Abstracts
BACKGROUND:
Intra-articular injections of local anesthetics are commonly used to enhance post-operative analgesia following orthopedic surgery as arthroscopic surgeries. Nevertheless, recent reports of severe complications due to the use of intra-articular local anesthetic have raised concerns.
OBJECTIVES:
The study aims to assess use of vitamin C in reducing adverse effects of the most commonly employed anesthetics - ropivacaine, bupivacaine and lidocaine - on human chondrocytes.
METHODS:
The chondrocyte viability following exposure to 0.5% bupivacaine or 0.75% ropivacaine or 1.0% lidocaine and/or vitamin C at doses 125, 250 and 500 µM was determined by LIVE/DEAD assay and annexin V staining. Expression levels of caspases 3 and 9 were assessed using antibodies by Western blotting. Flow cytometry was performed to analyze the generation of reactive oxygen species.
RESULTS:
On exposure to the local anesthetics, chondrotoxicity was found in the order ropivacaine < bupivacaine < lidocaine. Vitamin C effectively improved the reduced chondrocyte viability and decreased the raised apoptosis levels following exposure to anesthesia. At higher doses, vitamin C was found efficient in reducing the generation of reactive oxygen species and as well down-regulate the expressions of caspases 3 and 9.
CONCLUSIONS:
Vitamin C was observed to effectively protect chondrocytes against the toxic insult of local anesthetics ropivacaine, bupivacaine and lidocaine.
Chondrocytes; Vitamin C; Local anesthetics; Ropivacaine; Bupivacaine; Lidocaine
JUSTIFICATIVA:
Injeções de anestésicos locais por via intra-articular são comumente usadas para melhorar a analgesia no período pós-operatório de cirurgia ortopédica como artroscopia. No entanto, relatos recentes de complicações graves devido ao uso de anestésico local por via intra-articular causou preocupações.
OBJETIVOS:
O objetivo do estudo foi avaliar o uso de vitamina C para reduzir os efeitos adversos dos anestésicos mais comumente usados (ropivocaína, bupivacaína e lidocaína) sobre condrócitos humanos.
MÉTODOS:
A viabilidade dos condrócitos após a exposição à bupivacaína a 0,5% ou ropivacaína a 0,75% ou lidocaína a 1,0% e/ou vitamina C em doses de 125, 250 e 500 µM foi determinada pelo ensaio Vivo/Morto e coloração com anexina V. Os níveis de expressão das caspases 3 e 9 foram avaliados com o uso de anticorpos pela técnica Western blotting. Citometria de fluxo foi feita para analisar a geração de espécies reativas ao oxigênio.
RESULTADOS:
Na exposição aos anestésicos locais, condrotoxicidade foi encontrada na seguinte ordem: ropivacaína < bupivacaína < lidocaína. A vitamina C efetivamente melhorou a redução da viabilidade dos condrócitos e diminuiu os níveis elevados de apoptose após a exposição à anestesia. Em doses mais altas, a vitamina C foi eficiente para reduzir a geração de espécies reativas ao oxigênio e assim regular negativamente a expressão das caspases 3 e 9.
CONCLUSÕES:
Observamos que a vitamina C foi eficaz na proteção dos condrócitos contra a agressão tóxica dos anestésicos locais ropivacaína, bupivacaína e lidocaína.
Condrócitos; Vitamina C; Anestésicos locais; Ropivacaína; Bupivacaína; Lidocaína
Introduction
Local anesthetics are commonly employed in various clinical settings for either prevention or for symptomatic pain relief. During orthopedic practice, they are administered by many routes as spinal, epidural or intra-articular for post-operative pain relief, or as a modality in the treatment in osteoarthritis. Multiple clinical studies have shown that intra-articular injections of local anesthetics have high success rates when used for post-operative analgesia.11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91.,22. Piper SL, Kramer JD, Kim HT, et al. Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39:2245-53.and33. Dragoo JL, Braun HJ, Kim HJ, et al. The in vitro chondro-toxicity of single-dose local anesthetics. Am J Sports Med. 2012;40:794-9.
