecológica Michelin (n = 48 spp.) (Camurugi et al., 2010CAMURUGI, F.; LIMA, T.M.; MERCÊS, E.A. & JUNCÁ, F.A. 2010. Anuros da Reserva Ecológica da Michelin, Município de Igrapiúna, Estado da Bahia, Brasil. Biota Neotropica, 10(2):305-312.), Serra da Jibóia and Serra do Timbó (n = 53 spp.) (Juncá, 2006JUNCÁ, F.A. 2006. Diversidade e uso de hábitat por anfíbios anuros em duas localidades de Mata Atlântica, no norte do Estado da Bahia. Biota Neotropica, 6(2):1-8.), and RPPN Serra Bonita (n = 80 spp.) (Dias et al., 2014DIAS, I.R.; MEDEIRO, T.T.; VILA NOVA, M.T. & SOLÉ, M. 2014. Amphibians of Serra Bonita, southern Bahia: a new hotpoint within Brazil’s Atlantic Forest hotspot. Zookeys, 449:105-130.), all in the state of Bahia, have a higher amphibian diversity.
Conservation status of amphibians at the Serra do Urubu mountain range
The majority of the species with occurrence at the Serra do Urubu are classified as least concern by the (IUCN, 2014) (76%, N = 35). Only five species are classified as data deficient: Chiasmocleis alagoana, Gastrotheca pulchra, Boana freicanecae, Phyllodytes edelmoi and P. gyrinaethes. However, according to the Brazilian National List of Endangered species Chiasmocleis alagoana (endangered) P. gyrinaethes (critically endangered) and Hylomantis granulosa (vulnerable) are at risk of extinction. (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.). This gives the Serra do Urubu mountain range the status of the region with most threatened amphibian species in the northeastern Atlantic Forest.
Some species had their conservation status evaluated by the IUCN red list almost ten years ago. This is the case for Gastrotheca fissipes classified as “Least concern”. This was considered to be a common species, with a wide geographic distribution from the coast zone up to 700 meters altitude, between the states of Pernambuco and Espírito Santo (Carnaval & Peixoto, 2004CARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004b. Gastrotheca fissipes. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. Available at: Available at: www.iucnredlist.org
. Access in: 05/11/2013.
www.iucnredlist.org...
b). However, today it is known that at least two recently described species were confused with G. fissipes. G. megacephala which corresponds to the Atlantic Forest populations from South Bahia to Espírito Santo (Izecksohn et al., 2009IZECKSOHN, E.; CARVALHO-E-SILVA, S.P. & PEIXOTO, O.L. 2009. Sobre Gastrotheca fissipes (boulenger, 1888), com a descrição de uma nova espécie (Amphibia, Anura, Amphignathodontidae). Arquivos do Museu Nacional, Rio de Janeiro, 67(2):81-91.); and G. recava, the most generalist species, occurring from southeastern Bahia, south from the Reconcavo Bahiano (Teixeira Jr. et al., 2012TEIXEIRA JR., M.; DAL VECHIO, F.; RECODER, R.S.; CARNAVAL, A.C.; STRANGAS, M.; DAMASCENO, R.P.; SENA, M.A. & RODRIGUES, M.T. 2012. Two new species of marsupial tree-frogs genus Gastrotheca Fitzinger, 1843 (Anura, Hemiphractidae) from the Brazilian Atlantic Forest. Zootaxa, 3437:1-23.). Therefore G. fissipes is restricted to the Pernambuco Endemism Center, in the states of Pernambuco and Alagoas (Izecksohn et al., 2009IZECKSOHN, E.; CARVALHO-E-SILVA, S.P. & PEIXOTO, O.L. 2009. Sobre Gastrotheca fissipes (boulenger, 1888), com a descrição de uma nova espécie (Amphibia, Anura, Amphignathodontidae). Arquivos do Museu Nacional, Rio de Janeiro, 67(2):81-91.; Teixeira Jr. et al., 2012TEIXEIRA JR., M.; DAL VECHIO, F.; RECODER, R.S.; CARNAVAL, A.C.; STRANGAS, M.; DAMASCENO, R.P.; SENA, M.A. & RODRIGUES, M.T. 2012. Two new species of marsupial tree-frogs genus Gastrotheca Fitzinger, 1843 (Anura, Hemiphractidae) from the Brazilian Atlantic Forest. Zootaxa, 3437:1-23.). (Moura et al., 2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.) mentioned the occurrence of the species at the Parque Nacional de Dois Irmãos (200 hectares), municipality of Recife; Usina de São José (323 hectares), municipality of Igarassú; Brejo dos Cavalos (359 hectares), municipality of Caruaru; and Fazenda Buriti (110 hectares), municipality of Brejo da Madre de Deus; and found a population of this species at the Complexo Pedra do Cruzeiro (630 hectares), RPPN Frei Caneca, municipality of Jaqueira. The only documented record from the State of Alagoas is from the Estação Ecológica de Murici (Peixoto et al., 2003PEIXOTO, O.L.; CARAMASCHI, U. & FREIRE, E.M.X. 2003. Two new species of Phyllodytes (Anura: Hylidae) from the state of Alagoas, northeastern Brazil. Herpetologica, 59:235-246.), Conservation Unit with 6.116 hectares.
Despite the (IUCN, 2016INTERNATIONAL UNION FOR CONSERVATION OF NATURE - IUCN. 2016. The IUCN Red List of Threatened Species. version 2016.2. Available at: Available at: www.iucnredlist.org
. Access in: 01/12/2016.
www.iucnredlist.org...
) and (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.) consider the species to be out of risk, the updated information on its restricted geographic distribution, and the habitat severely fragmented and disconnected in its occurrence area < 10.000 km2, with a higher risk of habitat loss, makes this species feasible to be considered as Vulnerable B1ab (iii, iv).
Boana freicanecae, considered as data deficient by (IUCN, 2014), also has a very restricted distribution, it is only found at the type locality, in the Mata do Quengo region, at RPPN Frei Caneca, State of Pernambuco (Carnaval & Peixoto, 2004CARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004c. Hylomantis granulosa. In: The IUCN Red List of Threatened Species. Version 2014.3. Available at: Available at: www.iucnredlist.org
. Access in: 02/02/2015.
www.iucnredlist.org...
a; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.; Moura et al., 2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.), and at Estação Ecológica de Murici, State of Alagoas (Cardoso et al., 2006CARDOSO, M.C.S.; CRUZ, C.A.G.; LIMA, M.G. & SKUK, G. 2006. Geographic distribution: Hypsiboas freicanecae. Herpetological Review, 37(4):489.). The global geographic distribution of this species is less than 5.000 km², in isolated and fragmented areas. The presence of the fungi Batrachochytrium dendrobatidis in both areas of species occurrence (Carnaval et al., 2006CARNAVAL, A.C.O.Q.; PUSCHENDORF, R.; PEIXOTO, O.L.; VERDADE, V.K. & RODRIGUES, M.T. 2006. Amphibian chytrid fungus broadly distributed in the Brazilian Atlantic Rain Forest. Ecohealth, 3:41-48.; Lisboa et al., 2013LISBOA, B.S.; NEVES, J.M.M.; NASCIMENTO, F.A.C.; TAVARES-BASTO, L. & MOTT, T. 2013. New records of Batrachochytrium dendrobatidis in the Atlantic forest of Northeastern Brazil. North-western Journal of Zoology, 9(1):210-213.), is another factor that can threaten the species survival. We believe that B. freicanecae should be classified as critically endangered B1ab (iii, iv).
