Abstract
Background:
Local anesthetics (LAs) are generally considered as safe, but cytotoxicity has been reported for several local anesthetics used in humans, which is not well investigated. In the present study, the cytotoxicity of lidocaine, ropivacaine and the combination of lidocaine and ropivacaine were evaluated on human melanoma cell lines. Melphalan, a nitrogen mustard alkylating agent, was used as a control agent for comparison of cytotoxic activity.
Methods:
Melanoma cell lines, A375 and Hs294T, were exposed to 1 h to different concentrations of above agents. Cell-viability after exposure was determined by flow cytometry.
Results:
Investigated LAs showed detrimental cytotoxicity on studied melanoma cell lines in time- (p < 0.001), concentration- (p < 0.001), and agent dependant. In both A375 and Hs294T cell lines, minimum cell viability rates were found after 72 h of exposure to these agents. Lidocaine 2% caused a reduction of vital cells to 10% ± 2% and 14% ± 2% in A375 and Hs294T, respectively after 72 h of exposure. Ropivacaine 0.75% after 72 h reduced viable cells to 15% ± 3% and 25% ± 3% in A375 and Hs294T, respectively. Minimum cell viability after 72 h exposure to the combination was 10% ± 2% and 18% ± 2% in A375 and Hs294T, respectively. Minimum cell viability after 72 h exposure to melphalan was 8% ± 1% and 12% ± 2%, in A375 and Hs294T, respectively.
Conclusion:
LAs have cytotoxic activity on human melanoma cell lines in a time-, concentration- and agent-dependant manner. Apoptosis in the cell lines was mediated through activity of caspases-3 and caspases-8.
KEYWORDS
Lidocaine; Ropivacaine; Cytotoxicity; Aminoamide local anesthetics; Melanoma cell lines; Flow cytometry
Resumo
Justificativa:
Os anestésicos locais (ALs) são geralmente considerados como seguros, mas citotoxicidade foi relatada em vários anestésicos locais usados em seres humanos, a qual não é bem investigada. No presente estudo, a citotoxicidade de lidocaína e ropivacaína e da combinação de lidocaína e ropivacaína foi avaliada em linhagens celulares de melanoma humano. Melfalano, um agente alquilante de mostarda nitrogenada, foi usado como um agente de controle para a comparação da atividade citotóxica.
Métodos:
Linhagens celulares de melanoma, A375 e Hs294T foram expostas por uma hora a concentrações diferentes dos agentes mencionados acima. A viabilidade celular após a exposição foi determinada por citometria de fluxo.
Resultados:
Os ALs investigados mostraram citotoxicidade prejudicial nas linhagens celulares de melanoma estudadas dependente do tempo (p < 0,001), da concentração (p < 0,001) e do agente. Em ambas as linhagens de células A375 e Hs294T, níveis mínimos de viabilidade celular foram encontrados após 72 horas de exposição a esses agentes. Lidocaína a 2% provocou uma redução das células vitais para 10% ± 2% e 14% ± 2% em A375 e Hs294T, respectivamente, após 72 horas de exposição. Ropivacaína a 0,75% após 72 horas reduziu as células viáveis para 15% ± 3% e 25% ± 3%, em A375 e Hs294T, respectivamente. A viabilidade celular mínima após exposição de 72 horas para a combinação foi de 10% ± 2% e 18% ± 2% em A375 e Hs294T, respectivamente. A viabilidade celular mínima após exposição de 72 horas ao melfalano foi de 8% ± 1% e 12 ± 2, em A375 e Hs294T, respectivamente.
Conclusão:
Os ALs têm atividade citotóxica em linhagens de celulares de melanoma humano de modo dependente do tempo, da concentração e do agente. A apoptose nas linhagens celulares foi mediada por meio da atividade das caspases-3 e caspases-8.
PALAVRAS-CHAVE
Lidocaína; Ropivacaína; Citotoxicidade; Anestésicos locais do grupo amino-amida; Linhagens celulares de melanoma; Citometria de fluxo
Introduction
A little is known about chemotherapy and anesthetics in general. In perioperative and ambulatory settings local anesthetics are administered as intra-articular injections.11 Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84. Aminoamide local anesthetics exhibit their activity mainly by blocking the impulse conduction of nerve axons in a reversible manner. Lidocaine and ropivacaine both belong to amino amide class of local anesthetics with the former having antiarrhythic activity (class-1b). In general, local anesthetics (LAs) prevent or relieve pain by binding to specific receptor sites on the sodium (Na+) channels in nerves and blocking the movement of ions through these pores. Both the chemical and pharmacologic properties of individual local anesthetic drugs determine their clinical properties.22 Kobayashi K, Ohno S, Uchida S, et al. Cytotoxicity and type of cell death induced by local anesthetics in human oral normal and tumor cells. Anticancer Res. 2012;32:2925-33. Local anesthetics including lidocaine applied topically have been shown to produce good pain control in patients with oral or rectal cancer. Lidocaine and ropivacaine are used at a concentration of 1.5% or 2.0% and 0.5% or 0.75%, respectively, for surgical anaesthesia with reduced cardiotoxicty and CNS toxicity.11 Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84.,33 Sung CM, Hah YS, Kim JS, et al. Cytotoxic effects of ropivacaine, bupivacaine, and lidocaine on rotator cuff tenofibroblasts. Am J Sports Med. 2014;42:2888-96.,44 Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18. These LAs are widely used for pain control in patients with head and neck cancer, to inhibit the metastasis and relapse of tumours and to reduce surgical stress induced inhibition of natural killer (NK) cell activity. However, both in vitro and in vivo studies have shown cytotoxicity towards several cultured cells.55 Dregalla RC, Lyons NF, Reischling PD, et al. Amide-type local anesthetics and human mesenchymal stem cells: clinical implications for stem cell therapy. Stem Cells Transl Med. 2014;3:365-74.,66 Fedder C, Beck-Schimmer B, Aguirre J, et al. In vitro exposure of human fibroblasts to local anesthetics impairs cell growth. Clin Exp Immunol. 2010;162:280-8. Previous publications have reported that single dose injection of 1% lidocaine may have significant chondrotoxic activity. Cytotoxicity of lidocaine on mesenchymal stem cells, human oral and tumour cells, corneal endothelial cells and rotor cuff tenofibroblasts has been reported previously.77 Sakaguchi M, Kuroda Y, Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth Analg. 2006;102:1103-7.
8 Kim M, Lee YS, Mathews HL, et al. Induction of apoptotic cell death in a neuroblastoma cell line by dibucaine. Exp Cell Res. 1997;231:235-41.
