Abstract
Background:
Adequate preoperative fasting is critical in preventing pulmonary aspiration of gastric content. We proposed to study the sonographic gastric content dynamics after the ingestion of liquid or solid food in healthy volunteers and confront it with current guidelines for preoperative fasting times.
Methods:
We performed a prospective, crossover, evaluator-blinded study involving 17 healthy volunteers of both sexes. Each participant fasted for 10 h and was subjected to a baseline gastric ultrasound, intake of 400 mL of coconut water or a 145 g, 355 kcal meat sandwich, and sonographic gastric evaluations after 10 min and every hour until the stomach was completely empty.
Results:
At baseline, all subjects had an empty stomach. At 10 min, gastric content [mean + standard deviation (SD)] was 240.4 + 69.3 and 248.2 + 119.2 mL for liquid and solid foods, respectively (p > 0.05). Mean + SD gastric emptying times were 2.5 + 0.7 and 4.5 + 0.9 h for liquid and solid foods, respectively (p < 0.001). For the drink, the stomach was completely empty in 59% and 100% of the subjects after two and four hours, and for the sandwich, 65% and 100% of the subjects after four and seven hours, respectively.
Conclusions:
Sonographic gastric dynamics for coconut water and a meat sandwich resulted in complete gastric emptying times higher and lower, respectively, than those suggested by current guidelines for preoperative fasting.
KEYWORDS
Ultrasound; Gastric content; Gastric dynamics; Preoperative fasting
Resumo
Justificativa:
O jejum pré-operatório adequado é fundamental para prevenir a aspiração pulmonar do conteúdo gástrico. Nossa proposta foi avaliar a dinâmica ultrassonográfica do conteúdo gástrico após a ingestão de alimentos líquidos ou sólidos em voluntários sadios e confrontá-la com as diretrizes atuais para os períodos de jejum no pré-operatório.
Métodos:
Um estudo prospectivo, cruzado e avaliador-cego foi feito com 17 voluntários saudáveis de ambos os sexos. Cada participante jejuou por 10 horas e foi submetido a uma ultrassonografia gástrica na fase basal, ingestão de 400 mL de água de coco ou 355 g de sanduíche de carne e avaliações gástricas ultrassonográficas foram feitas após 10 minutos e a cada hora até o estômago estar completamente vazio.
Resultados:
Na fase basal, todos os participantes estavam com o estômago vazio. Aos 10 minutos, o conteúdo gástrico [média + desvio-padrão (DP)] foi de 240,4 + 69,3 e 248,2 + 119,2 mL para alimentos líquidos e sólidos, respectivamente (p > 0,05). Os tempos médios de esvaziamento gástrico + DP foram de 2,5 + 0,7 e 4,5 + 0,9 horas para alimentos líquidos e sólidos, respectivamente (p < 0,001). Para a bebida, o estômago ficou completamente vazio em 59% e 100% dos sujeitos após duas e quatro horas; para o sanduíche, o estômago ficou completamente vazio em 65% e 100% dos sujeitos após quatro e sete horas, respectivamente.
Conclusões:
A dinâmica ultrassonográfica do volume gástrico para água de coco e sanduíche de carne resultou em tempos totais de esvaziamento gástrico maiores e menores, respectivamente, do que os sugeridos pelas diretrizes atuais para o jejum pré-operatório.
PALAVRAS-CHAVE
Ultrassom; Conteúdo gástrico; Dinâmica gástrica; Jejum pré-operatório
Introduction
Literature has shown that long periods of preoperative fasting are unpleasant, as patients may experience thirst, hunger, anxiety, postoperative nausea and vomiting, sleepiness and tiredness.11 Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344-50.
2 Tosun B, Yava A, Acikel C. Evaluating the effects of preoperative fasting and fluid limitation. Int J Nurs Pract. 2015;21:156-65.
3 Tsutsumi R, Kakuta N, Kadota T, et al. Effects of oral carbohydrate with amino acid solution on the metabolic status of patients in the preoperative period: a randomized, prospective clinical trial. J Anesth. 2016;30:842-9.-44 Sada F, Krasniqi A, Hamza A, et al. A randomized trial of preoperative oral carbohydrates in abdominal surgery. BMC Anesthesiol. 2014;14:93. Considering that major complications and deaths directly related to anesthesia are rare,55 Pignaton W, Braz JR, Kusano PS, et al. Perioperative and anesthesia-related mortality: an 8-year observational survey from a tertiary teaching hospital. Medicine (Baltimore). 2016;95:e2208. and perioperative mortality in patients from low-, -middle- and high-income countries may be considered very low,66 International Surgical Outcomes Study G. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016;117:601-9. quality indicators in anesthesia and perioperative medicine may consider not only the surgical results but all those other aspects of patients' perioperative experiences.
