Abstracts
Background and objectives:
The aim of this study was to evaluate the effects of remote ischemic preconditioning by brief ischemia of unilateral hind limb when combined with dexmedetomidine on renal ischemia-reperfusion injury by histopathology and active caspase-3 immunoreactivity in rats.
Methods:
28 Wistar albino male rats were divided into 4 groups. Group I (Sham, n = 7): Laparotomy and renal pedicle dissection were performed at 65th minute of anesthesia and the rats were observed under anesthesia for 130min. Group II (ischemia-reperfusion, n = 7): At 65th minute of anesthesia bilateral renal pedicles were clamped. After 60 min ischemia 24 h of reperfusion was performed. Group III (ischemia-reperfusion + dexmedetomidine, n = 7): At the fifth minute of reperfusion (100 μg/kg intra-peritoneal) dexmedetomidine was administered with ischemia-reperfusion group. Reperfusion lasted 24 h. Group IV (ischemia-reperfusion + remote ischemic preconditioning + dexmedetomidine, n = 7): After laparotomy, three cycles of ischemic preconditioning (10 min ischemia and 10 min reperfusion) were applied to the left hind limb and after 5 min with group III.
Results:
Histopathological injury scores and active caspase-3 immunoreactivity were significantly lower in the Sham group compared to the other groups. Histopathological injury scores in groups III and IV were significantly lower than group II (p = 0.03 and p = 0.05). Active caspase-3 immunoreactivity was significantly lower in the group IV than group II (p = 0.01) and there was no significant difference between group II and group III (p = 0.06).
Conclusions:
Pharmacologic conditioning with dexmedetomidine and remote ischemic preconditioning when combined with dexmedetomidine significantly decreases renal ischemia- reperfusion injury histomorphologically. Combined use of two methods prevents apoptosis via active caspase-3.
Kidney; Ischemia-reperfusion injury; Dexmedetomidine; Caspase-3; Ischemic preconditioning; Apoptosis
Justificativa e objetivos:
Avaliar os efeitos do pré-condicionamento isquêmico remoto, mediante breve isquemia de membro posterior unilateral, em combinação com dexmedetomidina em lesão de isquemia-reperfusão renal por meio de histopatologia e imunorreatividade da caspase-3 ativa em ratos.
Métodos:
Foram divididos em quatro grupos 28 ratos machos albinos Wistar. Grupo I (Sham cirurgia controle], n = 7): laparotomia e dissecção do pedículo renal foram feitas em 65 minutos de anestesia e os ratos foram observados sob anestesia por 130 minutos. Grupo II (isquemia-reperfusão, n = 7): no 65° minuto de anestesia, os pedículos renais bilaterais foram pinçados; após 60 minutos de isquemia, foi feita reperfusão de 24 horas. Grupo III (isquemia-reperfusão + dexmedetomidina, n = 7): no quinto minuto de reperfusão, dexmedetomidina (100 mg/kg intraperitoneal) foi administrada ao grupo com isquemia-reperfusão. A reperfusão durou 24 horas. Grupo IV (isquemia-reperfusão + pré-condicionamento isquêmico remoto + dexmedetomidina, n = 7): após a laparotomia, três ciclos de pré-condicionamento isquêmico (10minutos de isquemia e 10minutos de reperfusão) foram aplicados no membro posterior esquerdo e depois de cincominutos ao grupo III.
Resultados:
Os escores de lesão histopatológica e imunorreatividade da caspase-3 ativa foram significativamente menores no grupo Sham em comparação com os outros. Os escores de lesão histopatológica dos grupos III e IV foram significativamente menores do que os do II (p = 0,03 e p = 0,05). A imunorreatividade da caspase-3 foi significativamente menor no grupo IV do que no II (p = 0,01) e não houve diferença significante entre os grupos II e III (p = 0,06).
Conclusões:
O condicionamento farmacológico com dexmedetomidina e o pré-condicionamento isquêmico remoto em combinação com dexmedetomidina diminuem de modo significante a lesão de isquemia-reperfusão renal histomorfologicamente. O uso combinado dos dois métodos previne a apoptose via caspase-3 ativa.
Rim; Lesão de isquemia-reperfusão; Dexmedetomidine; Caspase-3; Pré-condicionamento isquêmico; Apoptose
Introducción y objetivos:
El objetivo de este estudio fue evaluar los efectos del precondicionamiento isquémico remoto mediante breve isquemia del miembro posterior unilateral en combinación con la dexmedetomidina en la lesión de isquemia-reperfusión renal por medio de histopatología e inmunoreactividad de la caspasa-3 activa en ratones.
Métodos:
28 ratones machos albinos Wistar fueron divididos en 4 grupos. Grupo I (Sham cirugía control], n =7): se realizó laparotomia y disección del pediculo renal en 65 min de anestesia y los ratones fueron observados bajo anestesia durante 130min. Grupo II (isquemia-reperfusión, n = 7): en el sexagésimo quinto minuto de anestesia, los pídiculos renales bilaterales fueron pinzados; después de 60min de isquemia, se realizaron 24h de reperfusión. Grupo III (isquemia-reperfusión + dexmedetomidina, n = 7): al quinto minuto de reperfusión, la dexmedetomidina (100 μg/kg intraperitoneal) fue administrada en el grupo con isquemia-reperfusión; la reperfusión duró 24 h. Grupo IV (isquemia-reperfusión + precondicionamiento isquémico remoto + dexmedetomidina, n=7): después de la laparotomía, se aplicaron 3 ciclos de precondicionamiento isquémico (10 min de isquemia y 10 min de reperfusión) en el miembro posterior izquierdo y después de 5 min en el grupo in.
Resultados:
Las puntuaciones de lesión histopatológica e inmunoreactividad de la caspasa-3 activa fueron significativamente menores en el grupo Sham en comparación con los otros grupos. Las puntuaciones de lesión histopatológica de los grupos III y IV fueron significativamente menores que las del grupo II (p = 0,03 y p = 0,05). La inmunorreactividad de la caspasa-3 fue significativamente menor en el grupo IV que en el grupo II (p = 0,01) y no hubo diferencia significativa entre los grupos II y III (p = 0,06).
Conclusiones:
El condicionamiento farmacológico con dexmedetomidina y precondicionamiento isquémico remoto en combinación con dexmedetomidina disminuyen de modo significativo la lesión de isquemia-reperfusión renal histomorfológicamente. El uso combinado de los 2 métodos previene la apoptosis via caspasa-3 activa.