9 Unami A, Shinohara Y, Ichikawa T, et al. Biochemical and microarray analyses of bupivacaine-induced apoptosis. J Toxicol Sci. 2003;28:77-94.
10 Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007.
11 Lee HT, Xu H, Siegel CD, et al. Local anesthetics induce human renal cell apoptosis. Am J Nephrol. 2003;23:129-39.
12 Nakamura K, Kido H, Morimoto Y, et al. Prilocaine induces apoptosis in osteoblastic cells. Can J Anaesth. 1999;46:476-82.-1313 Shen Q, Tian F, Jiang P, et al. EGCG enhances TRAIL-mediated apoptosis in human melanoma A375 cell line. J Huazhong Univ Sci Technol Med Sci. 2009;29:771-5. Ropivacaine, a long-acting aminoamide local anesthetic, inhibits influx of sodium ions reversibly and thereby blocks impulse conduction in nerve fibres. Ropivacaine has been reported to demonstrate reduced potential for CNS and cardiotoxicity and is used more frequently for local anesthesia and in the management of labour pain and postoperative pain. In addition to local anesthetic activity, ropivacaine has also been reported to inhibit platelet aggregation and antibacterial activity in in vitro studies. Ropivacaine was also reported to be cytotoxicity on mesenchymal cell lines at a concentration of 0.5%. Neurotoxicity of local anesthetics was associated with their apoptosis.1414 Ross BK, Coda B, Heath CH. Local anesthetic distribution in a spinal model: a possible mechanism of neurologic injury after continuous spinal anesthesia. Reg Anesth. 1992;17:69-77.
15 Arai T, Hoka S. Neurotoxicity of intrathecal local anesthetics. J Anesth. 2007;21:540-1.
16 Kishimoto T, Bollen AW, Drasner K. Comparative spinal neurotoxicity of prilocaine and lidocaine. Anesthesiology. 2002;97:1250-3.-1717 Kamiya Y, Ohta K, Kaneko Y. Lidocaine-induced apoptosis and necrosis in U937 cells depending on its dosage. Biomed Res. 2005;26:231-9.,1010 Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007.,1818 Boselli E, Duflo F, Debon R, et al. The induction of apoptosis by local anesthetics: a comparison between lidocaine and ropivacaine. Anesth Analg. 2003;96:755-6. Despite the long use of aminoamide local anesthetics in several complications, there is inadequate information about cytotoxic activity of these agents and this need to be studied in detail. The cytotoxic activity of these commonly used LAs on melanoma has not yet been studied extensively. We hypothesized that these regularly used amino amide LAs have cytotoxic effects on human melanoma, the most deadly form of skin cancer, in a dose-, time-, and agent-specific manner. In this study, besides evaluating different concentrations of the above stated local anesthetics individually we have also investigated the combination of both the compounds to evaluate cytotoxic potential. The primary purpose of the study was to investigate whether these commonly used LAs have cytotoxic effects on the melanoma cell lines. In this study, effect of pH of local anesthetics on cell death of melanoma cell lines was also studied. Caspase activity test was performed to confirm whether the cell death was caused by apoptosis. Melphalan, a nitrogen mustard alkylating agent, is a chemotherapy drug that acts by alkylating DNA nucleotide guanine. Melphalan was reported to be cytotoxic on several cell lines including melanoma and therefore is used as a standard agent to evaluate cytotoxic potential and used as comparator.1919 Bauer TW, Gutierrez M, Dudrick DJ, et al. A human melanoma xenograft in a nude rat responds to isolated limb perfusion with TNF plus melphalan. Surgery. 2003;133:420-8.,2020 Hansson J, Berhane K, Castro VM, et al. Sensitization of human melanoma cells to the cytotoxic effect of melphalan by the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991;51:94-8.
Materials and methods
Materials
Lidocaine, ropivacaine, and melphalan (137-58-6; 98717-15-8 and 148-82-3, respectively) were purchased from Sigma-Aldrich, Germany. All these drugs and reagents were preservative free and dissolved in buffered saline solution (pH 7.0). 3-[4,5-Dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chem. Ind., St. Louis, MO, USA.
Cell culture
Human malignant melanoma cell line A375 was purchased from Institute of Cell Biology, Shanghai Institute for Biological Science, Chinese Academy of Science, China. The cell line was cultured in Dulbecco's Modified Eagle's Medium (DMEM) culture medium (Life Technologies, Carlsbad, CA, USA). The cell line was supplemented with 4 mM glutamine, the dipeptide l-alanyl-l-glutamine that prevents degradation and ammonia build up in both adherent and suspension cultures, and 5% fetal bovine serum (Life Technologies). Hs294T, human melanoma cell line (Institute of Cell Biology, Shanghai Institute for Biological Science, Chinese Academy of Science, China) was cultured in DMEM culture medium (Lonza, Basel, Switzerland) supplemented with 2 mM glutamine (Life Technologies) and 5% fetal bovine serum (Life Technologies). The normal human dermal fibroblast (NHDF; Lonza) cell line was cultured in MEMα culture medium (Lonza) supplemented with 2 mM glutamine (Life Technologies) and 10% fetal bovine serum (Life Technologies). Cells were grown to confluence and transferred to 48 well Plates 48 h before experimental treatment.
All the media contained 0.1 mg/mL streptomycin, 100 U/mL penicillin, and 0.25 µg/mL amphotericin B (Lonza). The cells were cultured at 37 °C in a humid atmosphere saturated with 5% CO2 at 95% relative humidity.
