Abstract
This paper investigates the butterfly fauna of the ‘Serra do Rola-Moça’ State Park, Minas Gerais, Brazil. We evaluate i) the seasonal variation of species richness and composition; and ii) the variation in composition of the local butterfly assemblage among three sampling sites and between the dry and rainy seasons. Sampling was carried out monthly between November 2012 and October 2013, using entomological nets. After a total sampling effort of 504 net hours, 311 species were recorded. One of them is endangered in Brazil, and eight are probable new species. Furthermore, two species were new records for the region and eight considered endemic of the Cerrado domain. There was no significant difference in species richness between the dry and the rainy seasons, however the species composition varies significantly among sampling sites. Due to its special, heterogeneous environment, which is home to a rich butterfly fauna, its preservation is important for the conservation of the regional butterfly fauna.
Key-Words
Actinote quadra; Atlantic Rainforest; Butterfly richness; Cerrado; New records
INTRODUCTION
The Atlantic Rainforest and the Cerrado domains present variable landscape structure (Tabarelli et al., 2005Tabarelli, M.; Pinto, L.P.; Silva, J.M.; Hirota, M.M. & Bedê, L.C. 2005. Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade, 1: 132-138.; Emery et al., 2006Emery, E.O.; Brown Jr., K.S. & Pinheiro, C.E.G. 2006. As borboletas (Lepidoptera, Papilionoidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 50: 85-92.), being considered threatened and important biodiversity hotspots (Mittermeier et al., 2004Mittermeier, R.A.; Gil, P.R.; Hoffmann, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J. & Da Fonseca, G.A.B. 2004. Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. Agrupación Sierra Madre, Conservation International.). The heterogeneous vegetation in those floristic domains is habitat for a diverse fauna of butterflies (Emery et al., 2006Emery, E.O.; Brown Jr., K.S. & Pinheiro, C.E.G. 2006. As borboletas (Lepidoptera, Papilionoidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 50: 85-92.). Although high butterfly species richness has been recorded in different areas of the Atlantic Rainforest and the Cerrado, as well as in transition areas between those domains, inventories in the various vegetation types in those ecosystems still need to be conducted (Carneiro et al., 2008Carneiro, E.; Mielke, O.H.H. & Casagrande, M.M. 2008. Inventários de borboletas no Brasil: estado da arte e modelo de áreas prioritárias para pesquisa com vista à conservação. Natureza e Conservação, 6(2): 68-90.).
The butterfly fauna of the Brazilian Cerrado is still poorly known, even in southern and southeastern Brazil, where many faunistic inventories have been done (Carneiro et al., 2008Carneiro, E.; Mielke, O.H.H. & Casagrande, M.M. 2008. Inventários de borboletas no Brasil: estado da arte e modelo de áreas prioritárias para pesquisa com vista à conservação. Natureza e Conservação, 6(2): 68-90.).
Some important butterfly inventories were done in the Cerrado in the Federal District and in the state of Minas Gerais (Brown Jr. & Mielke, 1967aBrown Jr., K.S. & Mielke, O.H.H. 1967a. Lepidoptera of the Central Brazil Plateau. I. Preliminary list of Rhopalocera: introduction, Nymphalidae, Libytheidae. Journal of the Lepidopterists’ Society, 21(2): 77-106., 1967bBrown Jr., K.S. & Mielke, O.H.H. 1967b. Lepidoptera of the Central Brazil Plateau. I. Preliminary list of Rhopalocera (continued): Lycaenidae, Pieridae, Papilionidae, Hesperiidae. Journal of the Lepidopterists’ Society, 21(3): 145-168., 1968Brown Jr., K.S. & Mielke, O.H.H. 1968. Lepidoptera of the Central Brazil Plateau. III. Partial list for the Belo Horizonte area, showing the character of the south-eastern “blend zone”. Journal of the Lepidopterists’ Society, 22(3): 147-157.; Emery et al., 2006Emery, E.O.; Brown Jr., K.S. & Pinheiro, C.E.G. 2006. As borboletas (Lepidoptera, Papilionoidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 50: 85-92.; Mielke et al., 2008Mielke, O.H.H.; Emery, E.O. & Pinheiro, C.E.G. 2008. As borboletas Hesperiidae (Lepidoptera, Hesperioidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 52: 283-288.; Motta, 2002Motta, P.C. 2002. Butterflies from the Uberlândia region, Central Brazil: Species list and biological comments. Brazilian Journal of Biology, 62: 151-163.). Few inventories were focused on the butterfly fauna of non-urban fragments of Atlantic Rainforest in Minas Gerais (Brown Jr. & Mielke, 1968Brown Jr., K.S. & Mielke, O.H.H. 1968. Lepidoptera of the Central Brazil Plateau. III. Partial list for the Belo Horizonte area, showing the character of the south-eastern “blend zone”. Journal of the Lepidopterists’ Society, 22(3): 147-157.; Brown Jr. & Freitas, 2000Brown Jr., K.S. & Freitas, A.V.L. 2000. Atlantic rainforest Butterflies: indicators for landscape conservation. Biotropica, 32: 934-956.; Ebert, 1969Ebert, H. 1969. On the frequency of butterflies in eastern Brazil, with a list of the butterfly fauna of Poços de Caldas, Minas Gerais. Journal of the Lepidopterists’ Society, 23(3): 1-48.; Silva et al., 2010Silva, A.R.M.; Guimarães, M.P.M.; Vitalino, R.F.; Bagni, A.S.; Martins, Y.E.; Cordeiro, A.M. & Oliveira, E.G. 2010. Borboletas frugívoras do Parque Estadual do Rio Doce/MG. MG.BIOTA, 3(4): 5-21.), although a significative portion of the Brazilian butterfly diversity occurs in this domain (Iserhard et al., 2018Iserhard, C.A.; Uehara-Prado, M.; Marini-Filho, O.J.; Duarte, M. & Freitas, A.V.L. 2018. Fauna da Mata Atlântica: Lepidoptera-borboletas. In: Monteiro-Filho, E.L.A & Conte, C.E. (Orgs.). Revisões em zoologia da Mata Atlântica. Curitiba, Editora UFPR. p. 57-102.).