Riñó; Lesión de isquemia-reperfusión; Dexmedetomidina; Caspasa-3; Precondicionamiento isquémico; Apoptosis
Introduction
Ischemic acute renal injury is a clinical syndrome with high mortality and morbidity. Due to its high energy requirements and the complex network of the renal microvasculature, the kidney is highly sensitive to ischemia-reperfusion (IR) injury.11 . Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-74.–44 . Nigwekar SU, Kandula P, Hix JK, et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413-23. The mechanisms of renal IR injury are multifactorial, including hypoxia, free-radical damage, and local and systemic inflammatory responses.55 . Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133-8. Reperfusion of ischemic renal tissue induces complex cellular conditions and the death of renal cells, due to apoptosis.66 . Tsutsui H, Sugiura T, Hayashi K, et al. Moxonidine prevents ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol. 2009;603:73-8.
Apoptosis is a form of genetically programmed cell death. Two main pathways play a role in epithelial apoptosis. The first, known as the extrinsic or death receptor pathway, is stimulated by TNF-α-family molecules bound to CD95 ligand (Fas ligand = CD95) through extracellular signals. This pathway combines with the second pathway, the intrinsic or mitochondrial pathway, via caspase-3 activation, which includes cytochrome c and the Bcl-2 family of proteins, and together, they enhance apoptosis.77 . Yazici P, Alizadehshargh S, Akdogan G. Apoptosis: regulatory molecules, relationships with diseases and apoptosis detection methods. Turkiye Klinikleri J Med Sci. 2009;29:1677-86.
Renal tubular cell apoptosis is a result of renal injury and a primary and major contributor to IR pathophysiology. Both inflammation and apoptosis coexist in renal IR injury. During hypoxia, caspase activity increases as a result of intracellular Ca2+ accumulation. Caspase becomes activated in ischemic tissues and is an indicator of cell death.88 . Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Immune Endocr Metabol Disord. 2005;5:269-87. These changes, which can be observed in tubular cells, may cause the loss of brush borders of proximal tubular cells, and spill out from the basement membrane of the cells into the tubular lumen, with eventual tubule obstruction.99 . Noiri E, Gailit J, Sheth D, et al. Cyclic RGD peptides ameliorate ischemic acute renal failure in rats. Kidney Int. 1994;46:1050-8.,1010 . Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486-91.
Among the methods used to reduce the effects of IR injury, remote ischemic preconditioning (RIPC) and pharmacological conditioning are the most commonly used.1111 . Tapuria N, Kumar Y, Habib MM, et al. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury - a review. J Surg Res. 2008;150:304-30. In reviewing the English-language literature, no study was found regarding an experimental IR model to investigate the effects of using pharmacological conditioning with dexmedetomidine administered in combination with RIPC.
The aim of this experimental study was to investigate the renal effects of unilateral RIPC administered in combination with dexmedetomidine to the lower extremity histopathologically and to evaluate active caspase-3 immunoreactivity in a rat renal IR model.
Materials and methods
In total, 28 adult male Wistar albino rats, weighing 250-300g, were used. Until the beginning of the research, rats were kept at room temperature (21-22 °C) and 40-60% relative humidity on a 12/12-h light/dark cycle and were fed with standard pellet diet and water ad libitum. After approval by the Local Ethics Committee for Animal Experiments at our faculty, the study was carried out in the Multidisciplinary Laboratory of Animal Experiments. Anesthesia was provided with 50 mg/kg ketamine and 10 mg/kg xylazine hydrochloride administered intraperitoneally (ip). After anesthesia, the animals were divided into four groups.
Group I (Sham, n = 7): After laparotomy, the left and right renal pedicles were exposed at the 65th minute of anesthesia, and rats were kept under anesthesia for 130 min with no other intervention.
Group II (IR, n = 7): After 60 min of ischemia, the clamp was removed and reperfusion of the kidneys was allowed for 24 h.
Group III (IR + dexmedetomidine, n = 7): After 60 min of ischemia, the clamp was removed and 100 μg/kg dexmedetomidine (Precedex 100 μg/2 mL, Abbott Laboratories, IL, USA) was administered ip, and reperfusion of the kidneys was allowed for 24 h.
Group IV (IR + RIPC +dexmedetomidine, n = 7): Following a laparotomy, after 5 min of RIPC, and a subsequent 60 min of renal ischemia, the clamp was removed and 100 μg/kg dexmedetomidine (Precedex 100 μg/2 mL, Abbott Laboratories) was given and finally 24 h of reperfusion was performed (Fig. 1).
Schematic representation of the experimental protocol. I, ischemia; R, reperfusion; D, deksmedetomidine.
Exposing renal pedicles in group I, and initiating ischemia at the 65th minute of anesthesia in groups II and III, was intended to synchronize all the groups to the preconditioning time of group IV and to standardize the start of the procedures. Tissue samples were obtained after 24 h of reperfusion.
To protect the rats from hypothermia, the operating table was heated with a lamp heater throughout the study and rectal body temperature was measured with a probe and maintained at 3737.5 °C. Hourly subcutaneous saline solution, at 3 mL/kg dosage, was administered to prevent dehydration. During the waiting time, the abdomen was closed with a moist sterile pad and surgical forceps. In all groups, the left kidneys were removed under anesthesia for histomorphological analyses and the rats were sacrificed by a cardiac puncture exsanguination at the end of the study. The kidneys were fixed in 10% buffered formalin and embedded in paraffin wax, cut at 4-5 μm, and stained with hematoxylin and eosin for histological studies using light microscopy.
Renal IR model
The right and left renal pedicles were exposed after laparotomy. Total renal ischemia in the left and right renal pedicles was maintained with atraumatic microvascular clamp compression. Adequate occlusion was confirmed by a lack of pulsation in the renal pedicles and presence of pallor in the kidneys. After the ischemic period, the microvascular clamps were removed and reperfusion occurred.