On the other hand, anesthesiologists will inevitably be concerned about gastric content at the induction of anesthesia due to the severe effects of pulmonary aspiration.77 Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.,88 Bannister WK, Sattilaro AJ. Vomiting and aspiration during anesthesia. Anesthesiology. 1962;23:251-64. Proper preoperative fasting is critical for preventing this adverse event.99 Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859-68.
10 Sutherland AD, Stock JG, Davies JM. Effects of preoperative fasting on morbidity and gastric contents in patients undergoing day-stay surgery. Br J Anaesth. 1986;58:876-8.-1111 Vieira AM, Rios RC, Brandão ACA, et al. Water ingestion and residual gastric content evaluation in pediatric patients undergoing elective surgeries. Rev Bras Anestesiol. 1997;47:283-7. Several studies have sought to establish the appropriate duration of preoperative fasting to minimize pulmonary aspiration risk at the induction of anesthesia.1111 Vieira AM, Rios RC, Brandão ACA, et al. Water ingestion and residual gastric content evaluation in pediatric patients undergoing elective surgeries. Rev Bras Anestesiol. 1997;47:283-7.
12 Lewis P, Maltby JR, Sutherland LR. Unrestricted oral fluid until three hours preoperatively: effect on gastric fluid volume and pH. Can J Anaesth. 1990;37:S132.-1313 Sutherland AD, Maltby JR, Sale JP, et al. The effect of preoperative oral fluid and ranitidine on gastric fluid volume and pH. Can J Anaesth. 1987;34:117-21.
Current guidelines from American Society of Anesthesiologists1414 Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology 2017;126:376-93. and European Society of Anesthesiology1515 Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556-69. suggest that clear liquids may be ingested for up to 2 h before procedures requiring general anesthesia, regional anesthesia, or procedural sedation and analgesia. Clear liquids involve water, fruit juices without pulp, carbonated beverages, carbohydrate-rich nutritional drinks, clear tea, black coffee and should not include alcohol. They recommend that a light meal or nonhuman milk may be ingested for up to 6 h before elective procedures requiring any kind of anesthesia, sedation and analgesia and that additional fasting time (eight or more hours) may be needed in cases of patient intake of fried foods, fatty foods, or meat.
Considering these guidelines, it is clear that there are several options for liquid and solid meals to be drunk and eaten at the preoperative period. Coconut water is a tasty and very popular drink in several tropical countries. We consider it an option as a preoperative drink, being a carbohydrate and also a very economical drink. We are also considering a meat sandwich as a preoperative meal for healthy patients undergoing minor or medium surgeries, according to the preoperative fasting time suggested by current guidelines.
As ultrasound has become an option for evaluating qualitatively and quantitatively preoperative gastric content, being a non-invasive, inexpensive and safe method,1616 Perlas A, Davis L, Khan M, et al. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113:93-7.,1717 Bouvet L, Mazoit JX, Chassard D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;14:1086-92. we proposed to evaluate the ultrasound gastric content dynamics after the ingestion of either coconut water or a meat sandwich in healthy volunteers as possible sources of nutrients to be given to healthy patients during the preoperative period. The secondary objective was to confirm or confront the minimum fasting time required for adequate gastric emptying for liquid and solid foods, according to the suggestion of current guidelines.
Methods
After the registration in Platform Brazil on October 8, 2014, under the number CAAE 37137014.9.0000.5550, and the approval by the Research Ethics Committee of the Ophir Loyola Hospital, this study was conducted from February to July, 2015, in volunteers who were properly informed about the procedures and their risks, complied with the established procedures and signed the informed consent form. The abdominal ultrasound examination was performed in the Radiology and Diagnostic Imaging Unit of the Ophir Loyola Hospital.
This is a prospective, crossover study, with voluntary participants aged between 18 and 50 years, classified as physical status 1 or 2, according to the American Society of Anesthesiologists (ASA). Exclusion criteria were the presence of any medical condition that could delay gastric emptying: body mass index ≥35 kg m−2; diabetes; gastritis; gastroesophageal reflux disease; pyloric stenosis; chronic renal failure; achalasia; Zenker's diverticulum; multiple myeloma; systemic lupus erythematosus and other collagenosis; pregnancy; any previous surgery at the gastrointestinal system; and others. Participants were advised to not drink alcoholic beverages in the 24 h before the study.