Many studies demonstrate that the number of active butterfly species decreases during the dry season, mainly due to scarcity of larval food (Brown Jr. & Freitas, 2000Brown Jr., K.S. & Freitas, A.V.L. 2000. Atlantic rainforest Butterflies: indicators for landscape conservation. Biotropica, 32: 934-956.). In the rainy season, when more species are active, adult females easily find new, soft, nutrient-reach leaves to feed the caterpillars (Iserhard et al., 2013Iserhard, C.A.; Brown Jr., K.S. & Freitas, A.V.L. 2013. Maximized sampling of butterflies to detect temporal changes in tropical communities. Journal of Insect Conservation, 17: 615-622.). However, environmental characteristics, such as vegetation type, are more relevant than the temporal variation (dry and rainy seasons) in organizing species composition for Hesperiidae butterfly assemblages (Carneiro et al., 2014Carneiro, E.; Mielke, O.H.H.; Casagrande, M.M. & Fiedler, K. 2014. Community Structure of Skipper Butterflies (Lepidoptera, Hesperiidae) along Elevational Gradients in Brazilian Atlantic Rainforest Reflects Vegetation Type Rather than Altitude. Plos One, 9: 1-11.) and can also be relevant for the species composition of the other butterfly families.
The study of species diversity in different vegetation types is critical to planning and developing environmental protection and insect conservation actions (Lewinsohn et al., 2005Lewinsohn, T.M.; Freitas, A.V.L. & Prado, P.I. 2005. Conservação de invertebrados terrestres e seus habitats no Brasil. Megadiversidade, 1: 62-69.), and faunistic inventories are indispensable tools for recording and describing the local biodiversity, as well as for management and protection of remnants of the Cerrado, of the Atlantic Rainforest and of the transition areas between them.
Here, the butterfly fauna of a heterogeneous area in the border of the Cerrado and the Atlantic Rainforest domains, in the Brazilian state of Minas Gerais, is inventoried and its species richness and composition in different vegetation types, in the dry and rainy seasons, are analyzed.
MATERIAL AND METHODS
Study area
The study was conducted in the ‘Serra do Rola-Moça’ State Park (PESRM) (20°03’01”S, 44°00’20”W), located in the municipalities of Belo Horizonte, Brumadinho, Ibirité and Nova Lima in the state of Minas Gerais (Fig. 1). The park is in the region known as Quadrilátero Ferrífero (‘Iron Quadrangle’) in the southern Espinhaço Range, and is composed of different vegetation types of the Atlantic Rainforest domain (riparian and seasonal semi-deciduous forest) and of the Cerrado domain (cerrado sensu strictu [savanna], natural grasslands and rocky fields) (Drummond & Martins, 2007Drummond, G.M. & Martins, C.S. (Coord.). 2007. Plano de manejo do Parque Estadual da Serra do Rola Moça. Belo Horizonte. Available at: Available at: http://www.biodiversitas.org.br/planosdemanejo/pesrm/regiao.htm
. Access in: 02/07/2016.
http://www.biodiversitas.org.br/planosde...
).
Location of the study site, the ‘Serra do Rola-Moça’ State Park (PESRM), in Minas Gerais state, Brazil.
The park is a mountainous area with altitudes raging between 1,000 m and 1,430 m (Drummond & Martins, 2007Drummond, G.M. & Martins, C.S. (Coord.). 2007. Plano de manejo do Parque Estadual da Serra do Rola Moça. Belo Horizonte. Available at: Available at: http://www.biodiversitas.org.br/planosdemanejo/pesrm/regiao.htm
. Access in: 02/07/2016.
http://www.biodiversitas.org.br/planosde...
), under a tropical climate, with a dry season between April and September, and a rainy season between October and March, with an average total rainfall of 850 mm (Drummond & Martins, 2007Drummond, G.M. & Martins, C.S. (Coord.). 2007. Plano de manejo do Parque Estadual da Serra do Rola Moça. Belo Horizonte. Available at: Available at: http://www.biodiversitas.org.br/planosdemanejo/pesrm/regiao.htm
. Access in: 02/07/2016.
http://www.biodiversitas.org.br/planosde...
). Surrounding the park, there are mines and urban areas that directly affect the park’s environment (Drummond & Martins, 2007Drummond, G.M. & Martins, C.S. (Coord.). 2007. Plano de manejo do Parque Estadual da Serra do Rola Moça. Belo Horizonte. Available at: Available at: http://www.biodiversitas.org.br/planosdemanejo/pesrm/regiao.htm
. Access in: 02/07/2016.
http://www.biodiversitas.org.br/planosde...
).
Sampling and identification
Sampling was conducted between November 2012 and October 2013, in twelve transects, two in each of six different vegetational types: rocky field, Cerrado sensu strictu (savanna) and natural grassland (physiognomies of the Cerrado); riparian forests and seasonal semi-deciduous forest (Atlantic Rainforest); and Forest-Cerrado transition areas. The twelve transects are 1 km to 1.5 km away from each other and were chosen considering their accessibility and vegetation types. Due to low temperatures and rain, the sampling effort was reduced in March.
Entomological nets were used to capture adult butterflies, following the standard procedures established by Iserhard (2011Iserhard, C.A. 2011. Padronização dos métodos de captura de lepidópteros com puçá. In: Encontro Pan Lepidópteros e RedeLep. Brasília.). Samples were carried out twice a month, with three collectors sampling for 3.5 hours along each of two transects each day, between 09:00 and 16:30.
The butterflies were sacrificed by thorax compression and stored in entomological envelopes for posterior identification. The following information was recorded for each captured butterfly: date, time, local of collection, and collector. The specimens collected were identified and deposited in the Centro de Coleções Taxonômicas of the Universidade Federal de Minas Gerais (CCT-UFMG).