RIPC model
For the tourniquet effect of RIPC, a method that has been shown to be effective by perfusion scintigraphy and a laser meter was used.1212 . Kanoria S, Jalan R, Davies NA, et al. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762-8.,1313 . Duru S, Koca U, Oztekin S, et al. Antithrombin III pretreatment reduces neutrophil recruitment into lung and skeletal muscle tissues in the rat model of bilateral lower limb ischaemia and reperfusion: a pilot study. Acta Anaesthesiol Scand. 2005;49:1142-8. For this purpose, the left hind leg of the rat was bound with an elastic bandage (1 cm wide and 30 cm long) at the groin, applying pressure all around. Three cycles of 10 min of ischemia were performed followed by 10 min of reperfusion (65 min total). The cessation of blood flow was confirmed using a laser current meter (Laser Flo BPM2, Vasamedic, USA).
Histomorphological evaluation of renal tissue
Renal tissue sections after IR were evaluated by light microscopy by two histologists blinded to the animal groups in terms of structural changes in proximal tubules (tubular atrophy, loss of tubular brush border, vacuolization, tubular dilatation, cast formation), mononuclear cells (MNCs) infiltration, interstitial structural changes, renal corpuscle morphology, and necrotic and apoptotic cells.
The cross-sectional images were scored semi-quantitatively in terms of tubulointerstitial damage. Scoring was conducted as follows: 0 = not at all, 1 =0-25%, 2 = 26-45%, 3=46-75%, and 4 = 76-100%.1414 . Feng L, Xiong Y, Cheng F, et al. Effect of ligustrazine on ischemia-reperfusion injury in murine kidney. Transplant Proc. 2004;36:1949-51.
Immunohistochemical methods
Kidney tissues were fixed in 10% buffered formaldehyde, and after routine histological follow-up procedures, paraffin-embedded kidney tissues were cut into 3-μm thick sections with a microtome and collected on poly-L-lysine-coated slides.
Samples were stored in the oven at 60°C for 12 h. Then, a rat-specific anti-caspase-3 monoclonal antibody (RB-10287-R7 Labvision) was used to assess anti-caspase-3 immunoreactivity.
Endogenous peroxidase activity was blocked using a 3% solution of hydrogen peroxidase in alcohol. Lysine sections were treated with anti-caspase-3 antibody at 4 °C overnight and then incubated with a biotinylated secondary antibody for 30 min. After application of the Vector Elite ABC kit (Vector Laboratories Inc., Burlingame, USA), the antibody-biotin-avidin-peroxidase complex was visualized using 0.02% 3,3'-diaminobenzidine solution. Following counterstaining with Mayer's hematoxylin, image analysis was performed.