Concern about the detrimental effects of local anesthetics came to note when multiple case studies demonstrated severe toxicity to cartilage and chondrolysis after local anesthetic infusions into the glenohumeral joint post arthroscopy.44. Petty DH, Jazrawi LM, Estrada LS, et al. Glenohumeral chon-drolysis after shoulder arthroscopy: case reports and review of the literature. Am J Sports Med. 2004;32:509-15., 55. Kamath R, Strichartz G, Rosenthal D. Cartilage toxicity from local anesthetics. Skeletal Radiol. 2008;37:871-3., 66. Gomoll AH, Yanke AB, Kang RW, et al. Long-term effects of bupi-vacaine on cartilage in a rabbit shoulder model. Am J Sports Med. 2009;37:72-7.,77. Solomon DJ, Navaie M, Stedje-Larsen ET, et al. Gleno-humeral chondrolysis after arthroscopy: a systematic review of potential contributors and causal pathways. Arthroscopy. 2009;25:1329-42.and88. Anderson SL, Buchko JZ, Taillon MR, et al. Chondrolysis of the glenohumeral joint after infusion of bupivacaine through an intraarticular pain pump catheter: a report of 18 cases. Arthroscopy. 2010;26:451-61. As the evidence of chondrotoxicity due to the local anesthetics has been increasing, investigations on the use of other substances to either inhibit or reduce the toxicity are being done. In orthopedic surgeries, adding to the effects of local anesthetics limited healing potential of hyaline articular cartilage is also a well-known problem.99. Hepburn Walsh P, Mulhall KJ. The chondrotoxicity of local anaesthetics: any clinical impact? Joint Bone Spine. 2011;78:438-40.
Among local anesthetics that are often used for pain relief, lidocaine and bupivacaine are members of amide group, but the duration of action of lidocaine is about one-half as that of bupivacaine.1010. McLure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiol. 2005;71:59-74. Ropivacaine is a long acting amino amide member of the pipecoloxylidide group of local anesthetics and is a promising substitute for bupivacaine for spine anesthesia.11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91. However, recent studies have presented the deleterious effects of these anesthetics on chondrocytes.1111. Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7., 1212. Baker JF, Walsh PM, Byrne DP, et al. In vitro assessment of human chondrocyte viability after treatment with local anaes-thetic, magnesium sulphate or normal saline. Knee Surg Sports Traumatol Arthrosc. 2011;19:1043-6.,1313. Park J, Sutradhar BC, Hong G, et al. Comparison of the cyto-toxic effects of bupivacaine, lidocaine, and mepivacaine in equine articular chondrocytes. Vet Anaesth Analg. 2011;38: 127-33.and1414. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chon-drocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25:707-15. Lo et al.1414. Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chon-drocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25:707-15. demonstrated that bupivacaine, ropivacaine, and lidocaine have a negative effect in a dose- and duration-dependent manner on the viability of chondrocytes.
In vitro studies have shown that lidocaine and bupivacaine can have cytotoxic effects on neurons and myocytes, with cell death occurring by both necrosis and apoptosis. 1515. Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004;29:333-40.and1616. Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxic-ity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007. In chondrocytes, exposure to these anesthetics also leads to cell death by both necrosis and apoptosis in a dose- and duration-dependent manner. 11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91.and 1717. Dragoo JL, Korotkova T, Kim HJ, et al. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38:1154-9. Fedder et al.1818. Fedder C, Beck-Schimmer B, Aguirre J, et al. In vitro exposure of human fibroblasts to local anaesthetics impairs cell growth. Clin Exp Immunol. 2010;162:280-8. reported that exposure to ropivacaine, lidocaine and bupivacaine reduced the viability of fibroblasts which is linked to generation of reactive oxygen species (ROS). Apoptosis is a highly regulated process distinct from necrosis that occurs in response to trauma, toxins, cytokines, and pathogens. 1919. Costouros JG, Dang AC, Kim HT. Inhibition of chondro-cyte apoptosis in vivo following acute osteochondral injury.Osteoarthritis Cartilage. 2003;11:756-9.and2020. Takahashi T, Yamamoto H, Ogawa Y, et al. Role of apoptosis inhibition in various chondrocyte culture systems. Int J Mol Med. 2003;11:299-303. Thus, inhibition of chondrocyte apoptosis has been described as a prospective means to reduce chondrocyte loss. 2020. Takahashi T, Yamamoto H, Ogawa Y, et al. Role of apoptosis inhibition in various chondrocyte culture systems. Int J Mol Med. 2003;11:299-303.and2121. Rao A, Johnston TR, Harris AH, et al. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anes-thetics chondrocyte apoptosis after anesthetics. Am J Sports Med. 2014;42:50-8.