Phyllodytes edelmoi is considered data deficient by (IUCN, 2014). The species distribution is similar to P. gyrinaethes, occuring in sympatry at Serra do Urubu and Estação Ecológica de Muricí (Peixoto et al., 2003PEIXOTO, O.L.; CARAMASCHI, U. & FREIRE, E.M.X. 2003. Two new species of Phyllodytes (Anura: Hylidae) from the state of Alagoas, northeastern Brazil. Herpetologica, 59:235-246.; Roberto & Ávila, 2013ROBERTO, I.J. & ÁVILA, R.W. 2013. The advertisement call of Phyllodytes gyrinaethes Peixoto, Caramaschi & Freire, 2003 (Anura: Hylidae). Zootaxa, 3669:193-196.). However this species is not restricted to the forested areas, occurring at forest edges, and open areas from sea level up to 650 a.s.l elevation (Peixoto et al., 2003PEIXOTO, O.L.; CARAMASCHI, U. & FREIRE, E.M.X. 2003. Two new species of Phyllodytes (Anura: Hylidae) from the state of Alagoas, northeastern Brazil. Herpetologica, 59:235-246.). At the Serra do Urubu mountain range the species was commonly found at Pedra do Cruzeiro Region, in the terrestrial bromeliads and at the forest edge. Other records of this species were from Mata do Catolé, municipality of Maceio. The species is endemic to the Pernambuco Endemism Center, occurring in fragmented areas with higher risk of deforestation, and can be classified as Vulnerable B1ab (iii, iv).
Chiasmocleis alagoana, considered as endangered by (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.), occurs at the Engenho de Tapacurá, municipality of São Lourenço da Mata, Pernambuco State (Santos & Amorim, 2010SANTOS, S.P.L. & SANTOS, E.M. 2010. Hypsiboas exastis. Geographic Distribution. Herpetological Review, 41:375-375.), RPPN Pedra D’Antas, município de Jaqueira, Pernambuco, present study; Mata do Buraquinho, municipality of João Pessoa, State of Paraíba (Santana et al., 2008SANTANA, G.G.; VIEIRA, W.L.S.; PEREIRA-FILHO, G.A.; DELFIM, F.R.; LIMA, Y.C.C. & VIEIRA, K.S. 2008. Herpetofauna em um fragmento de Mata Atlântica no estado da Paraíba, região nordeste do Brasil. Biotemas, 21(1):75-84.), Mata do Catolé, municipality of Maceió and Mata do Cedro, municipality of Rio Largo, State of Alagoas Alagoas (Cruz et al., 1999CRUZ, C.A.G.; CARAMASCHI, U. & FREIRE, E.M.X. 1999. Occurrence of the genus Chiasmocleis (Anura: Microhylidae) in the state of Alagoas, north-eastern Brazil, with a description of a new species. Journal of Zoology, London, 249:123-126.). It’s an explosive breeder species, reproducing in ephemeral ponds inside the forest (Nascimento & Skuk, 2006NASCIMENTO, F.A.C. & SKUK, G.O. 2006. O girino de Chiasmocleis alagoana Cruz, Caramaschi & Freire, 1999 (Anura: Microhylidae). Biota Neotropica, 6(3):1-5.). At the Serra do Urubu mountain range only one individual was recorded, despite previous attempts to find the species in the region (Santos & Carnaval, 2002SANTOS, E.M. & CARNAVAL, A.C.O.Q. 2002. Anfíbios anuros do Estado de Pernambuco. In: Tabarelli, M. & Silva, J.M.C. (Orgs.). Diagnóstico da Biodiversidade de Pernambuco. Recife, Editora Massangana. p. 529-535.; Santos & Santos, 2011SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.) showing the rarity of this species in the region.
Hylomantis granulosa is also a rare species at the Serra do Urubu moutain range, despite the occurrence of the species in disturbed areas in the RPPN Pedra D’Antas, the species was associated with the vegetation along the rocky streams inside the forest, being more abundant in the most pristine areas, such as Mata do Quengo at the RPPN Frei Caneca. Despite the rarity of the species in the region, A. granulosa has a wider geographic distribution than previously thought, occurring in the states of Alagoas, Bahia and Pernambuco (Haddad et al., 2013HADDAD, C.F.B.; TOLEDO, L.F.; PRADO, C.P.A.; LOEBMANN, D.; GASPARINI, J.L. & SAZIMA, I. 2013. Guia dos anfíbios da Mata Atlântica: diversidade e biologia. São Paulo, Anolis Books.); its wider geographic distribution doesn’t match with the vulnerable category applied by the (MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.), so we agree with (Campos et al., 2013CAMPOS, F.S.; BRITO, D. & SOLÉ, M. 2013. Threatened Amphibians and their conservation status within the protected area network in Northeastern Brazil. Journal of Herpetology, 47:277-285.) and (Carnaval & Peixoto, 2004CARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004c. Hylomantis granulosa. In: The IUCN Red List of Threatened Species. Version 2014.3. Available at: Available at: www.iucnredlist.org
. Access in: 02/02/2015.
www.iucnredlist.org...
c) who considered this species conservation status to be of least concern.
Reptiles
The reptile assemblage of the RPPN Pedra D’Antas is composed of a mix of forested and open area species, with the presence of rare species and/or considered endemic to other sub-biogeographical regions in the Atlantic Forest (seeRoberto et al., 2014ROBERTO, I.J.; OLIVEIRA, C.R.; ARAUJO FILHO, J.A. & AVILA, R.W. 2014. Dipsas sazimai Fernandes, Marques & Argolo, 2010 (Squamata: Dipsadidae): Distribution extension and new state record. Check List, 10(1):209-210.).
Moura et al. (2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.) compiled data regarding the reptiles of the RPPN Frei Caneca, and found a total of 19 species (10 species of lizards and nine snakes). Gathering the data of photographic records (Gussoni, C.O. personal archive, pers. com.) we were able to identify seven additional species records for the RPPN Frei Caneca: the lizards Dactyloa punctata, Hemidactylus mabouia and Strobilurus torquatus; and the snakes: Crotalus durissus, Lachesis muta, Oxyrhopus petolarius and Xenodon sp. When we combined the species of reptiles recorded at RPPN Pedra D’Antas and RPPN Frei Caneca (Serra do Urubu mountain range) we have a total of 42 species (24 snakes, 16 lizards and two amphisbaenids). This is the second most diverse area of the herpetofauna of Pernambuco state, after the Estação Ecológica de Tapacurá, municipality of São Lourenço da Mata, with 47 species of reptiles (Moura et al., 2011).
Some species recorded at RPPN Pedra D’Antas deserve some consideration regarding their distribution and/or conservation status; discussed hereafter.