The positive staining rate was evaluated using an indicator of semi-quantitative scoring (1-4), in terms of density and distribution.
Exclusion criteria
Rats in need of resuscitation were excluded from the study.
Statistical analysis
The SPSS software (ver. 15.0; SPSS, Chicago, IL, USA) was used for statistical analyses. A Kruskal-Wallis analysis of variance was performed to analyze the data. The Mann-Whitney U-test was used for pair-wise comparisons of the groups. All data are presented as means ± standard deviations. p values <0.05 were considered to indicate statistical significance.
Results
In total, 28 rats were included in the study group. One rat in the IR group died during the reperfusion period, and was excluded from the study. Histomorphological and immunohistochemical injury scores of the study groups are presented in Tables 1 and 2.
Renal histomorphological injury scores
MNC infiltration. Histomorphological injury scores of the Sham group were significantly lower than those of the IR group (p = 0.01). Injury scores in the IR group were significantly higher than in the IR+ Dex and IR+ RIPC+ Dex groups (p = 0.04 and p = 0.04, respectively; Fig. 2).
Mononuclear cell infiltration scores detected by renal histomorphological examination. *Comparison of the IR group with the Sham group (p = 0.01). ≠Comparison of the IR + Dex and IR + RIPC + Dex groups with the IR group (p < 0.05).
Structural changes in the proximal tubules. Histomorphological injury scores of the Sham group were significantly lower than those of the IR, IR + Dex, and IR + RIPC + Dex groups (p <0.01, p = 0.02, and p = 0.02, respectively). The IR group displayed significantly higher scores than the IR + Dex and IR + RIPC + Dex groups (p = 0.05 and p = 0.05, respectively), while no significant difference was found between the IR + Dex and IR + RIPC + Dex groups (p = 1.00; Fig. 3).
Tubular change injury scores detected by renal histomorphological examination. *Comparison of the IR, IR + Dex and IR + RIPC + Dex groups with the Sham group (p < 0.01). ≠Comparison of the IR group with the IR + Dex and IR + RIPC + Dex groups (p = 0.05).
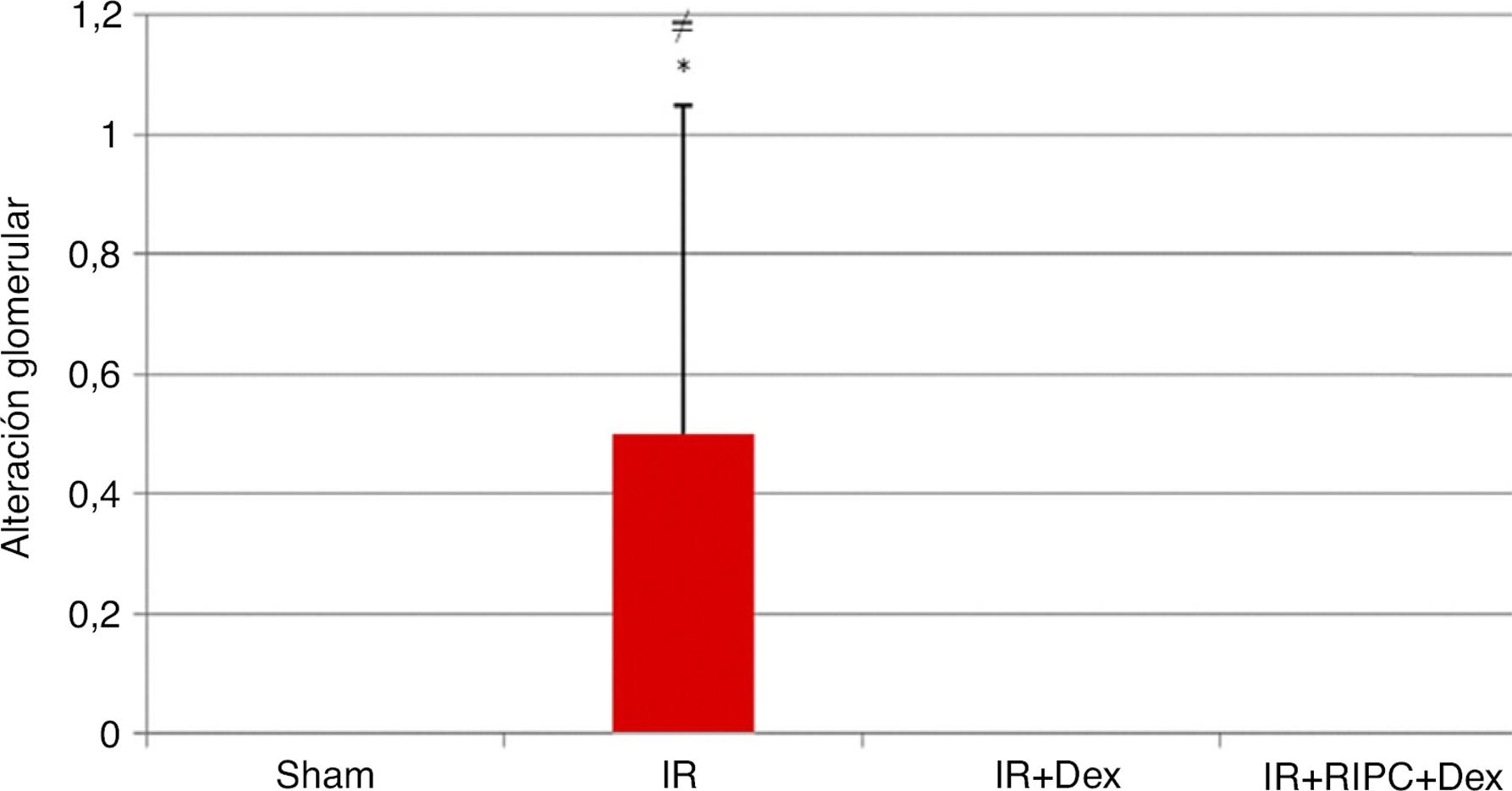
Glomerular changes. Histomorphological injury scores of the Sham group were significantly lower than those of the IR group (p = 0.04). The IR group displayed significantly higher scores than the IR + Dex and IR + RIPC + Dex groups (p = 0.04 and p = 0.04, respectively), while no significant difference was found between the IR + Dex and IR + RIPC + Dex groups (p = 1.00; Fig. 4).