Vitamins have been considered to be vital for living. Vitamin C, an exogenous water-soluble substance,2222. Gaby AR. Natural approaches to epilepsy. Altern Med Rev. 2007;12:9-24. is a cofactor in building blood vessels and takes the role of an antioxidant,2323. Gasiorowski A, Dutkiewicz J. Weight training and appropri-ate nutrient supplementation as an alternative method to pharmacological treatment in rehabilitation of post-myocardial infarction patients. Ann Agric Environ Med. 2012;19:333-8. by acting as an electron donor and reducing agent and thus preventing lipid, protein and DNA oxidation.2424. Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antiox-idant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18-35. There is growing recognition of the importance of nutritional factors in the maintenance of bone and joint health.2525. Okma-Keulen P, Hopman-Rock M. The onset of generalized osteoarthritis in older women: a qualitative approach. Arthritis Rheum. 2001;45:183-90. Bone matrix contains over 90% of protein as collagen2626. Termine JD. American Society for Bone and Mineral Research. Bone matrix proteins and the mineralization process. California: Kelseyville; 1990. and vitamin C is an essential cofactor for collagen formation and synthesis of hydroxyproline and hydroxylysine.2727. Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S-40S. Therefore, vitamin C may help in strengthening bone and prevent fractures. Experimentally induced deficiency of vitamin C in animals leads to impaired bone mass, cartilage and connective tissue.2828. Kipp DE, McElvain M, Kimmel DB, et al. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18:281-8. A 17-year follow-up study by Sahni et al.2929. Sahni S, Hannan MT, Gagnon D, et al. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture - a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int. 2009;20:1853-61. demonstrated that vitamin C supplementation decreases risk of hip fracture and osteoporosis. Thus considering the effects of vitamin C, the present study investigates if vitamin C inhibits chrondocyte loss and protects cartilage against the adverse effects of local anesthetics.
Methods
Chemicals and cells
Normal human chondrocytes were procured from Promocell, Germany and were cultured in chondrocyte growth medium (Promocell, Germany) under standard laboratory conditions (humidified atmosphere of 95% air and 5% CO2, 37 °C). Monolayer culture of chondrocytes has long been used as a method for assessing in vitro cell response to treatment. The cells at passage 5 were seeded at a density of 1033. Dragoo JL, Braun HJ, Kim HJ, et al. The in vitro chondro-toxicity of single-dose local anesthetics. Am J Sports Med. 2012;40:794-9. cells/cm2 into 96-well plates and cultured until 75-80% confluence. Upon reaching the desired confluence, the cells were exposed to local anesthetics. All chemicals used in the study were obtained from Sigma-Aldrich, MO, USA; otherwise, they are mentioned.
Anesthetic exposure
Chondrocytes were exposed for 1 h to 1 mL of local anesthetic solutions: 0.5% bupivacaine or 0.75% ropivacaine or 1.0% lidocaine,2121. Rao A, Johnston TR, Harris AH, et al. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anes-thetics chondrocyte apoptosis after anesthetics. Am J Sports Med. 2014;42:50-8.and3030. Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthet-ics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84. obtained from Sigma-Aldrich, MO, USA. All samples were treated using the same protocol. Specifically, culture medium was aspirated; 1 mL of 0.9% normal saline solution or anesthetic were added to each well; samples were incubated in 5% CO2 at 37 °C for 60 min; and the treatment solution was aspirated and fresh culture medium was added. Samples were returned to the incubator and chondrocyte viability was measured 24 h later. In separate experiments, following exposure to anesthesia, the cells were incubated in fresh medium supplemented with vitamin C (125 or 250 or 500 µM) for 24 h, following which the cells were assessed for viability.