The snake Xenopholis scalaris has a predominantly Amazonian distribution, occurring in Brazil, Bolivia, Colombia, Ecuador, French Guyana and Peru (Duellman, 1978DUELLMAN, W.E. 1978. The biology of an equatorial herpetofauna in Amazonian Ecuador. Miscellaneous Publications. Museum of Natural History, University of Kansas, 65:345-352.; Martins & Oliveira, 1999MARTINS, M. & OLIVEIRA, R.E. 1999. Natural history of forest snakes of the Manaus region, central Amazonia, Brazil. Herpetological Natural History, 6:78-150.; Duellman, 2005DUELLMAN, W.E. 2005. Cusco Amazónico: The lives of amphibians and reptiles in an Amazonian Rainforest. Ithaca, N.Y., Cornel University Press.; Jansen et al., 2009JANSEN, M.; ÁLVAREZ, L.C. & KÖHLER, G. 2009. Description of a new species of Xenopholis (Serpentes: Colubridae) from the Cerrado of Bolivia, with comments on Xenopholis scalaris in Bolivia. Zootaxa, 2222:31-45.; Ringler et al., 2010RINGLER, M.; URSPRUNG, E. & HÖLD, W. 2010. Predation on Allobates femoralis (Boulenger 1884; Anura: Aromobatidae) by the colubrid snake Xenopholis scalaris (Wucherer, 1861). Herpetology Notes, 3:301-304.). Even so the type locality is from a population at the Atlantic Forest of South Bahia, municipality of São João da Mata (Wucherer, 1861WUCHERER, O. 1861. Description of the new species of Elapomorphus from Brazil. Proceedings of the Zoological Society of London, 1:325-326.); it has been recorded in the Atlantic Forest of Bahia, São Paulo and Rio de Janeiro states (Wucherer, 1861WUCHERER, O. 1861. Description of the new species of Elapomorphus from Brazil. Proceedings of the Zoological Society of London, 1:325-326.; Zaher et al., 2011ZAHER, H.; BARBO, F.E.; MARTÍNEZ, P.S.; NOGUEIRA, C.; RODRIGUEZ, M.T. & SAWAYA, R.J. 2011. Reptiles from São Paulo state: current knowledge and perspectives. Biota Neotropica, 11(Suppl. 1):1-15.; Hamdan et al., 2015HAMDAN, B.; MACHADO, C. & CITELI, N.K. 2015. Filling gaps and a new state record of Xenopholis scalaris (Wucherer, 1861) (Serpentes: Dipsadidae). Check List, 11(5):1746.). The record in the RPPN Pedra D’Antas is the first for the Pernambuco Endemism Center, increasing the geographic distribution of the species to an area 501 km north from its type locality. The species is considered rare in the Atlantic Forest and deserves be included in regional red lists along this biome (Hamdan et al., 2015HAMDAN, B.; MACHADO, C. & CITELI, N.K. 2015. Filling gaps and a new state record of Xenopholis scalaris (Wucherer, 1861) (Serpentes: Dipsadidae). Check List, 11(5):1746.).
The record of Erythrolamprus aesculapii is the first for the state of Pernambuco. In northeastern Brazil the species was recorded previously in the states of Alagoas, Sergipe and Bahia (Uetz & Hõsek, 2014UETZ, P. & JIRÍ HOŠEK. 2014. The Reptile Database. Available at: Available at: www.reptile-database.org
. Access in: 08/12/2014.
www.reptile-database.org...
)
Sibynomorphus sp. is an undescribed species, closely related to S. neuwiedi (Franco, F.L., pers. com.), previously thought to occur at the Brejos de altitude of Bananeiras and Pau-Ferro in the state of Paraíba, and at Brejo dos Cavalos, state of Pernambuco (Pereira-Filho & Montigelli, 2011PEREIRA-FILHO, G.A. & MONTINGELLI, G.G. 2011. Check list of snakes from the Brejos de Altitude of Paraíba and Pernambuco, Brazil. Biota Neotropica, 11(3):145-150.), this is the first record of the species in the PEC.
Micrurus sp. corresponds to an undescribed species widely distributed in the Caatinga biome from sea level to about 800 meters of elevation (Guedes et al., 2014GUEDES, T.B.; NOGUEIRA, C. & MARQUES, O.A.V. 2014. Diversity, natural history, and geographic distribution of snakes in the Caatinga, northeasten Brazil. Zootaxa, 3863(1):1-93.).
Lachesis muta occurs mainly in primary forest but can occasionally be found in secondary disturbed forest (Campbell & Lamar, 2004CAMPBELL, J.A. & LAMAR, W.W. 2004. The venomous reptiles of the western hemisphere. Volume 1. Ithaca, N.Y., Cornell University Press.; Rodrigues et al., 2013RODRIGUES, R.; ALBUQUERQUE, R.L.; SANTANA, D.J.; LARANJEIRAS, D.O.; PRÓTAZIO, A.S.; FRANÇA, F.G.R. & MESQUITA, D.O. 2013b. Record of the occurence of Lachesis muta (Serpentes, Viperidae) in an Atlantic Forest fragment in Paraíba, Brazil, with comments on the species’ preservation status. Biotemas, 26(2):283-286.b). The populations of the Atlantic Forest occur in very fragmented areas, with a higher risk of extinction because of the isolation of the forest fragments (Alves et al., 2014ALVES, F.Q.; ARGÔLO, A.J.S. & CARVALHO, G.C. 2014. Reproductive biology of the bushmaster Lachesis muta (Serpentes: Viperidae) in the Brazilian Atlantic Forest. Phyllomedusa, 13(9):99-109.). At the RPPN Pedra D’Antas the species was found in secondary forest close to rocky outcrops inside de forest, being one of the most captured snakes in this study. This highlights the importance of this forest fragment for the conservation of the species in northeastern Brazil.
The individuals of Amerotyphlops arenensis found at RPPN Pedra D’Antas present a particular low number of dorsal scales (197-205), when compared to the specimens of the type locality, municipality of Areia, State of Paraiba (204-225) (Graboski et al., 2015GRABOSKI, R.; PEREIRA-FILHO, G.A.; SILVA, A.A.A.; PRUDENTE, A.L.C. & ZAHER, H. 2015. A new species of Amerotyphlops from Northeastern Brazil, with comments on distribution of related species. Zootaxa, 3920(3):443-452.), however the set of diagnostic characteristics agree with the description of A. arenensis, representing the second record outside the type locality, previously found by Roberto et al., (2015ROBERTO, I.J.; MELGAREJO, A. & ÁVILA, R.W. 2015. Répteis (Testudines, Squamata, Crocodylia) da Reserva Biológica de Pedra Talhada. In: Studer, A.; Nusbaumer, L. & Spichiger, N. (Eds.). Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas-Pernambuco, Brasil). Boissiera, 68:357-375.) in the municipality of Quebrangulo, REBIO Pedra Talhada, in the state of Alagoas.