Glomerular change injury scores detected by renal histomorphology. *Comparison of the IR group with the Sham group (p < 0.01). ≠Comparison of the IR group with the IR + Dex and IR + RIPC + Dex groups (p < 0.05).
Total histopathological injury score. The total histomorphological injury scores of the Sham group were significantly lower than those of the IR group (p = 0.003). Comparison of the IR group with the IR + Dex and IR + RIPC + Dex groups showed significantly higher scores than the IR group (p = 0.03 and p = 0.05, respectively), while the IR + Dex and IR + RIPC + Dex groups showed no significant difference (p = 0.79; Fig. 5).
Total histopathological injury scores detected by renal histomorphology. *Comparison of the IR group with the Sham group (p < 0.01). ≠Comparison of the IR group with the IR + Dex and IR + RIPC + Dex groups (p < 0.05).
Immunohistochemical injury score. Immunohistochemical staining scores of the IR group were significantly higher than in the Sham group (p = 0.001). No statistically significant difference was found between the IR and IR + Dex groups (p = 0.06).
In the comparison of injury scores between the IR + RIPC + Dex and IR groups, those of the IR + RIPC + Dex group were significantly lower (p = 0.01). The injury scores of the IR + Dex and IR + RIPC + Dex groups did not differ (p = 0.47; Fig. 6).
Renal immunohistochemical scoring. *Comparison of the IR group with the Sham group (p < 0.01). ≠Comparison of the IR group with the IR + Dex and IR + RIPC + Dex groups (p = 0.01).
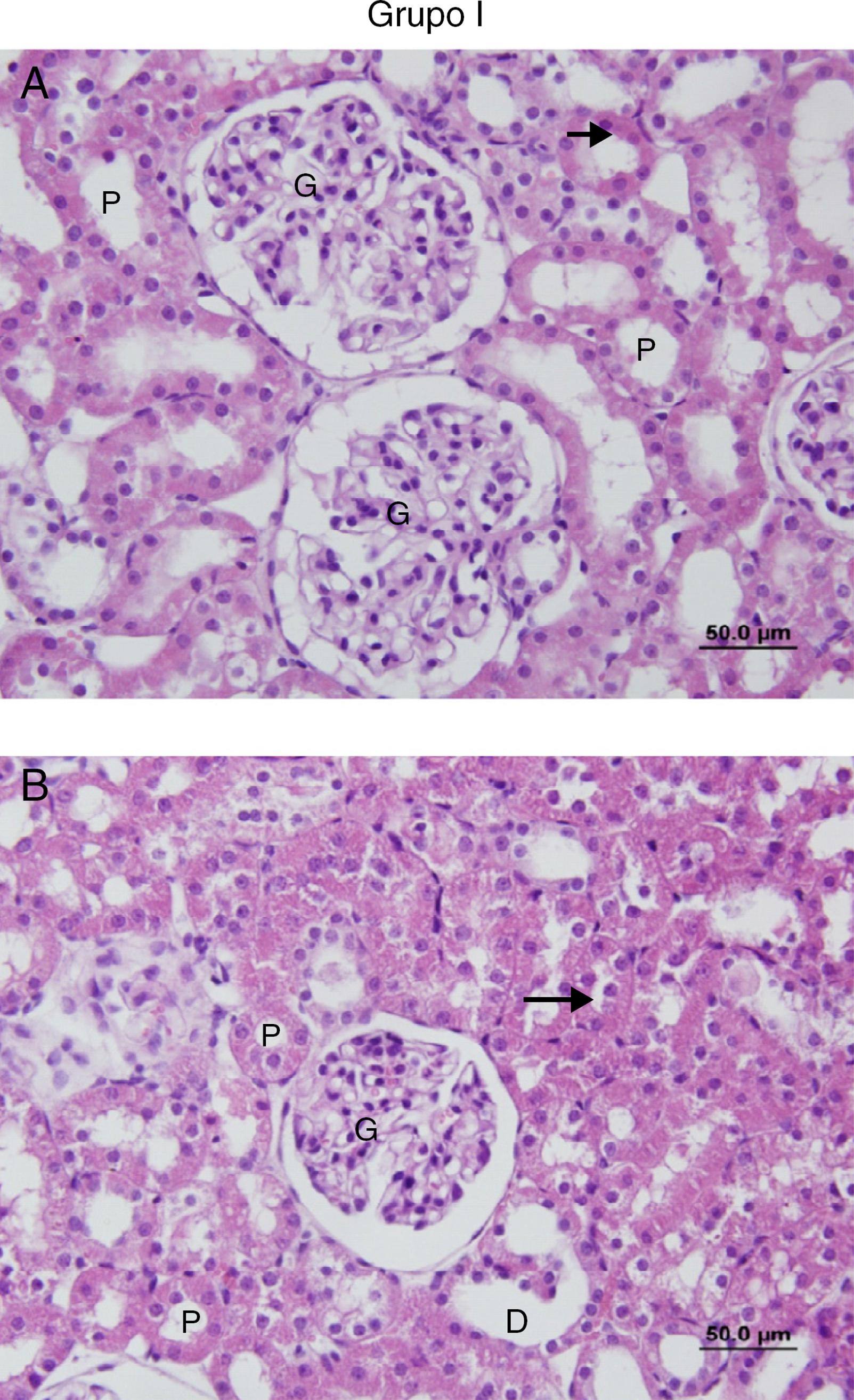
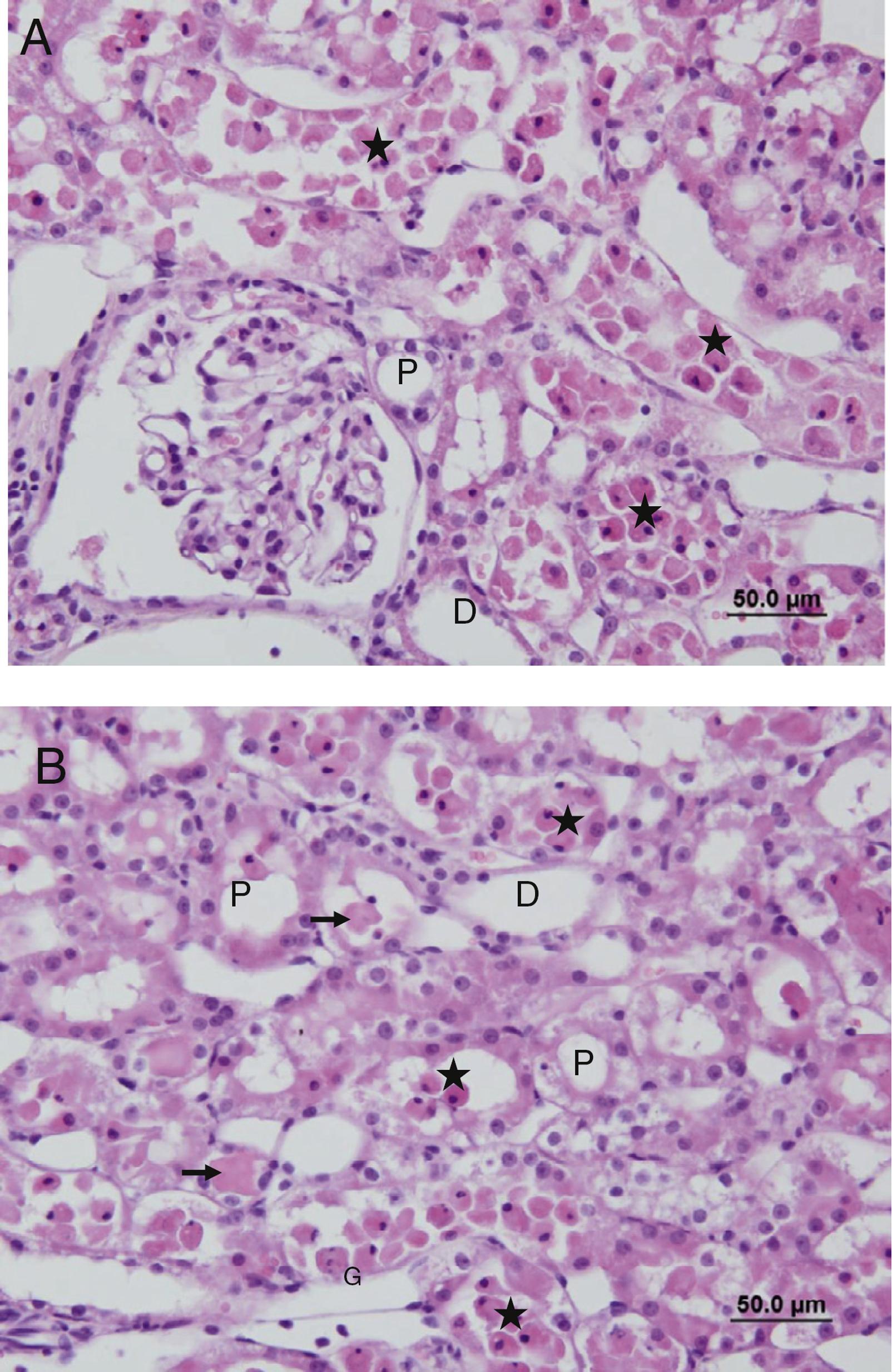
No cell infiltration or loss of brush border was observed in the sections of the Sham group (Fig. 7A and B). However, in the IR group, peritubular MNC infiltration, more prominent in the cortical area, and loss of brush border of the proximal tubule cells, tubular atrophy, tubular dilation, and vacuolation were observed. In some of the tubules, proteinaceous material accumulation, together with caste formation and cell debris in the tubule lumen, were observed (Fig. 8A and B).
(A and B) Sections of the Sham group. G, glomerular; P, the proximal tubule; D, the distal tubule: the brush border.
(A and B) Sections of the IR group. G, glomerular; P, the proximal tubule; D, the distal tubule, (→) accumulation of proteinaceous material in tubules, (⋆) poured into the lumen of tubular epithelial cells.
In the IR + Dex group, loss of the brush border of the tubule cells, tubular atrophy, tubular dilatation, vacuolization, and proteinaceous material accumulation and cell debris in the tubule lumen were observed, albeit to a lesser extent than in the IR group (Fig. 9A and B).