Apoptosis analysis
Chondrocytes exposed to anesthetics and/or vitamin C were rinsed with PBS and used for cell viability analysis. To measure apoptosis, the LIVE/DEAD cell viability (LIVE/DEAD cell viability kit, Invitrogen) assay was performed. The assay determines the intracellular esterase activity and plasma membrane integrity to assess the viability of cells. Chondrocytes, treated or untreated cells with anesthesia and/or vitamin C, were stained with 5 µmol/L ethidium homodimer and 5 µmol/L calcein-AM and incubated at 37 °C for 30 min. The cells were then analyzed under a Nikon labophot-2 fluorescence microscope. Live chondrocytes retain calcein-AM, a non-fluorescent polyanionic dye, and produce a green fluorescence through enzymatic (esterase) conversion. Further, ethidium homodimer enters damaged membranes of the dead cells and binds to nucleic acids thereby yielding a red fluorescence.
Apoptosis was also detected by measuring the loss of membrane asymmetry by assessing the binding properties of annexin V. The binding property of annexin V was evaluated using annexin V staining kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Annexin V antibody was conjugated with a Fluorescein isothiocyanate (FITC) dye. Briefly, 1 × 1066. Gomoll AH, Yanke AB, Kang RW, et al. Long-term effects of bupi-vacaine on cartilage in a rabbit shoulder model. Am J Sports Med. 2009;37:72-7. cells were exposed to bupivacine or ropivacaine or lidocaine for 1 h following treatment with vitamin C, or only those exposed to anesthetics were subjected to annexin V staining. The cells were washed in PBS, resuspended in 100 µL of binding buffer containing a FITC-conjugated anti-annexin V antibody, and then analyzed with flow cytometer (FACS Calibur, BD Biosciences).
Western blotting
To further analyze as to whether caspase activation was involved in the initiation of apoptosis following exposure to local anesthetics, western blot analysis was performed to determine the expression of caspase-3 and caspase-9 using respective antibodies (Cell Signaling Technology, Danvers, MA, USA). For isolation of total cellular proteins, cells were lysed in cell lysing buffer (Cell Signaling Technology, Danvers, MA, USA) and processed according to the manufacturer's instructions. Proteins were then fractionated by SDS-PAGE, electrotransferred to nitrocellulose membranes, blotted with respective antibodies and detected by enhanced chemiluminescence (GE Healthcare). The band signals of other proteins were normalized to those of ß-actin using anti-ß-actin at 1:2000 dilution (Cell Signaling Technology, Danvers, MA, USA).
Determination of reactive oxygen species (ROS)
The generation of intracellular ROS was measured by flow cytometry using 2',7'-dichlorofluorescein diacetate (DCFH-DA) staining. DCFH-DA is a non-fluorescent compound that can be enzymatically converted to DCF, a highly fluorescent compound, in the presence of ROS. Cells following exposure to anesthetic and/or vitamin C were further incubated with DCFH-DA (10 µM) for 30 min at 37 °C in dark. The cells were then washed twice with PBS and intensity of fluorescence was measured by flow cytometry.3131. Lu HF, Sue CC, Yu CS, et al. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol. 2004;42:1543-52.
Statistical analysis
The results are represented as mean ± SD. Values presenting p < 0.05 are considered significant as determined by one way analysis of variance (ANOVA). The analyses were performed using version 17.0 SPPSS package.