Analysis of cell apoptosis by flow cytometry
Lidocaine and ropivacaine form the group of aminoamide local anesthetics but differ in their analgesic potency, onset of action and duration of anaesthesia. Melanoma cells were exposed for 1 h to 1 mL of local anesthetics solutions with following concentrations: 0.03125%, 0.0625%, 0.125%, 0.25%, 0.5%, 1.0% and 2% of lidocaine, and 0.03125%, 0.0625%, 0.125%, 0.25%, 0.5%, and 0.75% of ropivacaine, melphalan (0.2%) and 0.03125%, 0.0625%, 0.125%, 0.25%, 0.5%, and 0.75% of lidocaine and ropivacaine combination. The concentration of test compounds ranged from 0.03125% to 2.0%. Control cells were treated with normal saline solution for 1 h. The treated solutions containing nonadherant melanoma cells were removed and centrifuged. Formed cell pellets were washed in buffered saline and returned to the respective wells with culture medium. After 24 and 72 h, cell viability was determined using flow cytometry. To determine the influence of pH on human melanoma cell lines, viability rates were analysed 24 and 72 h after a 1-h exposure to saline solution with pH of 7.4, 7.0, 6.0, 5.0, and 4.0. Following general procedure was used for analysis of melanoma cell viability: A375 cells (1 × 106 mL−1) were seeded into culture plates and were allowed to adhere and harvested after treatment with lidocaine ropivacaine and combination of lidocaine and ropivacaine for 24 h. Controls included unstained cells and untreated cells (i.e. not vehicle-treated). All processing was done on ice. At the individual time points culture medium was collected, pooled with the suspended cells and centrifuged for 5 min at 1200 rpm to make sure that all the cellular material was collected. The cell pellets were suspended in 500 µL 1× binding buffer at a density of approximately 1 × 106 mL−1. Samples were incubated with 1 µL Annexin V-FITC staining and 5 µL PI for exactly 5 min at room temperature in the dark and then samples were transferred to 5 mL FACS tubes and then measurement was carried out on a FACSCalibur cytometer (Becton Dickinson, USA). PI and V-FITC fluorescence were detected in the FL-1 (green) and FL-2 (red) channels, respectively, after correction to the spectral overlap between the two channels. CellQuest software (Becton Dickinson, Country) was employed to analyse the data.11 Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84.
Assay for caspase-3/8 activity
Caspase-3/8 activity was analysed at 12, 24 and 72 h after exposure to the local anesthetics using colorimetric assay kits (Keygen Co., China). Cells (1 × 106 well−1) were seeded into 6-well plates, and treated with different concentrations of lidocaine, ropivacaine or mixture of lidocaine and ropivacaine and melphalan for 12, 24 and 72 h. Caspase-3 and caspase-8 hydrolyze peptide substrate Ac-DEVD-PNA and Ac-IETD-PNA, respectively, which leads to the release of a p-nitroanilide (pNA) moiety. pNA concentration calculated according to the absorbency measured at a wavelength of 405 nm and calibration curve based on standard pNA solutions. All values obtained were expressed as pNA/mg of total protein. Further, all values obtained were normalized to the cell viability of control.1313 Shen Q, Tian F, Jiang P, et al. EGCG enhances TRAIL-mediated apoptosis in human melanoma A375 cell line. J Huazhong Univ Sci Technol Med Sci. 2009;29:771-5.
Statistical analysis
Each experiment was performed in triplicate and representative data were reported. One-way ANOVA was employed to measure mean values of statistical comparisons. Correlation of the variables was assessed by using bivariate correlation analysis. Differences with p < 0.05 were considered statistically significant and all p-values were determined by two-sided tests. The statistical analysis was performed by using SPSS 12.0 software.
Results
Analysis of cell viability using flow cytometry
In this study, lidocaine, ropivacaine and combination of lidocaine and ropivacaine demonstrated a concentration-, time- and agent-dependant cytotoxicity to human melanoma cells (A375, Hs294T) and the results obtained were comparable to melphalan, a nitrogen mustard alkylating agent, used as a standard agent for comparison (Figs. 1-6).
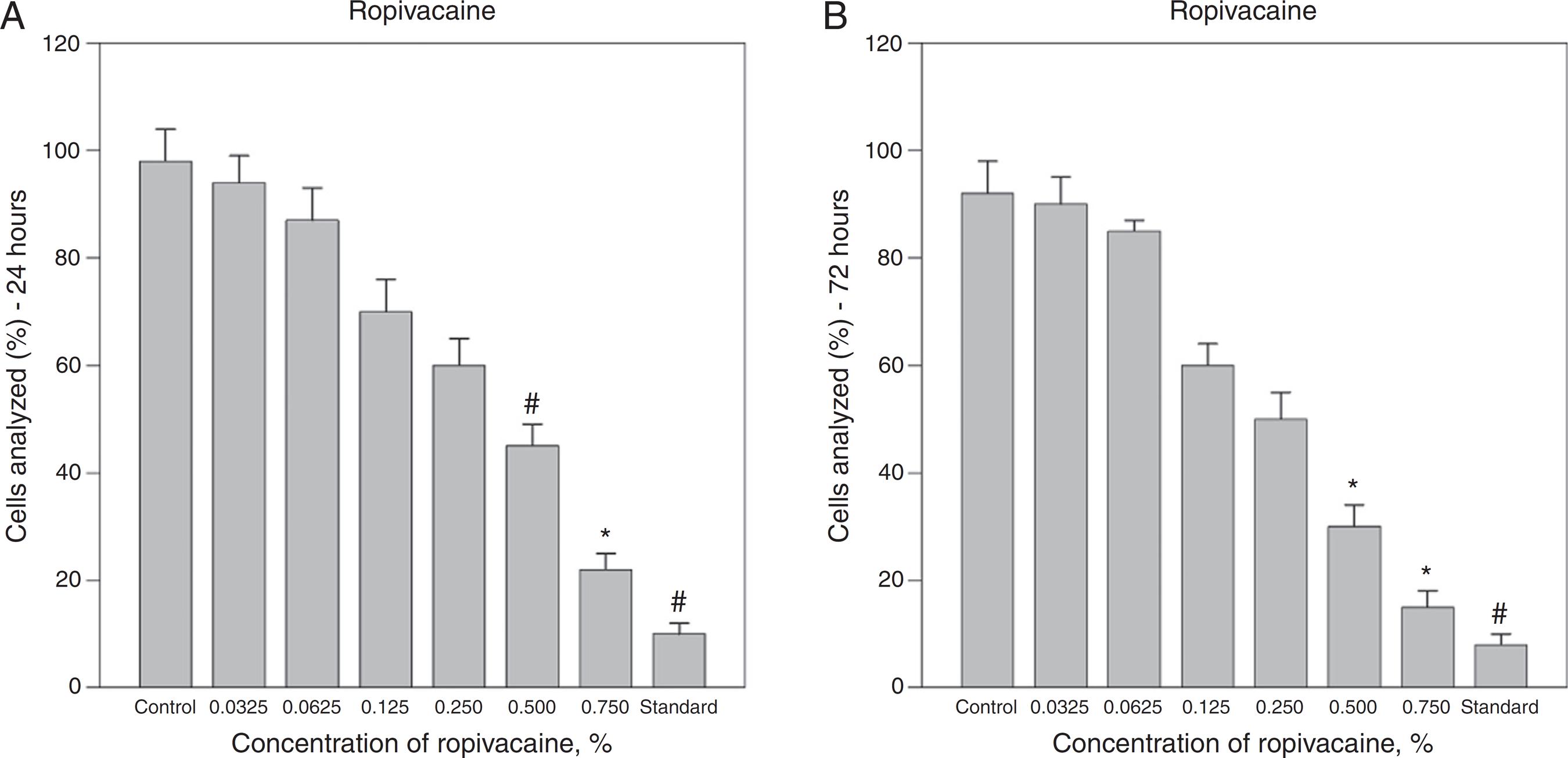
Effect of ropivacaine on A375 melanoma cell viability. A dose–response curve of cytotoxicity of ropivacaine was measured (A) 24 h and (B) 72 h after a 1 h exposure using flow cytometry. (A and B) Ropivacaine concentrations of 0.50% and 0.75% caused significant decreases in cell viability compared with control (saline solution) and were comparable with standard agent (melphalan 0.5%) after 24 and 72 h. The bars represent the mean values of experiments with standard error as error bars (*p < 0.05; # p < 0.01).