(A and B) Sections of the IR + Dex group. G, glomerular; P, the proximal tubule; D, the distal tubule, (➞) accumulation of proteinaceous material to be reduced compared to other groups.
Compared with the IR group, the IR + RIPC + Dex group displayed tubular atrophy, tubular dilatation, vacuolization, proteinaceous material accumulation and cell debris in the lumen of the tubules, and loss of the brush border of the tubule cells, albeit to a lesser extent. However, compared with the IR + Dex group, tubular changes were less marked (Fig. 10A and B).
(A and B) Sections of the IR + RIPC + Dex group. G, glomerular; P, proximal tubule; D, distal tubule, and (→) accumulation of proteinaceous to be reduced compared to other.
Immunohistochemical staining intensity was increased in the active caspase-3-positive cells of the IR group compared with the Sham group, while it was decreased in the cells of the IR + Dex and IR + RIPC + Dex groups compared with the IR group. Staining intensity in the active caspase-3-positive cells of the IR + RIPC + Dex group was decreased compared with the IR + Dex group (Fig. 11).
Active caspase-3 immunohistochemistry experimental groups of the sectional images of stained. (A) Sham, (B) IR, (C) IR + Dex, and (D) IR + RIPC + Dex ➩ active caspase-3 immunostaining indicate positive proximal tubule epithelial cells. (C and D) Sections of the positive active caspase-3 immunostaining were observed in the proximal tubule epithelial cells in the B group decreased.
Discussion
Acute renal failure occurring as a result of ischemia may be due to hypotension, hypovolemia, and hypoperfusion secondary to dehydration as well as IR damage in major cardiothoracic, vascular, and transplant surgeries.1515 . Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448-60.–1818 . Gu J, Pamela S, Hailin Z, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. IR injury is one of the most common causes of perioperative acute renal failure.1818 . Gu J, Pamela S, Hailin Z, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153.
Methods such as RIPC and pharmacological conditioning with dexmedetomidine, which are used to prevent or treat renal IR injury, have been shown to have positive effects in terms of IR injury in many previous studies.1111 . Tapuria N, Kumar Y, Habib MM, et al. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury - a review. J Surg Res. 2008;150:304-30.
In a literature search, we found no study that had evaluated the effects of combining these two methods for IR injury. This experimental study, using a rat model of renal IR, compared the effects of using dexmedetomidine alone or RIPC in combination with dexmedetomidine against renal apoptosis, assessing using caspase-3 immunoreactivity and histopathological injury scores.
According to our results, dexmedetomidine used alone or in combination with RIPC significantly reduced MNC infiltration, glomerulotubular changes, and total damage scores in IR injury. In both groups, all the scores except for tubular changes (p = 0.05) were similar to the scores of the Sham group. Significant decreases were detected in active caspase-3 immunoreactivity in the dexmedetomidine combined with RIPC group, suggesting that apoptosis, a major pathway of IR injury, could be reduced or prevented by this combination.
Various periods of ischemia and reperfusion were used in renal IR injury models in many studies.1919 . Kocoğlu H, Ozturk H, Ozturk H, et al. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70-4.–2222 . Hussein Ael-A, Shkeir AA, Sarhan ME, et al. Effects of combined erythropoietin and epidermal growth factor on renal ischaemia/reperfusion injury: a randomized experimental controlled study. BJU Int. 2011;107:323-8. The critical ischemic period is dependent on the organ, and ischemia lasting more than 5 min for the brain, and 15-20 min for the liver and kidney may cause neuronal death and infarction.2323 . Jaeschke H, Farhood A. Kupffer cell activation after no-flow ischemia versus hemorrhagic shock. Free Radic Biol Med. 2002;33:210-9. Williams et al.2020 . Williams P, Lopez H, Britt D, et al. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1-7. investigated the effects of IR injury in blood and tissue samples obtained after reperfusion following 45 min of renal ischemia, and reported that renal IR injury occurred earliest, at the fourth hour and peaked at 24 h.
Organ-protective effects of dexmedetomidine against IR damage have been shown in many tissues, such as the brain, heart, and kidneys.2424 . Sanders RD, Maze M. Alpha 2-adrenoceptor agonists. Curr Opin Investig Drugs. 2007;8:25-33.–2727 . Kuhmonen J, Pokorny J, Miettinen R, et al. Neuroprotective effects of dexmedetomidine in the gerbil hippocampus after transient global ischemia. Anesthesiology. 1997;87:371-7. The effects of dexmedetomidine in rat renal IR injury were investigated by Kocoğlu et al.1919 . Kocoğlu H, Ozturk H, Ozturk H, et al. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70-4. These researchers reported a significant decrease in scores for histopathological injury, detected at the 45th minute after ip administration of 100 μg/kg dexmedetomidine at the beginning of reperfusion. In our study, with the same dose of dexmedetomidine administered at the beginning of reperfusion, a renoprotective effect was achieved, as evidenced by significant reductions in the histological injury scores. This renoprotective effect was found not only during the early period after reperfusion injury but it continued, even at 24 h, which was considered the peak of IR injury.2020 . Williams P, Lopez H, Britt D, et al. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1-7.
Although the mechanism underlying the protective effect of dexmedetomidine in renal IR is unclear, it is considered to increase renal blood flow and glomerular filtration by reducing the release of noradrenaline.2828 . Taoda M, Adachi YU, Uchihashi Y, et al. Effect of dexmedetomidine on the release of 3H]-noradrenaline from rat kidney cortex slices: characterization of alpha2-adrenoceptor. Neurochem Int. 2001;38:317-22.
Villela et al.2929 . Villela NR, Nascimento PVN, Carvalho LR. Effects of dexmedetomidine on renal system and on vasopressin plasma levels. Experimental study in dogs. Rev Bras Anestesiol. 2005;55:429-40. reported that low-dose dexmedetomidine administration in anesthetized dogs reduced the urinary osmolality and plasma vasopressin level and caused free-water diuresis. In patients who had no renal disease but experienced thoracic surgery, by administering an infusion of dexmedetomidine, Frumento et al.3030 . Frumento RJ, Logginidou HG, Wahlander S, et al. Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery. J Clin Anesth. 2006;18:422-6. showed an improvement in renal function, including urine flow and glomerular filtration in the postoperative period.