The taxonomic status of the Enyalius catenatus populations of the PEC also deserves attention. Rodrigues et al., (2014RODRIGUES, M.T.; BERTOLOTTO, C.L.V.; AMARO, R.C.; YONEGAGA-YASSUDA, Y.; FREIRE, E.M.X. & PELLEGRINO, K.C.M. 2014. Molecular phylogeny, species limits and biogeography of the Brazilian endemic lizard genus Enyalius (Squamata: Leiosauridae): an example of the historical relationships between Atlantic Forests and Amazonia. Molecular Phylogenetics and Evolution, 81:137-146.) performed a phylogenetic and biogeographical analysis of Enyalius, and found the populations of Enyalis catenatus from the Atlantic Forest in Alagoas State to be closely related to Enyalius bibronii instead of to the populations of E. catenatus from Bahia. The authors mentioned that the populations from Alagoas may represent a candidate species, deserving additional analyses and evaluation of additional specimens from these populations. Based on these findings we provisionally refer the populations of E. catenatus at the Serra do Urubu mountain range as Enyalius aff. catenatus, pending further phylogenetic and taxonomic studies. Enyalis aff. catenatus was the most common lizard species found at RPPN Pedra D’Antas, occurring both in secondary and primary forest.
Ophiodes sp. is probably an undescribed taxon. Unfortunately we did not capture any specimens, but based on the photographic record (Gussoni, C.O. personal archive, pers. com.), the specimens from Pedra D’Antas have a similar color pattern to the individuals from Serra da Ibiapaba, state of Ceará (seeLoebmann & Haddad, 2010LOEBMANN, D. & HADDAD, C.F.B. 2010. Amphibians and reptiles from a highly diverse area of the Caatinga domain: composition and conservation implications. Biota Neotropica, 10(3):227-256.) and REBIO Pedra Talhada (Roberto et al., 2015ROBERTO, I.J.; MELGAREJO, A. & ÁVILA, R.W. 2015. Répteis (Testudines, Squamata, Crocodylia) da Reserva Biológica de Pedra Talhada. In: Studer, A.; Nusbaumer, L. & Spichiger, N. (Eds.). Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas-Pernambuco, Brasil). Boissiera, 68:357-375.). The collection of additional Ophiodes specimens from the humid forests of northeastern Brazil is still needed to assess the taxonomic status of these populations. The record of Ophiodes sp. is the first documented record from a locality within the State of Pernambuco. Moura et al., (2011MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.) mentioned the species occurrence in east Pernambuco, but didn’t provide any reference regarding the locality or voucher specimens for this record.
FINAL REMARKS
The situation of the herpetofauna species in the Pernambuco Endemism Center is worrying; this sub-region is the most threatened and fragmented in the Atlantic Forest (Ribeiro et al., 2009RIBEIRO, M.C.; METZGER, J.P.; MARTENSEN, A.C.; PONZONI, F.J. & HIROTA, M.M. 2009. The Brazilian Atlantic forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6):1141-1153.), suffering from high anthropic pressures (Tabarelli et al., 2005TABARELLI, M.; SIQUEIRA-FILHO, J.A. & SANTOS, A.M.M. 2005. A floresta atlântica ao norte do Rio São Francisco. In: Pôrto, K.C.; Almeida-Cortez, J.S. & Tabarelli, M. (Orgs.). Diversidade Biológica e Conservação da Floresta Atlântica ao norte do Rio São Francisco, Brasília, Ministerio do Meio Ambiente. p. 25-37 (Biodiversidade 14).). Studies on other faunal groups indicate that local population extinctions may result in less diverse communities.
Asfora & Pontes (2009ASFORA, P.H. & PONTES, A.R.M. 2009. The small mammals of the highly impacted North-eastern Atlantic Forest of Brazil, Pernambuco Endemism Center. Biota Neotropica, 9(1):31-35.), studying the small mammals in the fragments of Atlantic Forest in the PEC, found that the long history of deforestation in the region caused the extinction of the most specialized species, resulting in a less diverse community with many generalist species from open environments. A similar situation was found to exist for the large mammal assemblages at the RPPN Frei Caneca (Silva Jr. & Pontes, 2008SILVA JR., A.P. & PONTES, A.R.M. 2008. The effect of mega-fragmentation process on large mammal assemblages in the highly-threatened Pernambuco Endemism Centre, north-eastern Brazil. Biodiversity & Conservation, 17(6):1455-1464); the authors conclude that 50% of the medium-sized and large sized mammals were extinct in this fragment. The entire remaining mammal community has a population density, which lies below the viable minimum needed for long term survival.
Other alarming data are concern the bird species of the PEC, this region harbors 14 threatened taxa, 11 of them are endemic to this Atlantic Forest sub-region (Silveira et al., 2003SILVEIRA, L.F.; OLMOS, F. & LONG, A.J. 2003. Birds in the Atlantic Forest fragments in north-eastern Brazil. Cotinga, 20:32-46.; Silva et al., 2004SILVA, J.M.C.; SOUSA, M.C. & CASTELLETI, C.H.M. 2004. Areas of endemismo for passerine birds in the Atlantic Forest, South America. Global Ecology and Biogeography, 13:85-92.; Barnett & Buzzetti, 2014BARNETT, J.M. & BUZZETTI, D.R.C. 2014. A new species of Cichlocolaptes Reichenback 1853 (Furanriidae), the “gritador-do-nordeste”, an undescribed trace of the fading bird life of northeastern Brazil. Revista Brasileira de Ornitologia, 22(2):75-94.; Pereira et al., 2014PEREIRA, G.A.; DANTAS, S.M.; SILVEIRA, L.F.; RODA, S.A.; ALBANO, C.; SONNTAG, F.A.; LEAL, S.; PERIQUITO, M.C.; MALACCO, G.B. & LEES, A.C. 2014. Status of the globally threatened forest birds of northeast Brazil. Papéis Avulsos de Zoologia, 54(14):177-194.), and three of them were recently considered extinct in the wild. These are Cichlocolaptes mazarbarnetti, Glaucidium mooreorum and Philydor novaesi (Pereira et al., 2014PEREIRA-FILHO, G.A. & MONTINGELLI, G.G. 2011. Check list of snakes from the Brejos de Altitude of Paraíba and Pernambuco, Brazil. Biota Neotropica, 11(3):145-150.; MMA, 2014MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.). Cichlocolaptes mazarbarnetti and Philydor novaesi had a distribution similar to Boana freicanecae and Phyllodytes gyrinaethes, both these bird species are specialists in foraging in arboreal bromeliads. This illustrates the higher risk to specialized species of deforestation (Barnett & Buzzetti, 2014BARNETT, J.M. & BUZZETTI, D.R.C. 2014. A new species of Cichlocolaptes Reichenback 1853 (Furanriidae), the “gritador-do-nordeste”, an undescribed trace of the fading bird life of northeastern Brazil. Revista Brasileira de Ornitologia, 22(2):75-94.). For a series of important conservation actions proposed for the PEC (seePereira et al., 2014PEREIRA-FILHO, G.A. & MONTINGELLI, G.G. 2011. Check list of snakes from the Brejos de Altitude of Paraíba and Pernambuco, Brazil. Biota Neotropica, 11(3):145-150.).