Results
Vitamin C improves viability of chondrocytes following anesthetic exposure
Chondrocyte viability in response to anesthetic exposure was analyzed by LIVE/DEAD assay. After exposure of chondrocyte cultures to local anesthetics for 24 h, chondrotoxicity observed with 1% lidocaine was almost twice greater than 0.75% ropivacaine (Fig. 1). There was a marked decrease in cell viability percentage after exposure to lidocaine, ropivacaine or bupivacaine. Comparing equipotent concentrations of ropivacaine (0.75%) and bupivacaine (0.5%), the cell viability rates were significantly higher 24 h after treatment with 0.75% ropivacaine than with 0.5% bupivacaine. Chondrotoxicity was more pronounced on exposure to lidocaine followed by bupivacaine. Exposure to vitamin C following anesthesia resulted in a significant (p < 0.05) improvement in cell viability percentage. The viable chondrocyte counts increased with increasing concentrations of vitamin C. The 500 µM concentration caused a marked raise in chondrocyte viability when compared to lower doses (125 and 250 µM).
Influence of vitamin C on the cell viability of chondrocytes. Values are represented as mean ± SD; n = 6. *Represents p < 0.05 compared with control as determined by one way ANOVA.
Apoptosis of the chondrocytes following anesthesia exposure was also detected by measuring the loss of membrane asymmetry by annexin V staining. The apoptotic cell counts were markedly higher (p < 0.05) following anesthesia exposure. Ropivacaine at 0.75% caused considerably lower apoptosis as compared to 1% lidocaine and 0.5% bupivacaine. Incubation with vitamin C resulted in raised viability percentage with decreased apoptotic cell counts. Vitamin C at 500 µM concentration was more potent than lower doses in reducing apoptosis after exposure to local anesthetics (Fig. 2).
Chondrotoxicity induced by anesthetic exposure: (a) 0.5% bupivacaine; (e) 1% lidocaine; (i) 0.75% ropivacaine. Vitamin C on effect of anesthetics: (b) 0.5% bupivacaine + 125 µM vitamin C; (c) 0.5% bupivacaine + 250 µM vitamin C; (d) 0.5% bupivacaine + 500 µM vitamin C; (f) 1% lidocaine + 125 µM vitamin C; (g) 1% lidocaine + 250 µM vitamin C; (h) 1% lidocaine + 500 µM vitamin C; (j) 0.75% ropivacaine + 125 µM vitamin C; (k) 0.75% ropivacaine + 250 µM vitamin C; (l) 0.75% ropivacaine + 500 µM vitamin C. Apoptotic cell counts. Values are represented as mean ± SD; n = 6. *Represents p < 0.05 compared with control as determined by one way ANOVA.
Vitamin C suppresses apoptosis by down-regulating expression of caspase-3 and caspase-9
To evaluate the possible involvement of caspase activation in anesthesia induced chondrotoxicity, expression of caspase-3 and caspase-9 was determined 24 h following exposure to anesthesia. One hour exposure to anesthetics caused up-regulation of caspase-3 and caspase-9 in the order, lidocaine > bupivacaine > ropivacaine (Fig. 3). Thought ropivacaine at 0.75% caused raised expressions of caspases, it was found to be a non-significant raise as compared to control chondrocytes not exposed to anesthetics. Nevertheless, 1% lidocaine resulted in marked enhancement in the level of expressions indicating caspase cascade activation in apoptosis. Incubation with vitamin C for 24 h markedly reduced the expression of caspases in a dose-dependent manner that was in line with the results of the LIVE/DEAD assay and annexin V staining. Vitamin C at 250 and 500 µM were more effective in down-regulating the expressions of caspases 3 and 9.
Effect of vitamin C on caspase-3 (a and b) and caspase-9 (c and d) expressions in chondrocytes following anesthetic exposure. L1, control; L2, anesthetic alone; L3, anesthesia + 125 µM vitamin C; L4, anesthesia + 250 µM vitamin C; L5, anesthesia + 500 µM vitamin C. (b and d) Values are represented as mean ± SD; n = 6 (b and d). *Represents p < 0.05 compared with control as determined by one way ANOVA.