Seventeen volunteers were evaluated twice, in different days with a gap of at least 7 days, one time for the liquid ingestion and one time for the solid food ingestion, according to the following protocol: the day before the examination, the volunteers at the last meal at 10 pm, which was 10 h before baseline evaluation. All the subjects were then submitted to a gastric ultrasound evaluation by the radiologist (baseline) and were conducted to an appropriate room where they were assigned by draw, according to sealed opaque envelopes, to drink or eat the standard beverage or meal, respectively. The standard liquid consumed was two pre-packaged coconut water of 200 mL (total of 400 mL) that contained, per package of 200 mL, 45 kcal (189 kJ), 11 g of carbohydrates, 45 mg of sodium and 300 mg of potassium (Sococo S.A. Indústrias Alimentícias, Maceió/AL, Brazil). The standard solid food was a 145 g pre-packaged meat sandwich (Hot pocket X-burguer) containing 355 kcal with the following composition: 34 g of carbohydrates, 19 g of protein, 17 g of total fat, 6.6 g of saturated fat, 0.5 g of trans fat, 1.7 g of dietary fiber and 858 mg of sodium (Sadia, S.A., São Paulo/SP, Brazil). The investigators provided both foods to the volunteers. After the ingestion of the foods, the volunteers were conducted to the examination room where they were blinded evaluated by the radiologist, who performed the ultrasound gastric examination after 10 min and every hour until the stomach was considered completely empty.
In all volunteers and all the times, a single radiologist of the hospital who was properly certified and experienced in abdominal ultrasound examination performed the sonographic examinations. The examiner was unaware of the fasting time, the type of food consumed by the study participant and the time points of the study. The ultrasound machine was a Logiq P6 (GE Healthcare, Little Chalfont, United Kingdom) and images were obtained using a 2-6 MHz convex probe. After the examination, the radiologist used a form designed for the study to record the volume of the gastric contents and their physical (texture) characteristics. Each ultrasound was conducted in approximately 2 min and no more than three volunteers were scheduled for an exam per day to ensure compliance with the protocol. All volunteers adopted the right lateral decubitus position during the examination.
To estimate the gastric content, the cross-sectional area of the gastric antrum was determined, according to the formula employed by Bolondi and collaborators, which represents the area of an ellipse in cm2: CSA = (AP × CC × π)/4, where CSA (cross-sectional area) is the area of the ellipse, AP is the anteroposterior diameter (cm) and CC is the craniocaudal diameter (cm).1818 Bolondi L, Bortolotti M, Santi V, et al. Measurement of gastric emptying time by real- time ultrasonography. Gastroenterology. 1985;89:752-9. The image of the gastric antrum was obtained in the sagittal plane of the epigastric region in the area contiguous to the edge of the left liver lobe and at the level of the aorta.1919 Perlas A, Chan VW, Lupu CM, et al. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82-9.,2020 Arzola C, Perlas A, Siddiqui NT, et al. Bedside gastric ultrasonography in term pregnant women before elective cesarean delivery: a prospective cohort study. Anesth Analg. 2015;121:752-8.
To estimate gastric content volumes (mL), the two-dimensional measurement of the CSA obtained by ultrasound was transformed into a three-dimensional measurement. For this purpose, the researchers used the mathematical model validated by Perlas and collaborators: volume (mL) = 27 + (14.6 × CSA) − (1.28 × age).2121 Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357-63.
With the purpose of establishing a possible "risk stomach", we also calculated the volume of the gastric content on the basis of the volunteers' weight and assumed that 0.8 mL.kg−1 would characterize a higher risk for pulmonary aspiration.1717 Bouvet L, Mazoit JX, Chassard D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;14:1086-92.
Statistical analysis
The sample size was calculated considering a difference in gastric volume 1 h after the intake of liquid or solid food. With a difference of 90 mL and a standard deviation of 72 mL, a power of 95% and α equal to 0.05, 17 subjects in each intervention would be necessary for the study.
The statistical analysis of the crossover design included the effect of the treatment application sequence, treatment application order and their residuals. The variables evaluated were volume (mL), emptying time and the gastric volume on the basis of the volunteers' weight. When possible, the Student t-test was used to compare the interventions at each evaluation time. The software SAS, version 9.3, was used for the analysis.