At the Serra do Urubu mountain range the elaboration and implementation of a management plan for both Conservation Units is extremely important. It is necessary to design and implement a proper zonation of the area, defining areas of integral protection, especially at the Mata do Quengo, Complexo do Cruzeiro at RPPN Frei Caneca, and Sites 4 and 5 at RPPN Pedra D’Antas where the most threatened species of amphibians Hylomantis granulosa, Chiasmocleis alagoana, Gastrotheca fissipes, Boana freicanecae and Phyllodytes gyrinaethes are concentrated.
ACKNOWLEDGMENTS
We are grateful to the SAVE Brasil team for permitting our research at the RPPN Pedra D’Antas, and for all the conservation actions and efforts at the Serra do Urubu mountain range, which are essential for the conservation of these forest fragments of Atlantic Forest. To Zezito (José Antônio Vicente Filho) and his family for all the support and help at the RPPN Pedra D’Antas; To D.B de Oliveira for helping in the field surveys; To C.O. Gussoni for kindly sharing and providing photographic records of the Herpetofauna of the Serra do Urubu mountain range. To E.M. Santos for critically reviewing the early version of the manuscript. To Gerard Van Buurt and Erik Wild to reviewing the English version of the manuscript. To FUNCAP for a scholarship to IJR. RWA thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing research fellowship (PQ # 303622/2015-6). To ICMBio for collecting permits (license number: SISBIO 34734-1).
REFERENCES
- ALVES, F.Q.; ARGÔLO, A.J.S. & CARVALHO, G.C. 2014. Reproductive biology of the bushmaster Lachesis muta (Serpentes: Viperidae) in the Brazilian Atlantic Forest. Phyllomedusa, 13(9):99-109.
- ASFORA, P.H. & PONTES, A.R.M. 2009. The small mammals of the highly impacted North-eastern Atlantic Forest of Brazil, Pernambuco Endemism Center. Biota Neotropica, 9(1):31-35.
- BARNETT, J.M. & BUZZETTI, D.R.C. 2014. A new species of Cichlocolaptes Reichenback 1853 (Furanriidae), the “gritador-do-nordeste”, an undescribed trace of the fading bird life of northeastern Brazil. Revista Brasileira de Ornitologia, 22(2):75-94.
- BROWN, K.S.J.R. & BROWN, G.G. 1992. Habitat alteration and species loss in Brazilian forests. In: Whitmore, T.C. & Sayer, J.A. Tropical Deforestation and Species Extinction. London, Chapman & Hall. p. 119-142.
- CAMPBELL, J.A. & LAMAR, W.W. 2004. The venomous reptiles of the western hemisphere. Volume 1. Ithaca, N.Y., Cornell University Press.
- CAMPOS, F.S.; BRITO, D. & SOLÉ, M. 2013. Threatened Amphibians and their conservation status within the protected area network in Northeastern Brazil. Journal of Herpetology, 47:277-285.
- CAMURUGI, F.; LIMA, T.M.; MERCÊS, E.A. & JUNCÁ, F.A. 2010. Anuros da Reserva Ecológica da Michelin, Município de Igrapiúna, Estado da Bahia, Brasil. Biota Neotropica, 10(2):305-312.
- CANEDO, C. & HADDAD, C.F.B. 2012. Phylogenetic relationships within anuran clade Terrarana, with emphasis on the placement of Brazilian Atlantic rainforest frogs genus Ischnocnema (Anura: Brachycephalidae). Molecular Phylogenetics and Evolution, 65(2):610-620.
- CARDOSO, M.C.S.; CRUZ, C.A.G.; LIMA, M.G. & SKUK, G. 2006. Geographic distribution: Hypsiboas freicanecae. Herpetological Review, 37(4):489.
- CARNAVAL, A.C.; WALTARI, E.; RODRIGUES, M.T.; ROSAUER, D.; VANDERWALL, J.; DAMASCENO, R.; PRATES, I.; STRANGAS, M.; SPANOS, Z.; RIVERA, D.; PIE, M.R.; FIRKOWSKI, C.R.; BORNSCHEIN, M.R.; RIBEIRO, L.F. & MORITZ, C. 2014. Prediction of phylogeographic endemismo of an environmentally complex biome. Proceedings of the Royal Society B, Biological Science, London, 281:1-8.
- CARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004a. A new species of Hyla from Northeastern Brazil (Amphibia, Anura, Hylidae). Herpetologica, 60:387-395.
- CARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004b. Gastrotheca fissipes. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. Available at: Available at: www.iucnredlist.org Access in: 05/11/2013.
» www.iucnredlist.org - CARNAVAL, A.C.O.Q. & PEIXOTO, O.L. 2004c. Hylomantis granulosa. In: The IUCN Red List of Threatened Species. Version 2014.3. Available at: Available at: www.iucnredlist.org Access in: 02/02/2015.
» www.iucnredlist.org - CARNAVAL, A.C.O.Q.; PUSCHENDORF, R.; PEIXOTO, O.L.; VERDADE, V.K. & RODRIGUES, M.T. 2006. Amphibian chytrid fungus broadly distributed in the Brazilian Atlantic Rain Forest. Ecohealth, 3:41-48.
- CECHIN, S.Z. & MARTINS, M. 2000. Eficiência de armadilhas de queda (pitfall traps) em amostragens de anfíbios e répteis. Revista Brasileira de Zoologia, 17:729-740.
- COLWELL, R.K. 2006. Estimates: Statistical Estimation of Species Richness and Shared Species from Samples. Version 7.5. User’s Guide and Application. Available at: Available at: http://viceroy.eeb.uconn.edu/estimates Access in: 02/02/2015.
» http://viceroy.eeb.uconn.edu/estimates - COSTA, H.C. & BÉRNILS, R.S. 2014. Répteis brasileiros: Lista de espécies. Herpetologia Brasileira, 3(3):74-84.
- CRUMP, M.L. & SCOTT JR., N.J. 1994. Visual encounter surveys. In: Heyer, W.R.; Donnelly, M.A.; McDiarmid, R.W.; Hayek, L.A.C. & Foster, M.S. Measuring and monitoring biological diversity. Standard methods for amphibians. Washington & London, Smithsonian Institution Press. p. 17-39.
- CRUZ, C.A.G.; CARAMASCHI, U. & FREIRE, E.M.X. 1999. Occurrence of the genus Chiasmocleis (Anura: Microhylidae) in the state of Alagoas, north-eastern Brazil, with a description of a new species. Journal of Zoology, London, 249:123-126.
- DE SÁ, R.O.; GRANT, T.; CAMARGO, A.; HEYER, W.R.; PONSSA, M.L. & STANLEY, E. 2014. Systematics of the neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): Phylogeny, the relevance of non-molecular evidence, and species accounts. South American Journal of Herpetology, São Paulo, 9(S1):S1-S128.
- DIAS, I.R.; MEDEIRO, T.T.; VILA NOVA, M.T. & SOLÉ, M. 2014. Amphibians of Serra Bonita, southern Bahia: a new hotpoint within Brazil’s Atlantic Forest hotspot. Zookeys, 449:105-130.
- DUELLMAN, W.E. 1978. The biology of an equatorial herpetofauna in Amazonian Ecuador. Miscellaneous Publications. Museum of Natural History, University of Kansas, 65:345-352.