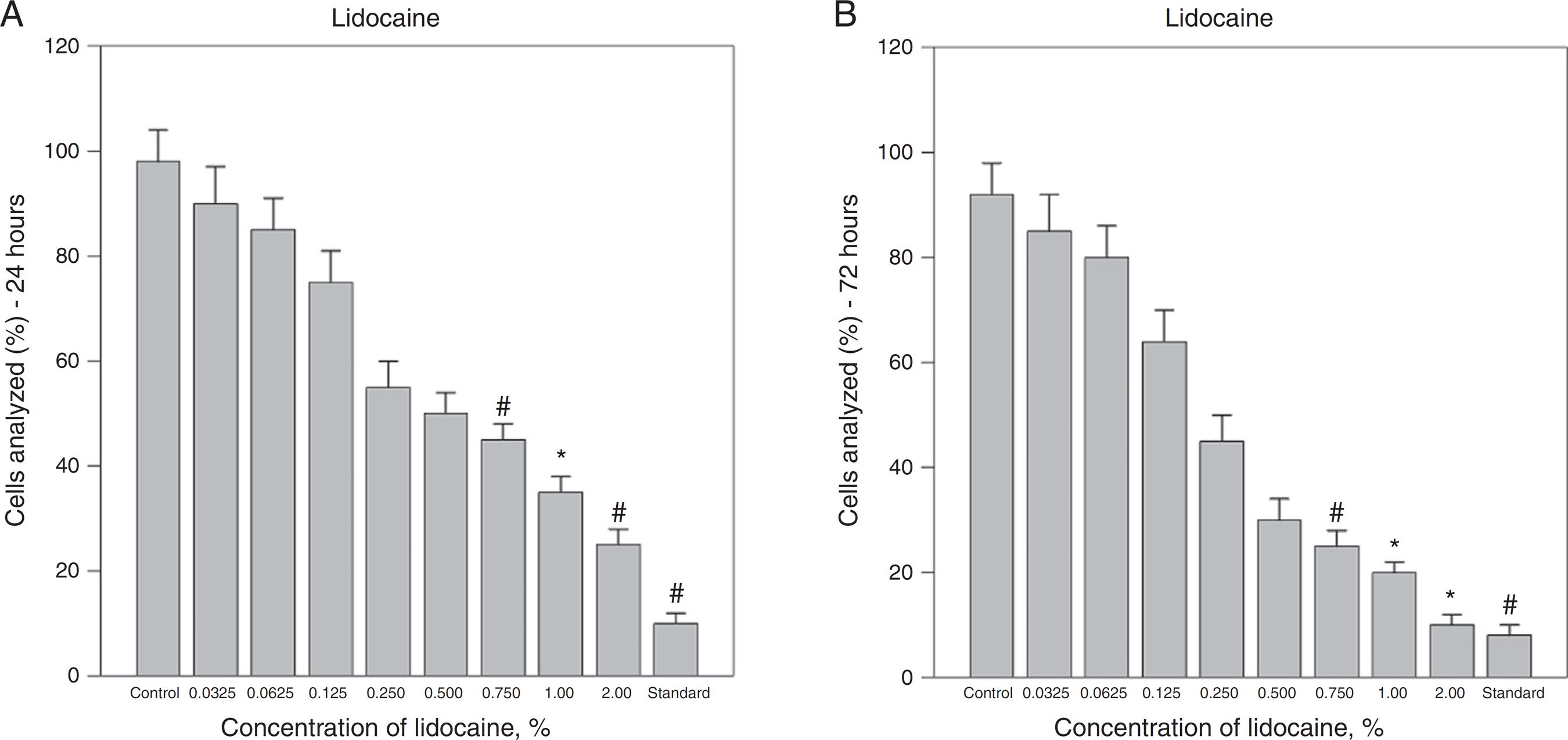
Effect of lidocaine on A375 melanoma cell viability. Lidocaine concentrations of 0.75% and higher caused significant decreases in cell viability compared with control (saline solution) and were cytotoxicity was comparable with standard agent (melphalan 0.5%) after 24 and 72 h. The bars represent the mean values of experiments with standard error as error bars (*p < 0.05; # p < 0.01; control, saline solution; standard, melphalan 0.5%).
Effect of combination of lidocaine and ropivacaine on A375 melanoma cell viability. Viability rates were significantly lowered by combination of lidocaine and ropivacaine concentrations greater than 0.25% and above 24 and 72 h after treatment. The bars represent the mean values of experiments with standard error as error bars (*p < 0.05; # p < 0.01; control, saline solution; standard, melphalan 0.5%).
Effects of ropivacaine on Hs294T melanoma cell viability. A dose–response curve of cytotoxicity of ropivacaine was measured 24 h (A) and 72 h (B) after a 1-h exposure using flow cytometry. Ropivacaine concentrations of 0.50% and 0.75% caused significant decreases in cell viability compared with controls (saline solution) and were comparable with standard (melphalan 0.5%) after 24 and 96 h. Viability assessments showed no relevant differences for lower concentrations. The bars represent the mean values of experiments with standard error as error bars (*p < 0.05; # p < 0.01).