One of the most likely mechanisms of the action of dexmedetomidine is protecting the kidney by inhibiting the surgical stress response and preventing adrenergic systemmediated vasoconstriction.3131 . Kulka PJ, Tryba M, Zenz M. Preoperative alpha 2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Crit Care Med. 1996;24:947-52.–3434 . Flacke JW, Bloor BC, Flacke WE, et al. Reduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary bypass surgery. Anesthesiology. 1987;67:11-9. It may also increase renal arterial vasodilation through direct vascular effects.1919 . Kocoğlu H, Ozturk H, Ozturk H, et al. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70-4. PKC has been reported to play an important role in ischemic preconditioning and to open sarcolemmal and mitochondrial ATP-dependent K+ channels by stimulating intracellular transduction pathways and inducing synthesis of protective cellular proteins. In a study of alternative signal transduction pathways, alpha 2B receptor agonists were shown to stimulate PKC activity and the production of inositol triphosphate in the distal renal collecting tubule cells, suggesting that alpha 2 agonists mimic cell protection by IPC.3535 . Gesek FA. Alpha2-adrenergic receptors activate phospholipase C in renal epithelial cells. Mol Pharmacol. 1996;50:407-14.,3636 . O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420-32. Gu et al.1818 . Gu J, Pamela S, Hailin Z, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153. investigated the mechanism of action of dexmedetomidine in vitro with a stabilized human renal proximal tubule cell culture deprived of both oxygen and glucose. They reported both a significant increase in phosphoAkt expression in cultured tubular cells after treatment with dexmedetomidine, dependent on the dose, and an alpha-2 adrenoreceptor effect. The phosphoAkt pathway ensures cell viability by inhibiting caspase-controlled intrinsic apoptotic pathways through phosphorylation of proapoptotic Bcl-2, which triggers cell death, and upregulation of anti-apoptotic Bcl-2 and Bcl-XL expression.
The neuroprotective effect of dexmedetomidine has been reported to be a result of the increase in anti-apoptotic Bcl-2 and Mdm-2 expression; this increase has been associated with a decrease in the levels of proapoptotic caspase-3 and Bax.3737 . Engelhard K, Werner C, Eberspacher E, et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyldaspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524-31. The Akt pathway is critical in recovering from renal IR. In this study, as an indicator of apoptosis, active caspase-3 immunoreactivity was evaluated. Activation of caspase-3 is the final step of apoptosis, which is common to the two major pathways of apoptosis and a definitive indicator of cell death. Dexmedetomidine administration was shown to decrease active caspase-3 immunoreactivity, but not significantly so (p = 0.06), compared with IR injury.
This statistically non-significant difference could be explained by the small number of study subjects. Ischemic preconditioning is a method applied mechanically or pharmacologically prior to target organ ischemia to reduce the level of subsequent IR injury. The aim in ischemic preconditioning is to apply ischemia and reperfusion to target organs in short intervals, to ensure that the target organ(s) can tolerate ischemia well. High energy demands and the intense microvascular network of the kidneys make them vulnerable to IR injury, which is considered a major cause of kidney damage in renal artery stenosis and renal microvascular surgery. Renal IR injury is the major cause of cardiovascular morbidity and mortality, and is associated with post-transplantation delay in graft function, and renal injury occurring in cardiac and aortic surgery and post-shock renal injury.11 . Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-74.–44 . Nigwekar SU, Kandula P, Hix JK, et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413-23.,3838 . Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42.,3939 . Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159-69.
Both experimental and clinical studies in the literature show that distant organ IPC may be protective for the kidney.4040 . Wever KE, Menting TP, Rovers M, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE. 2012;7:e32296.,4141 . Venugopal V, Laing CM, Ludman A, et al. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis. 2010;56:1043-9. Similar to our study, Wever et al.4040 . Wever KE, Menting TP, Rovers M, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE. 2012;7:e32296. investigated the effect of hind leg DIPC on renal IR injury. Differently, these authors compared the effect of administering DIPC either continuously or periodically, and also to one or two extremities. Although they performed three cycles of DIPC, they used I/R periods of 4/4min and investigated the eventual effects of 25 min of ischemia. They reported that molecule-1 expression, indicating renal tubular and renal injury, decreased significantly in the group with three cycles of I/R; moreover, this protection was not related to adenosine, one of the key elements in I/R injury. The underlying mechanism of DIPC and its transduction pathways are not yet completely understood. Both neurogenic pathways and biochemical transmitters may play roles in the mechanism of DIPC.4242 . Curtis FG, Vianna PT, Viero RM. Dexmedetomidine and S(+)-ketamine in ischemia and reperfusion injury in the rat kidney. Acta Cir Bras. 2011;26:202-6. These mechanisms may vary, depending on the target organ and applied preconditioning protocol. In the myocardial ischemia methods using renal DIPC, expression of NF-κB protein followed by the opening of K+ ATP channels have been reported to be important.4343 . Diwan V, Kant R, Jaggi AS, et al. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 2008;315:195-201.
Although renal IR injury is a common and important clinical problem, strategies to reduce IR injury are insufficient and new treatments are needed. In the literature, no report of both pharmacological and mechanical protection in renal IR injury in rats could be found.
Thus, two different methods used in rat renal IR injury, dexmedetomidine, widely reported to be effective, and DIPC, that has been shown to be effective in few studies,1111 . Tapuria N, Kumar Y, Habib MM, et al. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury - a review. J Surg Res. 2008;150:304-30. were combined and compared. Similar to dexmedetomidine, using dexmedetomidine and DIPC in combination prevented histopathological injury and improved IR injury scores, except tubular change scores, to levels close to the Sham group. At the same time, significant decreases in active caspase-3 immunoreactivity compared with the IR group suggested that using dexmedetomidine and DIPC in combination might prevent apoptosis. Using dexmedetomidine alone decreased active caspase-3 immunoreactivity, albeit non-significantly so, which suggests that these two methods operate via similar pathways. These two protective methods are likely to increase the effect of each other.
In our study, xylazine, an anesthetic agent having alpha 2-agonist activity, and ketamine4242 . Curtis FG, Vianna PT, Viero RM. Dexmedetomidine and S(+)-ketamine in ischemia and reperfusion injury in the rat kidney. Acta Cir Bras. 2011;26:202-6. suggested to have negative effects on IR injury, were used. Histopathological scores and active caspase-3 immunoreactivity were normal in the Sham group, which suggests that ketamine had no negative effect on our results.