- DUELLMAN, W.E. 2005. Cusco Amazónico: The lives of amphibians and reptiles in an Amazonian Rainforest. Ithaca, N.Y., Cornel University Press.
- FAIVOVICH, J. 2002. A cladistic analysis of Scinax (Anura: Hylidae). Cladistics, 18:367-393.
- FAIVOVICH, J.; HADDAD, C.F.B.; GARCIA, P.C.; FROST, D.R.; CAMPBELL, J.A. & WHEELER, W.C. 2005. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History, 294:1-240.
- FOUQUET, A.; CASSINI, C.S.; HADDAD, C.F.B.; PECH, N. & RODRIGUES, M.T. 2013. Species delimitation, patterns of diversification and historical biogeography of the Neotropical frog genus Adenomera (Anura, Leptodactylidae). Journal of Biogeography, 12250:1-16.
- FOUQUET, A.; LOEBMANN, D.; CASTROVIEJO-FISHER, S.; PADIAL, J.M.; ORRICO, V.G.D.; LYRA, M.L.; ROBERTO, I.J.; KOK, P.J.R.; HADDAD, C.F.B. & RODRIGUES, M.T. 2012. From Amazonia to the Atlantic Forest: molecular phylogeny of Phyzelaphryninae frogs reveals unexpected diversity and striking biogeographic pattern emphasizing conservation challenges. Molecular Phylogenetics and Evolution, 65(2):547-561.
- FREIRE, E.M.X.; CARAMASCHI, U. & GONCALVES, U. 2010. A new species of Dendrophidion (Serpentes: Colubridae) from the Atlantic Rain Forest of Northeastern Brazil. Zootaxa, 2719:62-68.
- FROST, D.R. 2016. Amphibian species of the world: an online Reference. version 5.6. Available at: Available at: http://research.amnh.org/vz/herpetology/amphibia Access in: 02/12/2016.
» http://research.amnh.org/vz/herpetology/amphibia - GONÇALVES, U.; TORQUATO, S.; SKUK, G. & SENA, G.A. 2012. A new species of Coleodactylus Parker, 1926 (Squamata: Sphaerodactylidae) from the Atlantic Forest of northeast Brazil. Zootaxa, 3204:20-30.
- GRABOSKI, R.; PEREIRA-FILHO, G.A.; SILVA, A.A.A.; PRUDENTE, A.L.C. & ZAHER, H. 2015. A new species of Amerotyphlops from Northeastern Brazil, with comments on distribution of related species. Zootaxa, 3920(3):443-452.
- GUEDES, T.B.; NOGUEIRA, C. & MARQUES, O.A.V. 2014. Diversity, natural history, and geographic distribution of snakes in the Caatinga, northeasten Brazil. Zootaxa, 3863(1):1-93.
- HADDAD, C.F.B. & PRADO, C.P.A. 2005. Reproductive modes in frogs and their unexpected diversity in the Atlantic Rain Forest of Brazil. Bioscience, 55(3):207-217.
- HADDAD, C.F.B.; TOLEDO, L.F.; PRADO, C.P.A.; LOEBMANN, D.; GASPARINI, J.L. & SAZIMA, I. 2013. Guia dos anfíbios da Mata Atlântica: diversidade e biologia. São Paulo, Anolis Books.
- HAMDAN, B.; MACHADO, C. & CITELI, N.K. 2015. Filling gaps and a new state record of Xenopholis scalaris (Wucherer, 1861) (Serpentes: Dipsadidae). Check List, 11(5):1746.
- HAMMER, O.; HARPER, D.A.T. & RYAN, P.D. 2001. Past: Paleontological statistics software package for education and data analysis. Available at: Available at: http://palaeo-electronica.org/2001_1/past/issue1_01.htm Access in: 02/02/2015.
» http://palaeo-electronica.org/2001_1/past/issue1_01.htm - INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA - IBGE. 1985. Atlas nacional do Brasil: região Nordeste. Rio de Janeiro, IBGE.
- INTERNATIONAL UNION FOR CONSERVATION OF NATURE - IUCN. 2016. The IUCN Red List of Threatened Species. version 2016.2. Available at: Available at: www.iucnredlist.org Access in: 01/12/2016.
» www.iucnredlist.org - IZECKSOHN, E.; CARVALHO-E-SILVA, S.P. & PEIXOTO, O.L. 2009. Sobre Gastrotheca fissipes (boulenger, 1888), com a descrição de uma nova espécie (Amphibia, Anura, Amphignathodontidae). Arquivos do Museu Nacional, Rio de Janeiro, 67(2):81-91.
- JANSEN, M.; ÁLVAREZ, L.C. & KÖHLER, G. 2009. Description of a new species of Xenopholis (Serpentes: Colubridae) from the Cerrado of Bolivia, with comments on Xenopholis scalaris in Bolivia. Zootaxa, 2222:31-45.
- JUNCÁ, F.A. 2006. Diversidade e uso de hábitat por anfíbios anuros em duas localidades de Mata Atlântica, no norte do Estado da Bahia. Biota Neotropica, 6(2):1-8.
- LISBOA, B.S.; NEVES, J.M.M.; NASCIMENTO, F.A.C.; TAVARES-BASTO, L. & MOTT, T. 2013. New records of Batrachochytrium dendrobatidis in the Atlantic forest of Northeastern Brazil. North-western Journal of Zoology, 9(1):210-213.
- LOEBMANN, D. & HADDAD, C.F.B. 2010. Amphibians and reptiles from a highly diverse area of the Caatinga domain: composition and conservation implications. Biota Neotropica, 10(3):227-256.
- MARTINS, M. & OLIVEIRA, R.E. 1999. Natural history of forest snakes of the Manaus region, central Amazonia, Brazil. Herpetological Natural History, 6:78-150.
- MINISTÉRIO DO MEIO AMBIENTE - MMA. 2014. Lista Nacional Oficial de espécies da fauna ameaçadas de extinção. In: Diário Oficial da União, Portaria 444.
- MOURA, G.J.B.; SANTOS, E.M.; ANDRADE, E.V.E. & FREIRE, E.M.X. 2011. Distribuição geográfica e caracterização ecológica dos anfíbios de Pernambuco. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 51-84.
- MYERS, N.; MITTERMEIER, R.A.; MITTERMEIER, C.G.; FONSECA, G.A.B. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403:853-858.
- NAPOLI, M.F.; ANANIAS, F.; FONSECA, P.M. & SILVA, A.P.Z. 2009. Morphological and karyotypic contributions for a better taxonomic definition of the frog Ischnocnema ramagii (Boulenger, 1888) (Anura, Brachycephalidae). South American Journal of Herpetology, São Paulo, 4(2):164-172.
- NASCIMENTO, F.A.C. & SKUK, G.O. 2006. O girino de Chiasmocleis alagoana Cruz, Caramaschi & Freire, 1999 (Anura: Microhylidae). Biota Neotropica, 6(3):1-5.
- NIMER, E. 1972. Climatologia da região nordeste do Brasil. Revista Brasileira de Geografia, 34:3-51.