Specimen identification was accomplished with aid of the taxonomic literature (Brown Jr., 1992Brown Jr., K.S. 1992. Borboletas da Serra do Japi: diversidade, habitats, recursos alimentares e variação temporal. In: Patrícia, L. & Morellato, C. (Eds.). História Natural da Serra do Japi: Ecologia e preservação de uma área florestal no Sudeste do Brasil. Campinas, FAPESP. p. 142-186.; Penz & Devries, 2002Penz, C.M. & Devries, P.J. 2002. Phylogenetic analysis of Morpho Butterflies (Nymphalidae, Morphinae): Implications for classification and natural history. American Museum Novitates, 3374: 1-33.; Willmott, 2003Willmott, K.R. 2003. The genus Adelpha: Its systematics, biology and biogeography (Lepidoptera: Nymphalidae: Limenitidini). Gainesville, Scientific Publishers .; Uehara-Prado et al., 2004Uehara-Prado, M.; Freitas, A.V.L.; Francini, R.B. & Brown Jr., K.S. 2004. Guia das Borboletas Frugívoras da Reserva Estadual do Morro Grande e da Região de Caucaia do Alto, Cotia (São Paulo). Biota Neotropica, 4: 1-25.; D’Abrera, 2005D’Abrera, B. 2005. World Butterflies. Hill House Publishers, London. (Reduced version of The Concise Atlas of the Butterflies of the World, 2001).; Garwood et al., 2009Garwood, K.; Lehman, R.; Carter, W. & Carter, G. 2009. Butterflies of Southern Amazon: A photography checklist of common species. Texas, McAllen, RiCalé Publishing.; Santos et al., 2011Santos, J.P.; Iserhard, C.A.; Teixeira, M.O. & Romanowski, H.P. 2011. Fruit-feeding butterflies guide of subtropical Atlantic Rainforest and Araucaria Moist Forest in State of Rio Grande do Sul, Brazil. Biota Neotropica, 11: 253-274.), as well as of the illustrated list of South American butterflies (Warren et al., 2017Warren, A.D.; Davis, K.J.; Stangeland, E.M.; Pelham, J.P. & Grishin, N.V. 2017. Illustrated Lists of American Butterflies. Available at: Available at: http://www.butterfliesofamerica.com
. Access in: 18/06/2018.
http://www.butterfliesofamerica.com...
). Specimens that could not be identified by the authors were taken for identification in the Laboratory of Studies of Neotropical Lepidoptera, of the Universidade Federal do Paraná (UFPR) (Hesperiidae), and Laboratory of Ecology and Systematics of Butterflies of the Universidade Estadual de Campinas (UNICAMP) (Riodinidae, Satyrinae and Ithomiinae [Nymphalidae]). The classification employed here were those of Lamas (2004Lamas, G. 2004. Atlas of Neotropical Lepidoptera. Checklist: Part 4A. Hesperioidea - Papilionoidea. Gainesville, Scientific Publishers.); Wahlberg et al. (2009Wahlberg, N.; Leneveu, J.; Kodandaramaiah, U.; Peña, C.; Nylin, S.; Freitas, A.V.L. & Brower, A.V.Z. 2009. Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proceedings of the Royal Society B: Biological Sciences, 276: 4295-4302.); for Hesperiidae, Warren et al. (2008Warren, A.D.; Ogawa, J.R. & Brower, A.V.Z. 2008. Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics, 24: 642-676., 2009Warren, A.D.; Ogawa, J.R. & Brower, A.V.Z 2009. Revised classification of the family Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Systematic Entomology, 34: 467-523.); and for Riodinidae, Seraphim et al. (2018Seraphim, N.; Kaminski, L.A.; Devries, P.J.; Penz, C.; Callaghan, C.; Wahlberg, N.; Silva-Brandão, K.L. & Freitas, A.V.L. 2018. Molecular phylogeny and higher systematics of the metalmark butterflies (Lepidoptera: Riodinidae). Systematic Entomology, 43: 407-425.).
Data analysis
The Generalized Linear Model (GLM) was used to compare the species richness of the dry and rainy seasons, with richness being the response and seasons the explanatory variables. GLM was used to compare the species richness (response variable) of the sampling sites (explanatory variable). All GLM were submitted to residual analyses to evaluate adequacy of the error distribution (Crawley, 2013Crawley, M.J. 2013. The R Book. London, John Wiley and Sons Ltd.). Non-metric multidimensional scaling (NMDS) analyses employing the Jaccard similarity index were employed to compare the species composition between seasons and between sampling sites. Analyses of similarities (ANOSIM) were performed to test for differences between seasons and sampling sites. All analyses were performed in R software (R Core Team, 2014R Core Team. 2014. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing. Available at: Available at: http://www.R-project.org
. Access in: 14/03/2016.
http://www.R-project.org...
) using gdata, vegan and RT4Bio packages. Sample coverage of species richness in each of the dry and rainy seasons and in both seasons were verified using the RStudio software (RStudio Team, 2015RStudio Team. 2015. RStudio: Integrated development for R (Version 1.1.453). Boston, MA. Available at: Available at: http://www.rstudio.com
. Access in: 06/07/2018.
http://www.rstudio.com...
) with vegan, iNEXT, ggplot2, devtools, gridExtra and grid packages.
RESULTS AND DISCUSSION
Species richness and composition
After a total sampling effort of 504 net hours, 1,638 individuals of 311 species were recorded. The richest family was Hesperiidae with 113 species (36.5%), followed by Nymphalidae with 98 species (31.5%), Riodinidae with 31 (10%), Lycaenidae with 29 (9.5%), Pieridae with 24 (7.5%) and Papilionidae with 16 species (5%) (Table 1).
Species collected in the dry and rainy seasons in environments of Cerrado domain (c), Atlantic Rainforest (f) and transition between Cerrado and Atlantic Rainforest (t).x = new record for the Quadrilátero Ferrífero; * = new record for the state of Minas Gerais; • = endemic species of the Cerrado. Numbers in parenthesis after suprageneric taxa are the numbers of species sampled in each taxon.
The number of species of Pieridae and Papilionidae may have been underestimated, because observed individuals were hard to catch with entomological nets. Moreover, the sampling period (between 09:00 and16:30) may have also influenced the number of species recorded for some taxa, as Brassolini (Nymphalidae) and Hesperiidae, which include some typically crepuscular species (Mielke & Casagrande, 1997Mielke, O.H.H. & Casagrande, M.M. 1997. Papilionoidea e Hesperioidea (Lepidoptera) do Parque Estadual do Morro do Diabo, Teodoro Sampaio, São Paulo, Brasil. Revista Brasileira de Zoologia, 14: 967-1001.) that were rarely sampled.