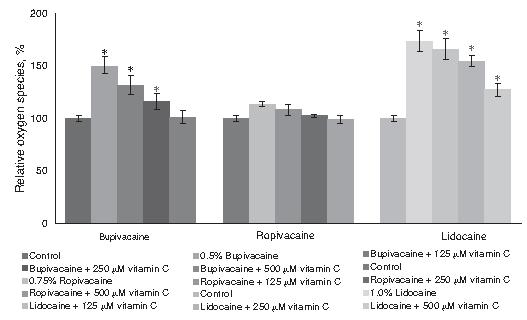
Influence of vitamin C on ROS generation
We determined the levels of ROS by flow cytometry using 2',7'-dichlorofluorescein diacetate (DCFH-DA) staining. Lidocaine, bupivacaine and ropivacaine caused a marked increase in ROS production. Lidocaine caused twice as much raised ROS generation as compared to ropivacaine and almost nearly 10% more than bupivacaine. This raise however was suppressed in chondrocytes incubated with vitamin C as against cells without vitamin C. Treatment with vitamin C significantly reduced ROS production in the order 125 < 250 < 500 µM (Fig. 4). The antioxidant capacity of vitamin C could have been responsible for the marked suppression of ROS.
Influence of vitamin C on ROS generation by flow cytometry. Values are represented as mean ± SD; n = 6. *Represents p < 0.05 compared with control as determined by one way ANOVA.
Discussion
Intra-articular injections of local anesthetics are frequently employed in peri-operative and ambulatory settings.3232. Koyonos L, Yanke AB, McNickle AG, et al. A randomized, prospective, double-blind study to investigate the effective-ness of adding DepoMedrol to a local anesthetic injection in postmeniscectomy patients with osteoarthritis of the knee. Am J Sports Med. 2009;37:1077-82. Intra-articular injections of local anesthetics enhance post-operative analgesia.11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91.,22. Piper SL, Kramer JD, Kim HT, et al. Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39:2245-53.and3333. Dragoo JL, Korotkova T, Kanwar R, et al. The effect of local anesthetics administered via pain pump on chondrocyte viabil-ity. Am J Sports Med. 2008;36:1484-8. Lidocaine, bupivacaine and ropivacaine are amide-type local anesthetics. Recent publications have suggested potential adverse effects of these three local anesthetics on articular chondrocytes in vitro.11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91.and1111. Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7.
Several studies have described the detrimental effects of local anesthetics on chondrocyte viability. Chu et al.3434. Chu CR, Izzo NJ, Papas NE, et al. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693-9. demonstrated severe toxicity of bupivacaine. The study evidenced on exposure to 0.5% bupivacaine and >99% of the bovine chondrocytes were found to be killed in all exposed cultures. Gomoll et al.3535. Gomoll AH, Kang RW, Williams JM, et al. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimen-tal model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813-9. reported marked histopathologic and metabolic changes in rabbit shoulders with continuous infusion of 0.25% bupivacaine with and without epinephrine. Lidocaine at 1% and 2% doses was reported to exhibit chondrocyte toxicity.1111. Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7. Further, chondrocyte toxicity was reported by Piper and Kim11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91. following exposure of human cartilage explants and chondrocytes to local anesthetics, bupivacaine and ropivacaine. In line with the previous reports, in our study, 0.5% bupivacaine, 0.75% ropivacaine and 1% lidocaine induced apoptosis and reduced the viability of chondrocytes. Lidocaine was observed to be potentially more toxic that bupivacaine and ropivacaine. Similar to earlier studies, ropivacaine was found to be less chondrotoxic than bupivacaine in human chondrocytes.11. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91.and3636. Farkas B, Kvell K, Czompoly T, et al. Increased chondrocyte death after steroid and local anesthetic combination. Clin Orthop Relat Res. 2010;468:3112-20. Vitamin C significantly improved cell viability of the chondrocytes. Studies have demonstrated the positive effect of vitamin C on bone health.2929. Sahni S, Hannan MT, Gagnon D, et al. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture - a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int. 2009;20:1853-61.