We did not show the effect of DIPC alone on apoptosis, which plays a key role in the mechanism of IR injury; this is a limitation of the present study. Other limiting factors are that oxidative stress and inflammatory mediators, which are also responsible for IR injury, and the neurogenic pathway, were not examined.
References
-
1Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-74.
-
2Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation. Lancet. 2004;364:1814-27.
-
3Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-40.
-
4Nigwekar SU, Kandula P, Hix JK, et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413-23.
-
5Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133-8.
-
6Tsutsui H, Sugiura T, Hayashi K, et al. Moxonidine prevents ischemia/reperfusion-induced renal injury in rats. Eur J Pharmacol. 2009;603:73-8.
-
7Yazici P, Alizadehshargh S, Akdogan G. Apoptosis: regulatory molecules, relationships with diseases and apoptosis detection methods. Turkiye Klinikleri J Med Sci. 2009;29:1677-86.
-
8Faubel S, Edelstein CL. Caspases as drug targets in ischemic organ injury. Immune Endocr Metabol Disord. 2005;5:269-87.
-
9Noiri E, Gailit J, Sheth D, et al. Cyclic RGD peptides ameliorate ischemic acute renal failure in rats. Kidney Int. 1994;46:1050-8.
-
10Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486-91.
-
11Tapuria N, Kumar Y, Habib MM, et al. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury - a review. J Surg Res. 2008;150:304-30.
-
12Kanoria S, Jalan R, Davies NA, et al. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762-8.
-
13Duru S, Koca U, Oztekin S, et al. Antithrombin III pretreatment reduces neutrophil recruitment into lung and skeletal muscle tissues in the rat model of bilateral lower limb ischaemia and reperfusion: a pilot study. Acta Anaesthesiol Scand. 2005;49:1142-8.
-
14Feng L, Xiong Y, Cheng F, et al. Effect of ligustrazine on ischemia-reperfusion injury in murine kidney. Transplant Proc. 2004;36:1949-51.
-
15Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448-60.
-
16Brezis M, Rosen S, Silva P, et al. Renal ischemia: a new perspective. Kidney Int. 1984;26:375-83.
-
17Caron A, Desrosiers RR, Beliveau R. Kidney ischemia reperfusion regulates expression and distribution of tubulin subunits, beta-actin and rho GTPases in proximal tubules. Arch Biochem Biophys. 2004;431:31-46.
-
18Gu J, Pamela S, Hailin Z, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153.
-
19Kocoğlu H, Ozturk H, Ozturk H, et al. Effect of dexmedetomidine on ischemia-reperfusion injury in rat kidney: a histopathologic study. Ren Fail. 2009;31:70-4.
-
20Williams P, Lopez H, Britt D, et al. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1-7.
-
21Fujii T, Takaoka M, Muraoka T, et al. Preventive effect of lcarnosine on ischemia/reperfusion-induced acute renal failure in rats. Eur J Pharmacol. 2003;474:261-7.
-
22Hussein Ael-A, Shkeir AA, Sarhan ME, et al. Effects of combined erythropoietin and epidermal growth factor on renal ischaemia/reperfusion injury: a randomized experimental controlled study. BJU Int. 2011;107:323-8.
-
23Jaeschke H, Farhood A. Kupffer cell activation after no-flow ischemia versus hemorrhagic shock. Free Radic Biol Med. 2002;33:210-9.
-
24Sanders RD, Maze M. Alpha 2-adrenoceptor agonists. Curr Opin Investig Drugs. 2007;8:25-33.
-
25Billings FT, Chen SW, Kim M, et al. Alpha 2-adrenergic agonists protect against radiocantrast-induced nephropathy in mice. Am J Physiol Renal Physiol. 2008;295:741-8.
-
26Ma D, Hossain M, Rajakumaraswamy N, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87-97.
-
27Kuhmonen J, Pokorny J, Miettinen R, et al. Neuroprotective effects of dexmedetomidine in the gerbil hippocampus after transient global ischemia. Anesthesiology. 1997;87:371-7.
-
28Taoda M, Adachi YU, Uchihashi Y, et al. Effect of dexmedetomidine on the release of 3H]-noradrenaline from rat kidney cortex slices: characterization of alpha2-adrenoceptor. Neurochem Int. 2001;38:317-22.
-
29Villela NR, Nascimento PVN, Carvalho LR. Effects of dexmedetomidine on renal system and on vasopressin plasma levels. Experimental study in dogs. Rev Bras Anestesiol. 2005;55:429-40.
-
30Frumento RJ, Logginidou HG, Wahlander S, et al. Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery. J Clin Anesth. 2006;18:422-6.
-
31Kulka PJ, Tryba M, Zenz M. Preoperative alpha 2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Crit Care Med. 1996;24:947-52.
-
32Helbo-Hansen S, Fletcher R, Lundberg D, et al. Clonidine and the sympatico-adrenal response to coronary artery by-pass surgery. Acta Anaesthesiol Scand. 1986;30:235-42.
-
33Kulka PJ, Tryba M, Zenz M. Dose-response effects of intravenous clonidine on stress response during induction of anesthesia in coronary artery bypass graft patients. Anesth Analg. 1995;80:263-8.
-
34Flacke JW, Bloor BC, Flacke WE, et al. Reduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary bypass surgery. Anesthesiology. 1987;67:11-9.
-
35Gesek FA. Alpha2-adrenergic receptors activate phospholipase C in renal epithelial cells. Mol Pharmacol. 1996;50:407-14.
-
36O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420-32.
-
37Engelhard K, Werner C, Eberspacher E, et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyldaspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524-31.
-
38Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-42.
-
39Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159-69.
-
40Wever KE, Menting TP, Rovers M, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE. 2012;7:e32296.
-
41Venugopal V, Laing CM, Ludman A, et al. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. Am J Kidney Dis. 2010;56:1043-9.
-
42Curtis FG, Vianna PT, Viero RM. Dexmedetomidine and S(+)-ketamine in ischemia and reperfusion injury in the rat kidney. Acta Cir Bras. 2011;26:202-6.
-
43Diwan V, Kant R, Jaggi AS, et al. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 2008;315:195-201.
Publication Dates
-
Publication in this collection
Nov-Dec 2014
History
-
Received
04 July 2013 -
Accepted
02 Jan 2014