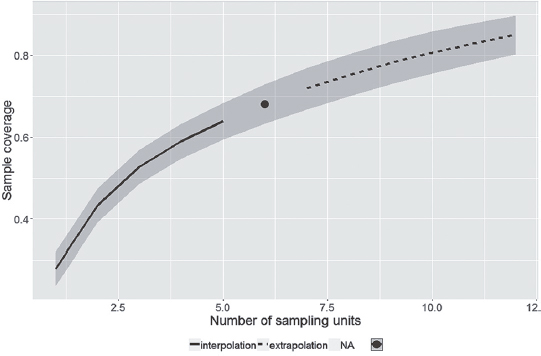
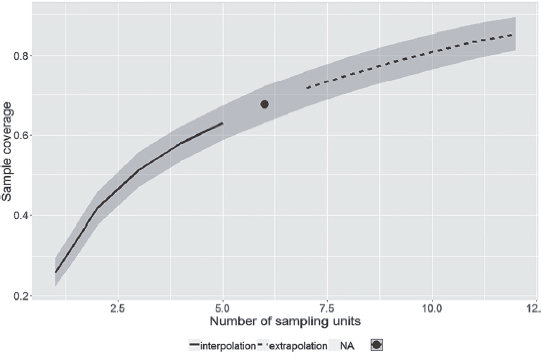
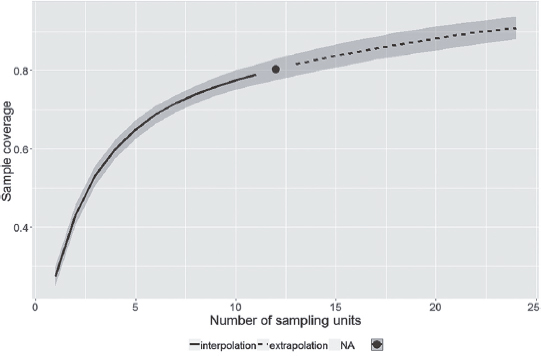
In the rainy season (October to March), 230 species were recorded, of which, 102 were sampled only in that season. In the dry season (April to September), 209 species were recorded, 81 of them unique to that season. A total of 128 species were sampled in both seasons. Sample coverage of species richness, in each season, reached almost 70% and indicates that more species could have been sampled (Figs. 2, 3). The sampling coverage of both seasons reached 80% (Fig. 4), but demonstrates that with more one year of sampling the coverage of species richness would tend to the estimated number. This suggests that the actual overlap between the faunas of the two seasons may be greater than the observed.
Sample coverage of species richness in rainy season, in the ‘Serra do Rola-Moça’ State Park, Minas Gerais state, Brazil.
Sample coverage of species richness in dry season, in the ‘Serra do Rola-Moça’ State Park, Minas Gerais state, Brazil.
Sample coverage of species richness in rainy and dry seasons, in the ‘Serra do Rola-Moça’ State Park, Minas Gerais state, Brazil.
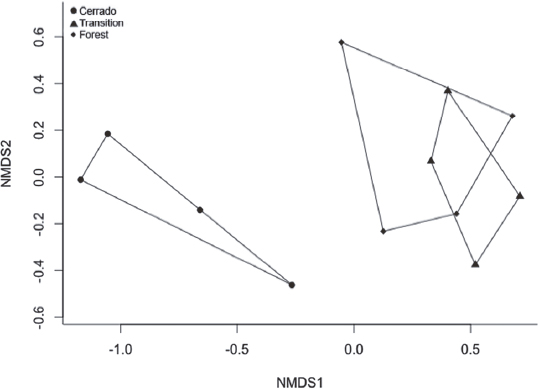
The species richness and composition of the butterfly assemblage did not vary significantly between the dry and the rainy seasons (GLM = Gaussian distribution, p = 0.79 and ANOSIM, p = 0.49). This result could be different maximizing the sample effort, either using bait trap for fruit-feeding species, and/or increasing the years of sampling. Pereira et al. (2017Pereira, G.C.N.; Coelho, M.S.; Beirão, M.V.; Braga, R.F. & Fernandes, G.W. 2017. Diversity of fruit-feeding butterflies in a mountaintop archipelago of rainforest. Plos One, 12(6): 1-20.), for instance, collected for two years and found statistical differences in fruit-feeding butterfly composition between dry and rainy seasons. On the other hand, significant differences were found when the composition of butterfly assemblages was compared among sampling sites, (ANOSIM, p = 0.005; Fig. 5), but not among their species richness (GLM = Gaussian distribution, p = 0.065).
Non-metric multidimensional scaling (NMDS) of the butterfly species composition in sampling sites of Cerrado domain, Atlantic Rainforest and Forest-Cerrado transition areas, in the ‘Serra do Rola-Moça’ State Park, Minas Gerais state, Brazil.
Among the 311 recorded species, eight are probably new, of which seven belong each to one of the following genera: Chalcone, Cogia, Cumbre, Cymaenes, Lucida, Moneuptychia, Phlebodes. A probably new species of Hesperiinae, however, could not be identified even to genus level. This specimen was collected in the Ibirité municipality, in riparian forest, at the rainy season.