Apoptosis can be induced through intrinsic and extrinsic pathways, both involving the activation of cellular caspases. Caspases are key enzymes involved in regulating the highly specific proteolytic cleavage of cellular proteins leading to cell death. Evidence suggests that caspase-3 may also be involved in DNA fragmentation.2121. Rao A, Johnston TR, Harris AH, et al. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anes-thetics chondrocyte apoptosis after anesthetics. Am J Sports Med. 2014;42:50-8. In our study, local anesthetics - lidocaine, ropivacaine and bupivacaine were observed to induce caspase expression levels which is indicative of induction of apoptosis in the chondrocytes via caspase activation. Perez-Castro et al. 1616. Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxic-ity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007. demonstrated activation of caspase-3/-7 in human neuroblastoma cells following incubation with lidocaine, ropivacaine and bupivacaine. Nevertheless, vitamin C suppressed the expression of caspases 3 and 9, markedly suggesting its anti-apoptotic efficacy.
The mechanisms that lead to the chondrotoxicity of local anesthetics are not completely understood. To analyze the involvement of ROS-mediated apoptosis, ROS generation in the chondrocytes was assessed. Marked raise in ROS generation following exposure to anesthetics was considerably normalized to almost normal levels on incubation of chondrocytes with various concentrations of vitamin C. This could be due to the potent antioxidant property of vitamin C. Vitamin C would have effectively prevented generation of ROS and/or neutralized ROS. However, we observed a striking correlation between cell viability, caspase expression and the levels of ROS production. Park et al.3737. Park CJ, Park SA, Yoon TG, et al. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84:852-7. have shown increased ROS concentration correlating with cell death of Schwann cells after incubation with bupivacaine. In our study, ROS concentrations increased upon exposure to lidocaine and bupivacaine, but non-significant levels were observed following ropivacaine exposure. Similar raise in ROS generation following exposure to local anesthetics was reported by Grishko et al.3838. Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lido-caine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18.
To summarize, we have shown that 0.75% ropivacaine, though toxic to human chondrocyte cells following 60 min exposure, is considerably on the lower toxic range as against 0.5% bupivacaine and 1% lidocaine. Lidocaine at 1% concentration presents more chondrotoxicity among the anesthetics studied. Enhanced ROS production evidenced in our study suggests that chondrotoxicity could be possibly due to ROS and ROS-mediated apoptosis. However, incubation with vitamin C significantly offered chondrocyte protection, which we could possibly be attributed to its antioxidant capacity.
Conclusion
Vitamin C was able to markedly protect the condrocytes against the toxic effects of local anesthetics - ropivacaine, bupivacaine and lidocaine.
References
-
1Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986-91.
-
2Piper SL, Kramer JD, Kim HT, et al. Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39:2245-53.
-
3Dragoo JL, Braun HJ, Kim HJ, et al. The in vitro chondro-toxicity of single-dose local anesthetics. Am J Sports Med. 2012;40:794-9.
-
4Petty DH, Jazrawi LM, Estrada LS, et al. Glenohumeral chon-drolysis after shoulder arthroscopy: case reports and review of the literature. Am J Sports Med. 2004;32:509-15.
-
5Kamath R, Strichartz G, Rosenthal D. Cartilage toxicity from local anesthetics. Skeletal Radiol. 2008;37:871-3.
-
6Gomoll AH, Yanke AB, Kang RW, et al. Long-term effects of bupi-vacaine on cartilage in a rabbit shoulder model. Am J Sports Med. 2009;37:72-7.
-
7Solomon DJ, Navaie M, Stedje-Larsen ET, et al. Gleno-humeral chondrolysis after arthroscopy: a systematic review of potential contributors and causal pathways. Arthroscopy. 2009;25:1329-42.
-
8Anderson SL, Buchko JZ, Taillon MR, et al. Chondrolysis of the glenohumeral joint after infusion of bupivacaine through an intraarticular pain pump catheter: a report of 18 cases. Arthroscopy. 2010;26:451-61.
-
9Hepburn Walsh P, Mulhall KJ. The chondrotoxicity of local anaesthetics: any clinical impact? Joint Bone Spine. 2011;78:438-40.
-
10McLure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiol. 2005;71:59-74.