- PASSOS, P.; FERNANDES, R.; BÉRNILS, R.S. & MOURA-LEITE, J.C. 2010. Taxonomic revision of the Brazilian Atlantic Forest Atractus (Reptilia: Serpentes: Dipsadidae). Zootaxa, 2364:1-63.
- PEIXOTO, O.L.; CARAMASCHI, U. & FREIRE, E.M.X. 2003. Two new species of Phyllodytes (Anura: Hylidae) from the state of Alagoas, northeastern Brazil. Herpetologica, 59:235-246.
- PEREIRA, G.A.; DANTAS, S.M.; SILVEIRA, L.F.; RODA, S.A.; ALBANO, C.; SONNTAG, F.A.; LEAL, S.; PERIQUITO, M.C.; MALACCO, G.B. & LEES, A.C. 2014. Status of the globally threatened forest birds of northeast Brazil. Papéis Avulsos de Zoologia, 54(14):177-194.
- PEREIRA-FILHO, G.A. & MONTINGELLI, G.G. 2011. Check list of snakes from the Brejos de Altitude of Paraíba and Pernambuco, Brazil. Biota Neotropica, 11(3):145-150.
- PRANCE, G.T. 1982. Forest refuges: evidences from woody angiosperms. In: Prance, G.T. (Ed.). Biological diversification in the tropics. New York, Columbia University Press. p. 137-158.
- PYRON, R.A.; BURBRINK, F.T. & WIENS, J.J. 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionay Biology, 13:1-93.
- RANTA, P.; BLOM, T.; NIEMELÃ, J.; JOENSUU, E. & SIITTONEN, M. 1998. The fragmented Atlantic forest of Brazil: size, shape, and distribution of forest fragments. Biodiversity and Conservation, 7:385-403
- RIBEIRO, M.C.; METZGER, J.P.; MARTENSEN, A.C.; PONZONI, F.J. & HIROTA, M.M. 2009. The Brazilian Atlantic forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation, 142(6):1141-1153.
- RINGLER, M.; URSPRUNG, E. & HÖLD, W. 2010. Predation on Allobates femoralis (Boulenger 1884; Anura: Aromobatidae) by the colubrid snake Xenopholis scalaris (Wucherer, 1861). Herpetology Notes, 3:301-304.
- ROBERTO, I.J. & ÁVILA, R.W. 2013. The advertisement call of Phyllodytes gyrinaethes Peixoto, Caramaschi & Freire, 2003 (Anura: Hylidae). Zootaxa, 3669:193-196.
- ROBERTO, I.J.; MELGAREJO, A. & ÁVILA, R.W. 2015. Répteis (Testudines, Squamata, Crocodylia) da Reserva Biológica de Pedra Talhada. In: Studer, A.; Nusbaumer, L. & Spichiger, N. (Eds.). Biodiversidade da Reserva Biológica de Pedra Talhada (Alagoas-Pernambuco, Brasil). Boissiera, 68:357-375.
- ROBERTO, I.J.; OLIVEIRA, C.R.; ARAUJO FILHO, J.A. & AVILA, R.W. 2014. Dipsas sazimai Fernandes, Marques & Argolo, 2010 (Squamata: Dipsadidae): Distribution extension and new state record. Check List, 10(1):209-210.
- RÖDEL, M.O. & ERNST, R. 2004. Measuring and monitoring amphibian diversity in tropical forests. I. An evaluation of methods with recommendations for standardizations. Ecotropica, 10:1-14.
- RODRIGUES, K.C.; DELFIM, F.R.; CASTRO, C.S.S.; FRANÇA, F.G.R.; LEITE-FILHO, E.; MESQUITA, D.O.; OLIVEIRA, F.A.; SANTOS, A.C.A.; FERRARI, S.F. & VALENÇA-MONTENEGRO, M.M. 2013A. Strobilurus torquatus Wiegmann, 1834 (Squamata: Tropiduridae): New records from the Brazilian state of Paraíba and a geographic distribution map. Check List, 9(3):614-617.
- RODRIGUES, R.; ALBUQUERQUE, R.L.; SANTANA, D.J.; LARANJEIRAS, D.O.; PRÓTAZIO, A.S.; FRANÇA, F.G.R. & MESQUITA, D.O. 2013b. Record of the occurence of Lachesis muta (Serpentes, Viperidae) in an Atlantic Forest fragment in Paraíba, Brazil, with comments on the species’ preservation status. Biotemas, 26(2):283-286.
- RODRIGUES, M.T.; BERTOLOTTO, C.L.V.; AMARO, R.C.; YONEGAGA-YASSUDA, Y.; FREIRE, E.M.X. & PELLEGRINO, K.C.M. 2014. Molecular phylogeny, species limits and biogeography of the Brazilian endemic lizard genus Enyalius (Squamata: Leiosauridae): an example of the historical relationships between Atlantic Forests and Amazonia. Molecular Phylogenetics and Evolution, 81:137-146.
- RODRIGUES, M.T.; FREIRE, E.M.X.; PELLEGRINO, K.C.M. & SITES JR. J.W. 2005. Phylogenetic relantionships of a new genus and species of microteiid lizard from the Atlantic forest of north-eastern Brazil (Squamata, Gymnophthalmidae). Zoological Journal of the Linnean Society, 144:543-557.
- SANTANA, G.G.; VIEIRA, W.L.S.; PEREIRA-FILHO, G.A.; DELFIM, F.R.; LIMA, Y.C.C. & VIEIRA, K.S. 2008. Herpetofauna em um fragmento de Mata Atlântica no estado da Paraíba, região nordeste do Brasil. Biotemas, 21(1):75-84.
- SANTOS, A.M.M.; CAVALCANTI, D.R.; SILVA, J.M.C. & TABARELLI, M. 2007. Biogeographical relantionships among tropical forests in north-eastern Brazil. Journal of Biogeography, 34:437-446.
- SANTOS, E.M. & AMORIM, F.O. 2010. Geographic distribution: Chiasmocleis alagoana. Herpetological Review, 41(1):103.
- SANTOS, E.M. & CARNAVAL, A.C.O.Q. 2002. Anfíbios anuros do Estado de Pernambuco. In: Tabarelli, M. & Silva, J.M.C. (Orgs.). Diagnóstico da Biodiversidade de Pernambuco. Recife, Editora Massangana. p. 529-535.
- SANTOS, S.P.L. & SANTOS, E.M. 2009. Gastrotheca pulchra. Geographic distribution. Herpetological Review, 40:445-445.
- SANTOS, S.P.L. & SANTOS, E.M. 2010. Hypsiboas exastis. Geographic Distribution. Herpetological Review, 41:375-375.
- SANTOS, S.P.L. & SANTOS, E.M. 2011. Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, município de Jaqueira, estado de Pernambuco, Brasil. In: Moura, G.J.B.; Santos, E.M.; Oliveira, M.A. & Cabral, M.C.C. (Orgs.). Herpetologia no estado de Pernambuco. Brasília, IBAMA. p. 187-198.