Environmental variation
The NMDS indicates that grassland and forest environments in the PESRM have distinct faunas. Most species restricted to the Cerrado domain (Brown Jr. & Mielke, 1967bBrown Jr., K.S. & Mielke, O.H.H. 1967b. Lepidoptera of the Central Brazil Plateau. I. Preliminary list of Rhopalocera (continued): Lycaenidae, Pieridae, Papilionidae, Hesperiidae. Journal of the Lepidopterists’ Society, 21(3): 145-168.; Mielke et al., 2008Mielke, O.H.H.; Emery, E.O. & Pinheiro, C.E.G. 2008. As borboletas Hesperiidae (Lepidoptera, Hesperioidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 52: 283-288., 2012Mielke, O.H.H.; Carneiro, E. & Casagrande, M.M. 2012. Hesperiidae (Lepidoptera, Hesperioidea) from Ponta Grossa, Paraná, Brazil: 70 years of records with special reference to faunal composition of Vila Velha State Park. Revista Brasileira de Entomologia, 56(1): 59-66.) in our sample belong to Pyrginae (Hesperiidae) and Theclinae (Lycaenidae), especially to the genera Cogia and Strymon, respectively. According to the list presented by Mielke et al. (2012Mielke, O.H.H.; Carneiro, E. & Casagrande, M.M. 2012. Hesperiidae (Lepidoptera, Hesperioidea) from Ponta Grossa, Paraná, Brazil: 70 years of records with special reference to faunal composition of Vila Velha State Park. Revista Brasileira de Entomologia, 56(1): 59-66.), the species of Pyrginae also sampled in this study Chiomara basigutta (Plötz, 1884), Cogia calchas (Herrich-Schäffer, 1869), Heliopetes omrina (Butler, 1870) and Viola viollela (Mabille, 1898) have preference for natural grassland habitats. Moreover, Hesperiinae (Hesperiidae) and Satyrinae (Nymphalidae) are more abundant in grassland habitats (Casagrande et al., 2012Casagrande, M.M.; Dolibaina, D.R.; Carneiro, E.; Dias, F.M.S.; Leite, L.A.R. & Mielke, O.H.H. 2012. Borboletas (Hesperioidea e Papilionoidea) de Jaguariaíva, Paraná, Brasil: Inventário em um enclave de Cerrado meridional. In: Carpanezzi, O.T.B. & Campos, J.B. (Eds.). Coletânea de Pesquisas. Parques Estaduais de Vila Velha, Cerrado e Guartelá. Curitiba, Instituto Ambiental do Paraná. p. 295-308.), probably because larvae of Hesperiinae feed exclusively on monocots (Carneiro et al., 2014Carneiro, E.; Mielke, O.H.H.; Casagrande, M.M. & Fiedler, K. 2014. Community Structure of Skipper Butterflies (Lepidoptera, Hesperiidae) along Elevational Gradients in Brazilian Atlantic Rainforest Reflects Vegetation Type Rather than Altitude. Plos One, 9: 1-11.), and those of Satyrinae almost exclusively on monocots (Peña & Wahlberg, 2008Peña, N. & Wahlberg, C. 2008. Prehistorical climate change increased diversification of a group of butterflies. Biology Letters, 4: 274-278.), which are poorly represented in the forest environment. Despite the fact that those feeding habits justify their abundance in the Cerrado vegetation, in the PESRM, almost 50% of the species of Hesperiinae and 30% of the Satyrinae were found only in the transition areas.
More attention has been given to the butteflies of the Atlantic Rainforest than to those of the Cerrado (Brown Jr. & Freitas, 2000Brown Jr., K.S. & Freitas, A.V.L. 2000. Atlantic rainforest Butterflies: indicators for landscape conservation. Biotropica, 32: 934-956.; Uehara-Prado et al., 2007Uehara-Prado, M.; Brown Jr., K.S & Freitas, A.V.L. 2007. Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: comparison between a fragmented and a continuous landscape. Global Ecology and Biogeography, 16: 43-54.; Carneiro et al., 2008Carneiro, E.; Mielke, O.H.H. & Casagrande, M.M. 2008. Inventários de borboletas no Brasil: estado da arte e modelo de áreas prioritárias para pesquisa com vista à conservação. Natureza e Conservação, 6(2): 68-90.). However, as emphasized by Dolibaina et al. (2011Dolibaina, D.R.; Mielke, O.H.H. & Casagrande, M.M. 2011. Borboletas (Papilionoidea e Hesperioidea) de Guarapuava e arredores, Paraná, Brasil: um inventário com base em 63 anos de registros. Biota Neotropica, 11: 1-14.) and Carneiro et al. (2014), the characteristic species of natural grasslands must also be considered conservation priorities since this vegetation type is either under threat.
New record, endemic and threatened species
Among the species sampled in the PESRM, Hypoleria lavinia (Hewitson, [1855]) was known, until now, only from the Parque Estadual do Rio Doce (Silva et al., 2010Silva, A.R.M.; Guimarães, M.P.M.; Vitalino, R.F.; Bagni, A.S.; Martins, Y.E.; Cordeiro, A.M. & Oliveira, E.G. 2010. Borboletas frugívoras do Parque Estadual do Rio Doce/MG. MG.BIOTA, 3(4): 5-21.) and from Viçosa (Cruz et al., 2012Cruz, K.C.; Lelis, S.M.; Godinho, M.A.S.; Fonseca, R.S.; Ferreira, P.S.F. & Vieira, M.F. 2012. Species richness of anthophilous butterflies of an Atlantic Rainforest fragment in Southeastern. Revista Ceres, Viçosa, 59: 571-579.), both in the core of the Atlantic Rainforest, in the state of Minas Gerais. This is, therefore, a new record for the Quadrilátero Ferrífero. Panoquina peraea (Hewitson, 1866), previously known to occurs only in the states of Rio de Janeiro (Mielke & Casagrande, 2002Mielke, O.H.H. & Casagrande, M.M. 2002. Notas taxonômicas em Hesperiidae neotropicais, com descrições de novos taxa (Lepidoptera). Revista Brasileira de Zoologia, 19(1): 27-76.) and Bahia (Lima & Zacca, 2014Lima, J.N.R. & Zacca, T. 2014. Lista de Espécies de Borboletas (Lepidoptera: Hesperioidea e Papilionoidea) de uma Área de Semiárido na Região Nordeste do Brasil. EntomoBrasilis, 7: 33-40.), is a new record for Minas Gerais.
Actinote quadra (Schaus, 1902) (Nymphalidae), classified as vulnerable in the Red Book of Brazilian Threatened Fauna (Machado et al., 2008Machado, A.B.M., Drummond, G.M. & Paglia, A.P. 2008. Livro vermelho da fauna brasileira ameaçada de extinção. Volume 1. MMA, Brasília. Belo Horizonte, Fundação Biodiversitas.), was sampled in the rock field at an altitude of about 1,475 m (20°03’29”S, 44°00’06”W; municipality of Brumadinho, Minas Gerais). This new record for the species and other three records are best detailed in Gomes et al. (2014Gomes, V.; Lourenço, G.M.; Soldati, D.; Iserhard, C.A.; Souza, T.S.; Kaminski, L.A. & Freitas, A.V.L., 2014. New geographical records for the threatened butterfly Actinote quadra (Lepidootera: Nymphalidae: Heliconiinae). Journal of the Lepidopterists’ Society, 68: 289-292.). This species occurs in isolated populations in mountain regions in the states of Minas Gerais, São Paulo and Rio de Janeiro, especially in the Serra da Mantiqueira, usually in wet forests above 1,000 m (Freitas et al., 2009Freitas, A.V.L.; Francini, R.B. & Souza, T.S. 2009. Immature stages and natural history of the threatened butterfly Actinote quadra (Nymphalidae: Heliconiinae: Acraeini). Tropical Lepidoptera Research, 19: 83-88.; Freitas & Marini-Filho, 2011Freitas, A.V.L. & Marini-Filho, O.J. 2011. Plano de ação nacional para conservação dos Lepidópteros ameaçados de extinção. Brasília, ICMBio. (Série Espécies Ameaçadas).). Main threats to Actinote quadra are habitat degradation and destruction (Freitas & Marini-Filho, 2011Freitas, A.V.L. & Marini-Filho, O.J. 2011. Plano de ação nacional para conservação dos Lepidópteros ameaçados de extinção. Brasília, ICMBio. (Série Espécies Ameaçadas).) and this reinforces the importance of the PESRM for the preservation of the species, as well as for all species of this genus inhabiting the park, since they are all endemic of mountainous environments and frequently rare (Gomes et al., 2014Gomes, V.; Lourenço, G.M.; Soldati, D.; Iserhard, C.A.; Souza, T.S.; Kaminski, L.A. & Freitas, A.V.L., 2014. New geographical records for the threatened butterfly Actinote quadra (Lepidootera: Nymphalidae: Heliconiinae). Journal of the Lepidopterists’ Society, 68: 289-292.).