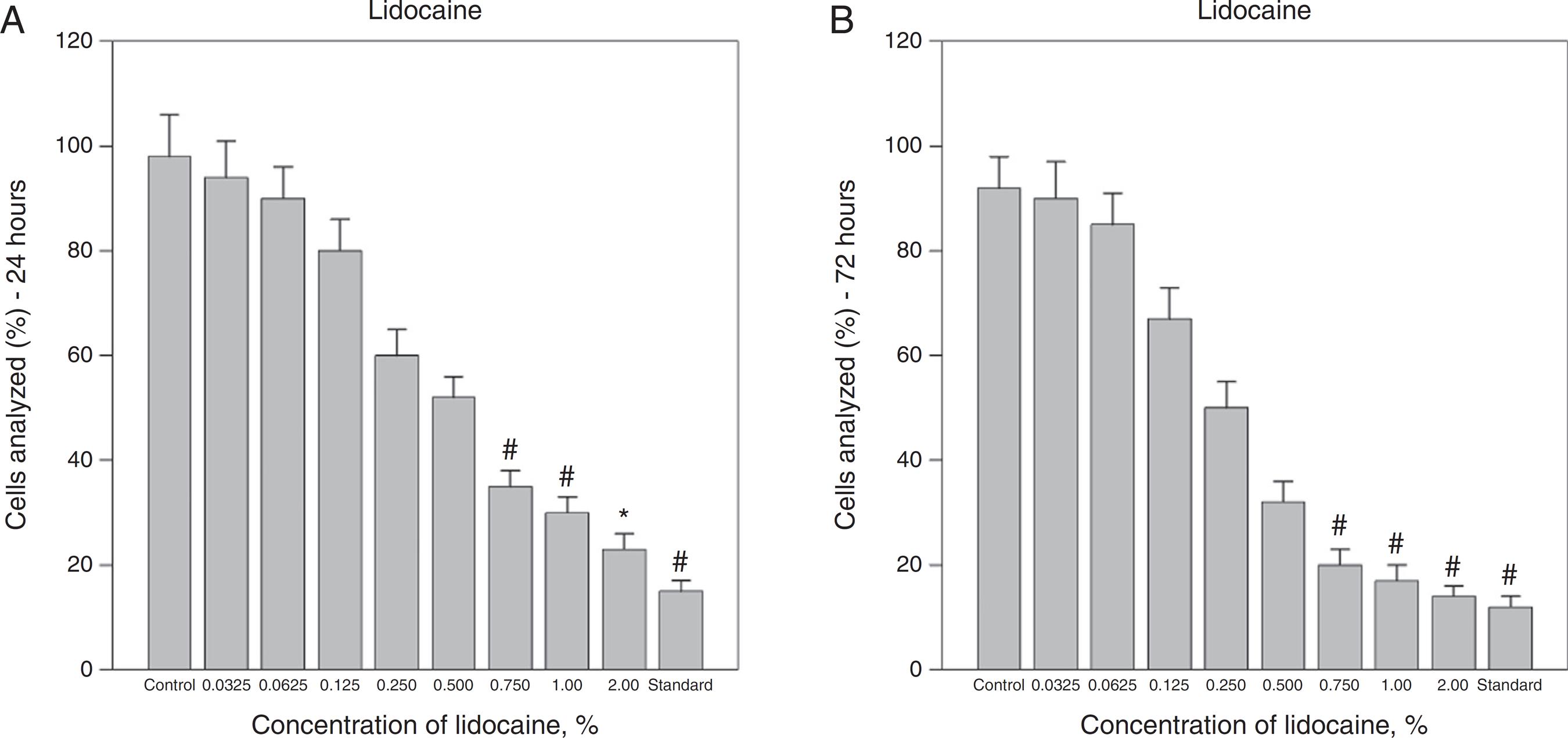
Effects of lidocaine on Hs294T melanoma cell viability. Treatment with 0.75% and higher concentrations of lidocaine significantly reduced viability after 24 and 72 h. The bars represent the mean values of experiments with standard error as error bars (*p < 0.05; # p < 0.01; control, saline solution; standard, melphalan 0.5%).
Effects of combination of lidocaine and ropivacaine on Hs294T melanoma cell viability. Viability rates were significantly lowered by combination of lidocaine and ropivacaine concentrations greater than 0.25% and above 24 and 72 h after treatment. The bars represent the mean values of experiments with standard error as error bars (*p < 0.05; # p < 0.01; control, saline solution; standard, melphalan 0.5%).
Exposure to increasing concentrations of lidocaine and ropivacaine resulted in a decreasing number of observable viable cells. Treatment with a combination of lidocaine and ropivacaine at a concentration of 0.25% and higher caused a significant decline of viable cells in both A375 and Hs294T cell lines. The fraction of apoptotic and necrotic cells increased. Minimum viability of cells was observed at a concentration of 0.75% and viability rates at this concentration were 15% ± 2% (p < 0.005) after 24 h and 10% ± 2% (p < 0.005) after 72 h, respectively (Fig. 3A and B). In A375 cell line, ropivacaine concentrations of 0.50% and greater caused significant decreases of viability and increased apoptosis and necrosis compared with a saline control at any of the study time points (Fig. 1A and B). The cytotoxicity of ropivacaine rose in a concentration-dependent manner. Ropivacaine 0.75% caused a reduction of vital cells to 22% ± 3% (p < 0.005) after 24 h and 15% ± 3% (p < 0.005) after 72 h, respectively.
Treatment with different concentrations of lidocaine also caused decline in the viability of A375 cell lines in a dose-dependent manner (Fig. 2A and B). Lidocaine at a concentration of 0.75% and higher caused reduction of the vital cells in A375 cell line and minimum viability of cells were 25% ± 2% (p < 0.001) and 10% ± 2% (p < 0.005) after 24 and 72 h, respectively. Melphalan was used as a standard agent and it caused a reduction of the vital cells to 10% ± 2% (p < 0.001) and 8% ± 1% (p < 0.001) after 24 and 72 h, respectively.
Similar results were obtained in Hs294T melanoma cell lines, further confirming the cytotoxic effect of these agents on melanoma cell lines (Figs. 4-6). In this study, A375 cell line was more sensitive to local anesthetics when compared with Hs294T cell line. In Hs294T cell line, exposure with combination of lidocaine and ropivacaine at concentration of 0.25% or greater caused cytotoxicity, and minimum viability rates were 20% ± 2% (p < 0.005) and 18% ± 2% (p < 0.005) after 72 h, respectively (Fig. 6A and B). Treatment with rising concentrations of lidocaine also demonstrated decline in the viability of Hs294T cell lines in a dose-dependent manner. Lidocaine at a concentration of 0.75% and higher caused reduction of the vital in A375 and minimum viability rates were 23% ± 5% (p < 0.005) and 14% ± 2% (p < 0.001) after 24 and 72 h, respectively (Fig. 5A and B).