-
11Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7.
-
12Baker JF, Walsh PM, Byrne DP, et al. In vitro assessment of human chondrocyte viability after treatment with local anaes-thetic, magnesium sulphate or normal saline. Knee Surg Sports Traumatol Arthrosc. 2011;19:1043-6.
-
13Park J, Sutradhar BC, Hong G, et al. Comparison of the cyto-toxic effects of bupivacaine, lidocaine, and mepivacaine in equine articular chondrocytes. Vet Anaesth Analg. 2011;38: 127-33.
-
14Lo IK, Sciore P, Chung M, et al. Local anesthetics induce chon-drocyte death in bovine articular cartilage disks in a dose- and duration-dependent manner. Arthroscopy. 2009;25:707-15.
-
15Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004;29:333-40.
-
16Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxic-ity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007.
-
17Dragoo JL, Korotkova T, Kim HJ, et al. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38:1154-9.
-
18Fedder C, Beck-Schimmer B, Aguirre J, et al. In vitro exposure of human fibroblasts to local anaesthetics impairs cell growth. Clin Exp Immunol. 2010;162:280-8.
-
19Costouros JG, Dang AC, Kim HT. Inhibition of chondro-cyte apoptosis in vivo following acute osteochondral injury.Osteoarthritis Cartilage. 2003;11:756-9.
-
20Takahashi T, Yamamoto H, Ogawa Y, et al. Role of apoptosis inhibition in various chondrocyte culture systems. Int J Mol Med. 2003;11:299-303.
-
21Rao A, Johnston TR, Harris AH, et al. Inhibition of chondrocyte and synovial cell death after exposure to commonly used anes-thetics chondrocyte apoptosis after anesthetics. Am J Sports Med. 2014;42:50-8.
-
22Gaby AR. Natural approaches to epilepsy. Altern Med Rev. 2007;12:9-24.
-
23Gasiorowski A, Dutkiewicz J. Weight training and appropri-ate nutrient supplementation as an alternative method to pharmacological treatment in rehabilitation of post-myocardial infarction patients. Ann Agric Environ Med. 2012;19:333-8.
-
24Padayatty SJ, Katz A, Wang Y, et al. Vitamin C as an antiox-idant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18-35.
-
25Okma-Keulen P, Hopman-Rock M. The onset of generalized osteoarthritis in older women: a qualitative approach. Arthritis Rheum. 2001;45:183-90.
-
26Termine JD. American Society for Bone and Mineral Research. Bone matrix proteins and the mineralization process. California: Kelseyville; 1990.
-
27Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S-40S.
-
28Kipp DE, McElvain M, Kimmel DB, et al. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18:281-8.
-
29Sahni S, Hannan MT, Gagnon D, et al. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture - a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int. 2009;20:1853-61.
-
30Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthet-ics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84.
-
31Lu HF, Sue CC, Yu CS, et al. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem Toxicol. 2004;42:1543-52.
-
32Koyonos L, Yanke AB, McNickle AG, et al. A randomized, prospective, double-blind study to investigate the effective-ness of adding DepoMedrol to a local anesthetic injection in postmeniscectomy patients with osteoarthritis of the knee. Am J Sports Med. 2009;37:1077-82.
-
33Dragoo JL, Korotkova T, Kanwar R, et al. The effect of local anesthetics administered via pain pump on chondrocyte viabil-ity. Am J Sports Med. 2008;36:1484-8.
-
34Chu CR, Izzo NJ, Papas NE, et al. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693-9.
-
35Gomoll AH, Kang RW, Williams JM, et al. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimen-tal model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813-9.
-
36Farkas B, Kvell K, Czompoly T, et al. Increased chondrocyte death after steroid and local anesthetic combination. Clin Orthop Relat Res. 2010;468:3112-20.
-
37Park CJ, Park SA, Yoon TG, et al. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84:852-7.
-
38Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lido-caine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18.
Publication Dates
-
Publication in this collection
Jan-Feb 2016
History
-
Received
16 Jan 2015 -
Accepted
26 Jan 2015