Eight species endemic or potentially endemic of the Cerrado domain, according to Mielke et al. (2008Mielke, O.H.H.; Emery, E.O. & Pinheiro, C.E.G. 2008. As borboletas Hesperiidae (Lepidoptera, Hesperioidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 52: 283-288.) and Pinheiro et al. (2010Pinheiro, C.E.G.; Malinov, I.K.; Emery, E.O. & Schmidt, K. 2010. Endemismos e conservação de borboletas (Lepidoptera: Papilionoidea e Hesperioidea) no bioma Cerrado. In: Diniz, I.V., Filho, J.M., Machado, R.B. & Cavalcanti, R.B. (Org.). Cerrado: conhecimento científico quantitativo como subsidio para as ações de conservação. Brasília, Thesaurus. p. 223-238.), were also found in the PESRM: Yphthimoides straminea (Butler, 1867), Cogia grandis Riley, 1921, Cogia cerradicola (Mielke, 1967), Lerema veadeira Mielke, 1968, Parides bunichus diodorus (Hopffer, 1865), Pseudoscada acilla quadrifasciata Talbot, 1928, Sarbia catomelaena Mabille & Boullet, 1908 and Sophista latifasciata (Spitz, 1930). However, some of them were found in the forest and/or transition environments. In fact, Murray & Prowell (2005Murray, D.L. & Prowell, D.P. 2005. Molecular phylogenetics and evolutionary history of the neotropical satyrine subtribe Euptychiina (Nymphalidae: Satyrinae). Molecular Phylogenetics and Evolution, 34: 67-80.) pointed out that many species of the genus Yphthimoides are endemic of the Brazilian Southeast Region and Freitas et al. (2012Freitas, A.V.L; Kaminski, L.A.; Mielke, O.H.H.; Barbosa, E.B. & Silva-Brandão, K.L. 2012. A new species of Yphthimoides (Lepidoptera: Nymphalidae: Satyrinae) from the southern Atlantic Rainforest region. Zootaxa, 3526: 31-44.) indicated that most of them are found both in the Atlantic Rainforest and in the Cerrado domain. This is due to the isolated forests patches in the Cerrado, as well as riparian forests that are found along the rivers in the Cerrado domain (Silva & Bates, 2002Silva, J.M.C. & Bates, J.M. 2002. Biogeographic patterns and conservation in the South American Cerrado: a tropical savanna hotspot. BioScience, 52(3): 225-234.; Werneck, 2011Werneck, F.P. 2011. The diversification of eastern South American open vegetation biomes: Historical biogeography and perspectives. Quaternary Science Reviews, 30: 1630-1648.). This means that butterflies species in the Cerrado domain can also inhabit forests.
The geological formation of the PESRM is very peculiar, composed by ferruginous field, savanna and forest. This is one of the few parks in Brazil where this rare environment is preserved. This and the fact that those fields are the natural habitat for many species of butterflies make the PESRM an especially important conservation area. The knowledge about the rich fauna of butterflies of Minas Gerais, mainly the new records and possible new species as well as endangered species provides important information for preservation actions and environmental conservation, especially on places with such a peculiar environment.
ACKNOWLEDGEMENTS
We are grateful to Diego Rodrigo Dolibaina (UFPR), André Victor Lucci Freitas and Lucas Augusto Kaminski (UNICAMP) for the identification of part of the material, and Marco Paulo Macedo Magalhães for checking the identification of some specimens. We thank Alessandro Lima, Cássio Montes, Felipe Freitas, Glória Soares, Isabela Oliveira, José Eustáquio, Mércia Araújo, Naíla Fernandes, Rayane Melo, Rodolfo Arantes, Stanley Franco, Thaís Cardoso and Walter Ávila for field assistance. We also thank Marina do Vale Beirão and Frederico Kirst for the help with statistical analyses, and Luciana Barçante Ferreira for critically reviewing this manuscript.
REFERENCES
- Brown Jr., K.S. 1992. Borboletas da Serra do Japi: diversidade, habitats, recursos alimentares e variação temporal. In: Patrícia, L. & Morellato, C. (Eds.). História Natural da Serra do Japi: Ecologia e preservação de uma área florestal no Sudeste do Brasil. Campinas, FAPESP. p. 142-186.
- Brown Jr., K.S. & Freitas, A.V.L. 2000. Atlantic rainforest Butterflies: indicators for landscape conservation. Biotropica, 32: 934-956.
- Brown Jr., K.S. & Mielke, O.H.H. 1967a. Lepidoptera of the Central Brazil Plateau. I. Preliminary list of Rhopalocera: introduction, Nymphalidae, Libytheidae. Journal of the Lepidopterists’ Society, 21(2): 77-106.
- Brown Jr., K.S. & Mielke, O.H.H. 1967b. Lepidoptera of the Central Brazil Plateau. I. Preliminary list of Rhopalocera (continued): Lycaenidae, Pieridae, Papilionidae, Hesperiidae. Journal of the Lepidopterists’ Society, 21(3): 145-168.
- Brown Jr., K.S. & Mielke, O.H.H. 1968. Lepidoptera of the Central Brazil Plateau. III. Partial list for the Belo Horizonte area, showing the character of the south-eastern “blend zone”. Journal of the Lepidopterists’ Society, 22(3): 147-157.