Exposure to increasing concentration of ropivacaine also caused decline in the viability of Hs294T cell line. Treatment with a concentration of 0.5% or greater caused reduction of the vital cells in the cell line and minimum viability rates were 32% ± 3% (p < 0.001) and 25% ± 3% (p < 0.005) after 24 and 72 h, respectively (Fig. 4A and B). Melphalan, standard cytotoxic agent, at a concentration of 0.2% caused a reduction of the vital cells to 15% ± 2% (p < 0.001) and 12% ± 2% (p < 0.001) after 24 and 72 h, respectively.
Effect of pH on cytotoxicity
Acidity of the local anesthetics could be one of the causes for cytotoxic effect and to evaluate the same the cell lines were treated with saline solutions with a pH ranging between 4.0 and 7.4. Melanoma cell lines’ (A375 and Hs294T) viability was not affected significantly after a 1-h exposure to saline solutions with pH of 4.0, 5.0, 6.0, 7.0 and 7.4 after 1, 4, and 7 days using flow cytometry. The ratios of vital cells are shown as percentage of the total cell number in different treatment groups (Fig. 7A and B).
Effects of pH on melanoma cell viability. Cell lines (A375 and Hs294T) were not affected significantly after a 1-h exposure to saline solutions with pH of 7.4, 7.0, 6.0, 5.0, and 4.0 after 1, 4, and 7 days using flow cytometry. The ratios of vital cells are shown as percentage of the total cell number in different treatment groups. The bars represent standard error as error bars.
Caspase-3/8 activity analysis
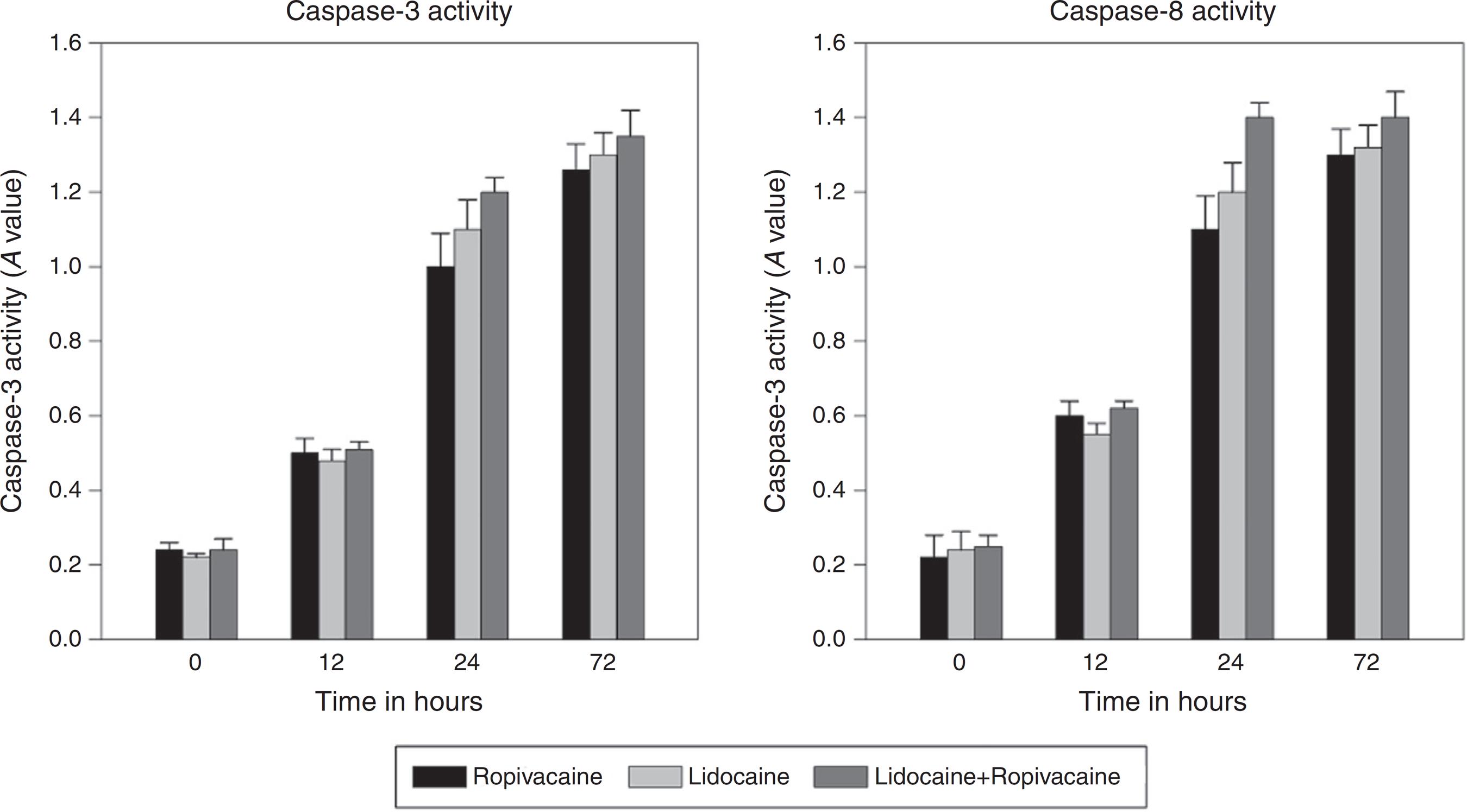
Apoptosis is mediated by a cascade of caspases or aspartate-specific cysteine proteases. Lidocaine, ropivacaine, and the combination of lidocaine and ropivacaine significantly increased the expression of activated caspase-3 and caspase-8 in a time-dependent manner in A375 cell line (Fig. 8 A and B). Peak levels were achieved in all of the local anesthetics rapidly after 12 h. At their peaks, all these agents showed significant higher level of caspase generation compared with the control group (p ≤ 0.004).
Effect of ropivacaine (0.75%), lidocaine (1.00%) and combination of ropivacaine and lidocaine (0.75%) on caspase-3 and caspase-8 activities.