- SILVA JR., A.P. & PONTES, A.R.M. 2008. The effect of mega-fragmentation process on large mammal assemblages in the highly-threatened Pernambuco Endemism Centre, north-eastern Brazil. Biodiversity & Conservation, 17(6):1455-1464
- SILVA, J.M.C. & CASTELLETI, C.H.M. 2003. Status of the biodiversity of the Atlantic Forest of Brazil. In: Galindo-Leal, C. & Câmara, I.G. (Eds.). The Atlantic Forest of South America: biodiversity status, threats, and outlook. Washington, CABS & Island Press. p. 31-42.
- SILVA, J.M.C.; SOUSA, M.C. & CASTELLETI, C.H.M. 2004. Areas of endemismo for passerine birds in the Atlantic Forest, South America. Global Ecology and Biogeography, 13:85-92.
- SILVA, S.T.; SILVA, U.G.; SENA, G.A.B. & NASCIMENTO, F.A.C. 2006. A biodiversidade da Mata Atlântica alagoana: anfíbios e répteis. In: Moura, F.B.P.M. (Org.). A Mata Atlântica em Alagoas. Maceio, EDUFAL. p. 65-76.
- SILVEIRA, L.F.; OLMOS, F. & LONG, A.J. 2003. Birds in the Atlantic Forest fragments in north-eastern Brazil. Cotinga, 20:32-46.
- TABARELLI, M.; SIQUEIRA-FILHO, J.A. & SANTOS, A.M.M. 2005. A floresta atlântica ao norte do Rio São Francisco. In: Pôrto, K.C.; Almeida-Cortez, J.S. & Tabarelli, M. (Orgs.). Diversidade Biológica e Conservação da Floresta Atlântica ao norte do Rio São Francisco, Brasília, Ministerio do Meio Ambiente. p. 25-37 (Biodiversidade 14).
- TEIXEIRA JR., M.; DAL VECHIO, F.; RECODER, R.S.; CARNAVAL, A.C.; STRANGAS, M.; DAMASCENO, R.P.; SENA, M.A. & RODRIGUES, M.T. 2012. Two new species of marsupial tree-frogs genus Gastrotheca Fitzinger, 1843 (Anura, Hemiphractidae) from the Brazilian Atlantic Forest. Zootaxa, 3437:1-23.
- UETZ, P. & JIRÍ HOŠEK. 2014. The Reptile Database. Available at: Available at: www.reptile-database.org Access in: 08/12/2014.
» www.reptile-database.org - VELOSO, H.P.; RANGEL-FILHO, A.L.R. & LIMA, J.C.A. 1991. Classificação da vegetação brasileira adaptada a um sistema universal. Rio de Janeiro, IBGE.
- VILELA, B.; LIMA, M.G.; GONÇALVES, U. & SKUK, G.O. 2011. Siphlophis compressus (Daudin, 1803) (Squamata: Dipsadidae): First records for the Atlantic forest north of the São Francisco river, northeastern Brazil. Cuadernos de Herpetologia, 25(1):23-24.
- WUCHERER, O. 1861. Description of the new species of Elapomorphus from Brazil. Proceedings of the Zoological Society of London, 1:325-326.
- ZAHER, H.; BARBO, F.E.; MARTÍNEZ, P.S.; NOGUEIRA, C.; RODRIGUEZ, M.T. & SAWAYA, R.J. 2011. Reptiles from São Paulo state: current knowledge and perspectives. Biota Neotropica, 11(Suppl. 1):1-15.
-
1
Editor Responsável: Marcelo Duarte
-
Publicado com o apoio financeiro do Programa de Apoio às Publicações Científicas Periódicas da USP
-
3
Os periódicos Papéis Avulsos de Zoologia e Arquivos de Zoologia estão licenciados sob uma Licença CC-BY da Creative Commons.
APPENDIX 1
Amphibians voucher specimens
Adenomera cf. hylaedactyla (URCA-H 552, 4157); Chiasmocleis alagoana (URCA-H 5092); Dendropsophus branneri (URCA-H 4171,4174,4181,5094,5122); D. elegans (URCA-H 4158, 5119, 6232, 6233); D. haddadi (URCA-H 6542); D. minutus (URCA-H 6227, 6235, 6238, 6339, 6243); D. oliveirai (URCA-H 5093, 5128); Gastrotheca fissipes (URCA-H 6539, 6545); Boana albomarginata (URCA-H 4141, 6236); B. atlantica (URCA-H 554); B. crepitans (URCA-H 547, 5113); B. exastis (URCA-H 551); B. freicanecae (URCA-H 6220, 6223, 6224); B. semilineata (URCA-H 4143, 4145, 4151, 4166); Leptodactylus cf. latrans (URCA-H 5106, 6221); L. troglodytes (URCA-H 5114, 6544); Lithobates palmipes (URCA-H 4147, 4148, 5078, 5080); Phyllodytes edelmoi (URCA-H 4169, 4175, 5089, 5091); P. gyrinaethes (URCA-H 4146, 4160, 4163, 4167); Pithecopus nordestinus (URCA-H 4149); Physalaemus cuvieri (URCA-H 6543); Pristimantis sp. (URCA-H 4153, 4159, 4164, 4165, 4173, 4176); Proceratophrys renalis (URCA-H 4138, 4139, 4142); Pseudopaludicola mystacalis (URCA-H 4172); Rhinella crucifer (URCA-H 4144, 4154, 4268); R. granulosa (URCA-H 562); Scinax auratus (URCA-H 4156, 4170, 4179); S. eurydice (URCA-H 5079, 5081, 5083); S. fuscomarginatus (URCA-H 5134); S. x-signatus (URCA-H 5115-5117); S. clade ruber (URCA-H 6540); S. nebulosus (URCA-H 4162, 5086, 6234, 6237).
APPENDIX 2
Reptile voucher specimens
Amphisbaena alba (URCA-H 6207); A. pretrei (URCA-H 6209); Dactyloa punctata (URCA-H 4183, 5064, 5069); Atractus potschi (URCA-H 5136); Corallus hortulanus (URCA-H 5105); Dipsas sazimai (URCA-H 5097); Dryadosaura nordestina (URCA-H 5135, 6222); Enyalius aff. catenatus (URCA-H 4131, 4133-4134, 5059-5063); Gymnodactylus darwinii (URCA-H 4180, 5118, 5120); Imantodes cenchoa (URCA-H 5101); Lachesis muta (URCA-H 4152); Leptodeira annulata (URCA-H 6546); Mabuya nigropunctata (URCA-H 5066-5068); Micrurus sp. (URCA-H 5076); Micrurus lemniscatus carvalhoi (URCA-H 5098); Oxyrhopus petolarius (URCA-H 5102, 6229); Polychrus marmoratus (URCA-H 4137, 6212); Pseudoboa nigra (URCA-H 5104); Sibynomorphus sp. (URCA-H 4135, 5100); Taeniophallus affinis (URCA-H 4136); Tantilla melanocephala (URCA-H 5077); Tropidurus hispidus (URCA-H 4132, 4150); T. semitaeniatus (URCA-H 5070); Amerotypyphlops arenensis (URCA-H 5074, 5103, 5137); Xenopholis scalaris (URCA-H 5099, 6210, 6211).
Publication Dates
-
Publication in this collection
2017
History
-
Received
24 July 2017 -
Accepted
15 Sept 2017