- Carneiro, E.; Mielke, O.H.H. & Casagrande, M.M. 2008. Inventários de borboletas no Brasil: estado da arte e modelo de áreas prioritárias para pesquisa com vista à conservação. Natureza e Conservação, 6(2): 68-90.
- Carneiro, E.; Mielke, O.H.H.; Casagrande, M.M. & Fiedler, K. 2014. Community Structure of Skipper Butterflies (Lepidoptera, Hesperiidae) along Elevational Gradients in Brazilian Atlantic Rainforest Reflects Vegetation Type Rather than Altitude. Plos One, 9: 1-11.
- Casagrande, M.M.; Dolibaina, D.R.; Carneiro, E.; Dias, F.M.S.; Leite, L.A.R. & Mielke, O.H.H. 2012. Borboletas (Hesperioidea e Papilionoidea) de Jaguariaíva, Paraná, Brasil: Inventário em um enclave de Cerrado meridional. In: Carpanezzi, O.T.B. & Campos, J.B. (Eds.). Coletânea de Pesquisas. Parques Estaduais de Vila Velha, Cerrado e Guartelá. Curitiba, Instituto Ambiental do Paraná. p. 295-308.
- Crawley, M.J. 2013. The R Book. London, John Wiley and Sons Ltd.
- Cruz, K.C.; Lelis, S.M.; Godinho, M.A.S.; Fonseca, R.S.; Ferreira, P.S.F. & Vieira, M.F. 2012. Species richness of anthophilous butterflies of an Atlantic Rainforest fragment in Southeastern. Revista Ceres, Viçosa, 59: 571-579.
- D’Abrera, B. 2005. World Butterflies. Hill House Publishers, London. (Reduced version of The Concise Atlas of the Butterflies of the World, 2001).
- Dolibaina, D.R.; Mielke, O.H.H. & Casagrande, M.M. 2011. Borboletas (Papilionoidea e Hesperioidea) de Guarapuava e arredores, Paraná, Brasil: um inventário com base em 63 anos de registros. Biota Neotropica, 11: 1-14.
- Drummond, G.M. & Martins, C.S. (Coord.). 2007. Plano de manejo do Parque Estadual da Serra do Rola Moça. Belo Horizonte. Available at: Available at: http://www.biodiversitas.org.br/planosdemanejo/pesrm/regiao.htm Access in: 02/07/2016.
» http://www.biodiversitas.org.br/planosdemanejo/pesrm/regiao.htm - Ebert, H. 1969. On the frequency of butterflies in eastern Brazil, with a list of the butterfly fauna of Poços de Caldas, Minas Gerais. Journal of the Lepidopterists’ Society, 23(3): 1-48.
- Emery, E.O.; Brown Jr., K.S. & Pinheiro, C.E.G. 2006. As borboletas (Lepidoptera, Papilionoidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 50: 85-92.
- Freitas, A.V.L. & Marini-Filho, O.J. 2011. Plano de ação nacional para conservação dos Lepidópteros ameaçados de extinção. Brasília, ICMBio. (Série Espécies Ameaçadas).
- Freitas, A.V.L.; Francini, R.B. & Souza, T.S. 2009. Immature stages and natural history of the threatened butterfly Actinote quadra (Nymphalidae: Heliconiinae: Acraeini). Tropical Lepidoptera Research, 19: 83-88.
- Freitas, A.V.L; Kaminski, L.A.; Mielke, O.H.H.; Barbosa, E.B. & Silva-Brandão, K.L. 2012. A new species of Yphthimoides (Lepidoptera: Nymphalidae: Satyrinae) from the southern Atlantic Rainforest region. Zootaxa, 3526: 31-44.
- Garwood, K.; Lehman, R.; Carter, W. & Carter, G. 2009. Butterflies of Southern Amazon: A photography checklist of common species. Texas, McAllen, RiCalé Publishing.
- Gomes, V.; Lourenço, G.M.; Soldati, D.; Iserhard, C.A.; Souza, T.S.; Kaminski, L.A. & Freitas, A.V.L., 2014. New geographical records for the threatened butterfly Actinote quadra (Lepidootera: Nymphalidae: Heliconiinae). Journal of the Lepidopterists’ Society, 68: 289-292.
- Iserhard, C.A. 2011. Padronização dos métodos de captura de lepidópteros com puçá. In: Encontro Pan Lepidópteros e RedeLep. Brasília.
- Iserhard, C.A.; Brown Jr., K.S. & Freitas, A.V.L. 2013. Maximized sampling of butterflies to detect temporal changes in tropical communities. Journal of Insect Conservation, 17: 615-622.
- Iserhard, C.A.; Uehara-Prado, M.; Marini-Filho, O.J.; Duarte, M. & Freitas, A.V.L. 2018. Fauna da Mata Atlântica: Lepidoptera-borboletas. In: Monteiro-Filho, E.L.A & Conte, C.E. (Orgs.). Revisões em zoologia da Mata Atlântica. Curitiba, Editora UFPR. p. 57-102.
- Lamas, G. 2004. Atlas of Neotropical Lepidoptera. Checklist: Part 4A. Hesperioidea - Papilionoidea. Gainesville, Scientific Publishers.
- Lewinsohn, T.M.; Freitas, A.V.L. & Prado, P.I. 2005. Conservação de invertebrados terrestres e seus habitats no Brasil. Megadiversidade, 1: 62-69.
- Lima, J.N.R. & Zacca, T. 2014. Lista de Espécies de Borboletas (Lepidoptera: Hesperioidea e Papilionoidea) de uma Área de Semiárido na Região Nordeste do Brasil. EntomoBrasilis, 7: 33-40.
- Machado, A.B.M., Drummond, G.M. & Paglia, A.P. 2008. Livro vermelho da fauna brasileira ameaçada de extinção. Volume 1. MMA, Brasília. Belo Horizonte, Fundação Biodiversitas.
- Mielke, O.H.H. & Casagrande, M.M. 1997. Papilionoidea e Hesperioidea (Lepidoptera) do Parque Estadual do Morro do Diabo, Teodoro Sampaio, São Paulo, Brasil. Revista Brasileira de Zoologia, 14: 967-1001.
- Mielke, O.H.H. & Casagrande, M.M. 2002. Notas taxonômicas em Hesperiidae neotropicais, com descrições de novos taxa (Lepidoptera). Revista Brasileira de Zoologia, 19(1): 27-76.