Discussion
Results from the conducted study supported the hypothesis that the examined aminoamide local anesthetics posses’ cytotoxic effect on human melanoma cell lines. Local anesthetics have been used in the dermenhysis, spinal anaesthesia, and topical anesthesia to relieve pain in patients with cancer. Lidocaine and ropivacaine are clinically used at a concentration of 1.5% or 2% and 0.5% or 0.75%, respectively, for surgical anesthesia.11 Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84.,33 Sung CM, Hah YS, Kim JS, et al. Cytotoxic effects of ropivacaine, bupivacaine, and lidocaine on rotator cuff tenofibroblasts. Am J Sports Med. 2014;42:2888-96. Ropivacaine has also been reported to be less cardio toxic and less central nervous toxicity than lidocaine and other commonly used local anesthetics. According to a pharmacodyanmic study of local anesthetics, ropivacaine has a longer action time and higher potency when compared with lidocaine. Furthermore, ropivacaine has a higher recovery rate from cardiac arrest than lidocaine.2121 Ali QE, Manjunatha L, Amir SH, et al. Efficacy of clonidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block: a prospective study. Indian J Anaesth. 2014;58:709-13. However, lidocaine and ropivacaine have been reported to possess cytotoxic activity.2222 Chlebowski RT, Block JB, Cundiff D, et al. Doxorubicin cytotoxicity enhanced by local anesthetics in a human melanoma cell line. Cancer Treat Rep. 1982;66:121-5. Studies have also been reported dose- and time-dependent cytotoxic effects of these local anesthetics on different cancers.2323 Lirk P, Berger R, Hollmann MW, et al. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth. 2012;109:200-7.
24 Malet A, Faure MO, Deletage N, et al. The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth Analg. 2015;120:589-96.-2525 Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7. But, not much research has been performed to evaluate the effect of lidocaine and ropivacaine, commonly used local anesthetics, on human melanoma cell lines and this driven the authors to carry out the present study. In addition, the combination study of two local anesthetics was also carried out to investigate the synergistic cytotoxic action on human melanoma cell lines. The combination of same class of local anesthetics, i.e. aminoamide, was devoid of any compatible issues and was found to possess synergistic activity and the cytotoxic effect from the combination were comparable with that of melphalan. However, in the present study ropivacaine has demonstrated cytotoxic activity when compared with normal saline solution at any concentration, but was less cytotoxic when compared with lidocaine, the combination and melphalan.
Several studies have shown cytotoxic effects of different concentrations of local anesthetics after different times of exposure and assessment.77 Sakaguchi M, Kuroda Y, Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth Analg. 2006;102:1103-7.
8 Kim M, Lee YS, Mathews HL, et al. Induction of apoptotic cell death in a neuroblastoma cell line by dibucaine. Exp Cell Res. 1997;231:235-41.
9 Unami A, Shinohara Y, Ichikawa T, et al. Biochemical and microarray analyses of bupivacaine-induced apoptosis. J Toxicol Sci. 2003;28:77-94.
10 Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007.
11 Lee HT, Xu H, Siegel CD, et al. Local anesthetics induce human renal cell apoptosis. Am J Nephrol. 2003;23:129-39.
12 Nakamura K, Kido H, Morimoto Y, et al. Prilocaine induces apoptosis in osteoblastic cells. Can J Anaesth. 1999;46:476-82.-1313 Shen Q, Tian F, Jiang P, et al. EGCG enhances TRAIL-mediated apoptosis in human melanoma A375 cell line. J Huazhong Univ Sci Technol Med Sci. 2009;29:771-5. On comparison with an identical experimental setting on cancer cell lines, human melanoma cell lines were more sensitive to local anesthetics causing more cell death after treatment with equal concentrations of local anesthetics for the same exposure and assessment times. After exposure to local anesthetics, immediate cell death might have been caused by necrosis, whereas the viability of cells decreased in a time-dependent manner over several hours. Published papers reported that membrane permeability and cytotoxicity was maximum when the lipophilicity, as determined by the octanol-water partition coefficient (log p) approached-3.2626 Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177:39-54.,2727 Ishihara M, Yokote Y, Sakagami H. Quantitative structure-cytotoxicity relationship analysis of coumarin and its derivatives by semiempirical molecular orbital method. Anticancer Res. 2006;26:2883-6. This finding was further supported by our work in which ropivacaine (log p = 2.91) and lidocaine (log p = 2.56) had shown cytotoxic effects on A375 and Hs294T melanoma cell lines. This result suggests that the cytoxicity of local anesthetics is very much related to their membrane permeability.
Acidity of the local anesthetics could be one of the causes for cytotoxic effects and to assess the same the cell lines were treated with saline solutions with a pH ranging between 4.0 and 7.4. Viability assessment showed no differences across the studied pH range and cytotoxic activity due to acidity of local anesthetics can be excluded in this study (Fig. 7A and B).
Caspase activity was evaluated to differentiate whether apoptosis or necrosis was responsible for cytotoxic activity.1313 Shen Q, Tian F, Jiang P, et al. EGCG enhances TRAIL-mediated apoptosis in human melanoma A375 cell line. J Huazhong Univ Sci Technol Med Sci. 2009;29:771-5. Caspases play an important role in apoptosis (programmed cell death), necrosis and inflammation. They are broadly classified by their roles in apoptosis (caspase-3, -6, -7, -8 and -9 in mammals) and in inflammation (caspases-1, -4, -5, and -12 in humans). Further, caspases involved in apoptosis are classified based on mechanism of action as either initiator caspases (caspases-8 and -9) or executioner caspases (caspases-3, -6, and -7). In the apoptotic cell, caspases-3 is activated by extrinsic (death ligand) and intrinsic (mitochondrial) pathways.2828 McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656.,2929 Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99-104. In this study, activity of both caspases-3 and caspases-8 was increased, suggesting the role of these caspases in the apoptosis regulation (Fig. 8A and B).
In this study, the method was designed to decrease and to approximately measure possible iatrogenic cell damage using an experimental setting in monolayer cultures. Local anesthetics exposure can impair cellular adherence to culture discs. So, non-adherent cells in the study were washed and returned back. Most commonly used instrument to measure cell viability is flow cytometry, so this was employed for accurate estimate of the viability.44 Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18.,2525 Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7.,3030 Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92:599-608.
Aminoamide local anesthetics used in this study are widely used to treat irritation, soreness, itchiness and are injected as a dental anaesthetic, or used as local anesthetics for minor surgery.3131 Neafsey PJ. Patching pain with lidocaine: new uses for the lidocaine 5% patch. Home Healthc Nurse. 2004;22:562-4.,3232 Di Croce DE, Trinks PW, de La CC. Amide-type local anesthetics action on the sarcoplasmic reticulum Ca-ATPase from fast-twitch skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:873-81. The studied local anesthetics, including combinations of lidocaine and ropivacaine, were more cytotoxic at higher concentrations than at lower concentrations. The investigated study suggests that lidocaine, ropivacaine and combination of lidocaine and ropivacaine can be further investigated for their anticancer properties for the treatment of melanoma patients, since presently available chemotherapeutic agents possess devastating side effects. The combination of lidocaine and ropivacaine, in particular, was found to be as cytotoxic as that of melphalan, a nitrogen mustard alkylating agent (10% vs. 8%, respectively; p < 0.01). The studied local anesthetics can further be investigated in combination with other anticancer agents or with other local anesthetics for synergistic activity on melanoma and other cancers. On the other hand, authors based on the results of this study recommend using commercially available low concentrations of the less cytotoxic local anesthetics, such as bupivacaine for treatment of skin and related diseases or using the concentration of these local anesthetics at which they were found to be less cytotoxic.