- Mielke, O.H.H.; Carneiro, E. & Casagrande, M.M. 2012. Hesperiidae (Lepidoptera, Hesperioidea) from Ponta Grossa, Paraná, Brazil: 70 years of records with special reference to faunal composition of Vila Velha State Park. Revista Brasileira de Entomologia, 56(1): 59-66.
- Mielke, O.H.H.; Emery, E.O. & Pinheiro, C.E.G. 2008. As borboletas Hesperiidae (Lepidoptera, Hesperioidea) do Distrito Federal, Brasil. Revista Brasileira de Entomologia, 52: 283-288.
- Mittermeier, R.A.; Gil, P.R.; Hoffmann, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J. & Da Fonseca, G.A.B. 2004. Hotspots revisited: Earth’s biologically richest and most endangered terrestrial ecoregions. Agrupación Sierra Madre, Conservation International.
- Motta, P.C. 2002. Butterflies from the Uberlândia region, Central Brazil: Species list and biological comments. Brazilian Journal of Biology, 62: 151-163.
- Murray, D.L. & Prowell, D.P. 2005. Molecular phylogenetics and evolutionary history of the neotropical satyrine subtribe Euptychiina (Nymphalidae: Satyrinae). Molecular Phylogenetics and Evolution, 34: 67-80.
- Peña, N. & Wahlberg, C. 2008. Prehistorical climate change increased diversification of a group of butterflies. Biology Letters, 4: 274-278.
- Penz, C.M. & Devries, P.J. 2002. Phylogenetic analysis of Morpho Butterflies (Nymphalidae, Morphinae): Implications for classification and natural history. American Museum Novitates, 3374: 1-33.
- Pereira, G.C.N.; Coelho, M.S.; Beirão, M.V.; Braga, R.F. & Fernandes, G.W. 2017. Diversity of fruit-feeding butterflies in a mountaintop archipelago of rainforest. Plos One, 12(6): 1-20.
- Pinheiro, C.E.G.; Malinov, I.K.; Emery, E.O. & Schmidt, K. 2010. Endemismos e conservação de borboletas (Lepidoptera: Papilionoidea e Hesperioidea) no bioma Cerrado. In: Diniz, I.V., Filho, J.M., Machado, R.B. & Cavalcanti, R.B. (Org.). Cerrado: conhecimento científico quantitativo como subsidio para as ações de conservação. Brasília, Thesaurus. p. 223-238.
- R Core Team. 2014. R: A language and environment for statistical computing. Vienna, R Foundation for Statistical Computing. Available at: Available at: http://www.R-project.org Access in: 14/03/2016.
» http://www.R-project.org - RStudio Team. 2015. RStudio: Integrated development for R (Version 1.1.453). Boston, MA. Available at: Available at: http://www.rstudio.com Access in: 06/07/2018.
» http://www.rstudio.com - Santos, J.P.; Iserhard, C.A.; Teixeira, M.O. & Romanowski, H.P. 2011. Fruit-feeding butterflies guide of subtropical Atlantic Rainforest and Araucaria Moist Forest in State of Rio Grande do Sul, Brazil. Biota Neotropica, 11: 253-274.
- Seraphim, N.; Kaminski, L.A.; Devries, P.J.; Penz, C.; Callaghan, C.; Wahlberg, N.; Silva-Brandão, K.L. & Freitas, A.V.L. 2018. Molecular phylogeny and higher systematics of the metalmark butterflies (Lepidoptera: Riodinidae). Systematic Entomology, 43: 407-425.
- Silva, A.R.M.; Guimarães, M.P.M.; Vitalino, R.F.; Bagni, A.S.; Martins, Y.E.; Cordeiro, A.M. & Oliveira, E.G. 2010. Borboletas frugívoras do Parque Estadual do Rio Doce/MG. MG.BIOTA, 3(4): 5-21.
- Silva, J.M.C. & Bates, J.M. 2002. Biogeographic patterns and conservation in the South American Cerrado: a tropical savanna hotspot. BioScience, 52(3): 225-234.
- Tabarelli, M.; Pinto, L.P.; Silva, J.M.; Hirota, M.M. & Bedê, L.C. 2005. Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade, 1: 132-138.
- Uehara-Prado, M.; Brown Jr., K.S & Freitas, A.V.L. 2007. Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic Forest: comparison between a fragmented and a continuous landscape. Global Ecology and Biogeography, 16: 43-54.
- Uehara-Prado, M.; Freitas, A.V.L.; Francini, R.B. & Brown Jr., K.S. 2004. Guia das Borboletas Frugívoras da Reserva Estadual do Morro Grande e da Região de Caucaia do Alto, Cotia (São Paulo). Biota Neotropica, 4: 1-25.
- Wahlberg, N.; Leneveu, J.; Kodandaramaiah, U.; Peña, C.; Nylin, S.; Freitas, A.V.L. & Brower, A.V.Z. 2009. Nymphalid butterflies diversify following near demise at the Cretaceous/Tertiary boundary. Proceedings of the Royal Society B: Biological Sciences, 276: 4295-4302.
- Warren, A.D.; Davis, K.J.; Stangeland, E.M.; Pelham, J.P. & Grishin, N.V. 2017. Illustrated Lists of American Butterflies. Available at: Available at: http://www.butterfliesofamerica.com Access in: 18/06/2018.
» http://www.butterfliesofamerica.com - Warren, A.D.; Ogawa, J.R. & Brower, A.V.Z 2009. Revised classification of the family Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Systematic Entomology, 34: 467-523.
- Warren, A.D.; Ogawa, J.R. & Brower, A.V.Z. 2008. Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics, 24: 642-676.
- Werneck, F.P. 2011. The diversification of eastern South American open vegetation biomes: Historical biogeography and perspectives. Quaternary Science Reviews, 30: 1630-1648.
- Willmott, K.R. 2003. The genus Adelpha: Its systematics, biology and biogeography (Lepidoptera: Nymphalidae: Limenitidini). Gainesville, Scientific Publishers .
-
Published with the financial support of the Committee of "Programa de Apoio às Publicações Científicas Periódicas da USP" (SIBi-USP)
Edited by
Publication Dates
-
Publication in this collection
25 Mar 2019 -
Date of issue
2019
History
-
Received
16 Mar 2018 -
Accepted
25 Oct 2018