Conclusion
The cytotoxic activity of the investigated aminoamide local anesthetics on melanoma cell lines (A375 and Hs294T) is dependent on concentration, agent and exposure time. Of the studied local anesthetics, ropivacaine was less cytotoxic when compared with lidocaine and the combination of lidocaine and ropivacaine. Apoptosis in the cell lines was mediated through activity of caspases-3 and caspases-8. Cell viability was not affected by the acidity of the studied local anesthetics. This study had few limitations. In this study, only two cell lines were investigated and this is an in vitro study. Although the study gives an idea of cytotoxic activity of these agents in in vitro settings, to confirm this activity clinical trials in human population are required.
References
-
1Breu A, Eckl S, Zink W, et al. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy. 2013;29:1676-84.
-
2Kobayashi K, Ohno S, Uchida S, et al. Cytotoxicity and type of cell death induced by local anesthetics in human oral normal and tumor cells. Anticancer Res. 2012;32:2925-33.
-
3Sung CM, Hah YS, Kim JS, et al. Cytotoxic effects of ropivacaine, bupivacaine, and lidocaine on rotator cuff tenofibroblasts. Am J Sports Med. 2014;42:2888-96.
-
4Grishko V, Xu M, Wilson G, et al. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92:609-18.
-
5Dregalla RC, Lyons NF, Reischling PD, et al. Amide-type local anesthetics and human mesenchymal stem cells: clinical implications for stem cell therapy. Stem Cells Transl Med. 2014;3:365-74.
-
6Fedder C, Beck-Schimmer B, Aguirre J, et al. In vitro exposure of human fibroblasts to local anesthetics impairs cell growth. Clin Exp Immunol. 2010;162:280-8.
-
7Sakaguchi M, Kuroda Y, Hirose M. The antiproliferative effect of lidocaine on human tongue cancer cells with inhibition of the activity of epidermal growth factor receptor. Anesth Analg. 2006;102:1103-7.
-
8Kim M, Lee YS, Mathews HL, et al. Induction of apoptotic cell death in a neuroblastoma cell line by dibucaine. Exp Cell Res. 1997;231:235-41.
-
9Unami A, Shinohara Y, Ichikawa T, et al. Biochemical and microarray analyses of bupivacaine-induced apoptosis. J Toxicol Sci. 2003;28:77-94.
-
10Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg. 2009;108:997-1007.
-
11Lee HT, Xu H, Siegel CD, et al. Local anesthetics induce human renal cell apoptosis. Am J Nephrol. 2003;23:129-39.
-
12Nakamura K, Kido H, Morimoto Y, et al. Prilocaine induces apoptosis in osteoblastic cells. Can J Anaesth. 1999;46:476-82.
-
13Shen Q, Tian F, Jiang P, et al. EGCG enhances TRAIL-mediated apoptosis in human melanoma A375 cell line. J Huazhong Univ Sci Technol Med Sci. 2009;29:771-5.
-
14Ross BK, Coda B, Heath CH. Local anesthetic distribution in a spinal model: a possible mechanism of neurologic injury after continuous spinal anesthesia. Reg Anesth. 1992;17:69-77.
-
15Arai T, Hoka S. Neurotoxicity of intrathecal local anesthetics. J Anesth. 2007;21:540-1.
-
16Kishimoto T, Bollen AW, Drasner K. Comparative spinal neurotoxicity of prilocaine and lidocaine. Anesthesiology. 2002;97:1250-3.
-
17Kamiya Y, Ohta K, Kaneko Y. Lidocaine-induced apoptosis and necrosis in U937 cells depending on its dosage. Biomed Res. 2005;26:231-9.
-
18Boselli E, Duflo F, Debon R, et al. The induction of apoptosis by local anesthetics: a comparison between lidocaine and ropivacaine. Anesth Analg. 2003;96:755-6.
-
19Bauer TW, Gutierrez M, Dudrick DJ, et al. A human melanoma xenograft in a nude rat responds to isolated limb perfusion with TNF plus melphalan. Surgery. 2003;133:420-8.
-
20Hansson J, Berhane K, Castro VM, et al. Sensitization of human melanoma cells to the cytotoxic effect of melphalan by the glutathione transferase inhibitor ethacrynic acid. Cancer Res. 1991;51:94-8.
-
21Ali QE, Manjunatha L, Amir SH, et al. Efficacy of clonidine as an adjuvant to ropivacaine in supraclavicular brachial plexus block: a prospective study. Indian J Anaesth. 2014;58:709-13.
-
22Chlebowski RT, Block JB, Cundiff D, et al. Doxorubicin cytotoxicity enhanced by local anesthetics in a human melanoma cell line. Cancer Treat Rep. 1982;66:121-5.
-
23Lirk P, Berger R, Hollmann MW, et al. Lidocaine time- and dose-dependently demethylates deoxyribonucleic acid in breast cancer cell lines in vitro. Br J Anaesth. 2012;109:200-7.
-
24Malet A, Faure MO, Deletage N, et al. The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth Analg. 2015;120:589-96.
-
25Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med. 2007;35:1621-7.
-
26Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177:39-54.
-
27Ishihara M, Yokote Y, Sakagami H. Quantitative structure-cytotoxicity relationship analysis of coumarin and its derivatives by semiempirical molecular orbital method. Anticancer Res. 2006;26:2883-6.
-
28McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656.
-
29Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99-104.
-
30Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92:599-608.
-
31Neafsey PJ. Patching pain with lidocaine: new uses for the lidocaine 5% patch. Home Healthc Nurse. 2004;22:562-4.
-
32Di Croce DE, Trinks PW, de La CC. Amide-type local anesthetics action on the sarcoplasmic reticulum Ca-ATPase from fast-twitch skeletal muscle. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:873-81.
Publication Dates
-
Publication in this collection
Nov-Dec 2016
History
-
Received
22 Feb 2015 -
Accepted
15 Apr 2015