Results
In total, 19 volunteers agreed to participate in the study. One was excluded due to hypothyroidism and another due to post-bariatric surgery. Thus, the sample consisted of 17 volunteers, of which 15 were classified as ASA 1 and two as ASA 2, with nine male and eight female participants. The mean ± standard deviation values for age, weight, height and body mass index were, respectively, 28.3 ± 3.5 years, 69.8 ± 15.7 kg, 166.9 ± 7.8 cm and 24.8 ± 4.0 kg m−2.
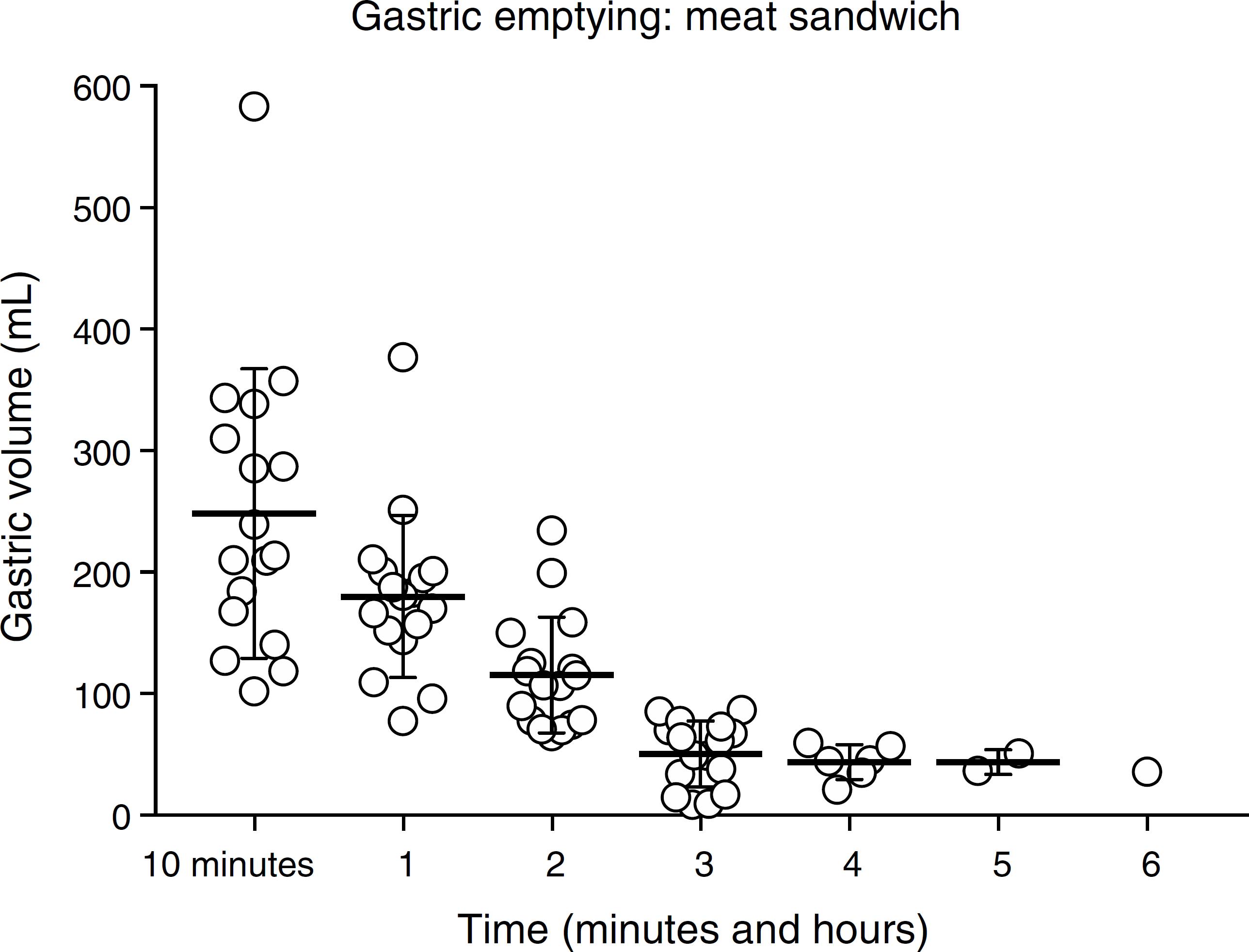
The values for gastric content and volumes are presented in Table 1. All volunteers had the stomach completely empty at baseline. The radiologist was capable of correctly affirm whether the gastric content was liquid or solid in 100% of his examinations. From 4 h on, the radiologist was only capable of measuring gastric volume for the solid food. Thus, the stomach was considered to be empty of the liquid ingested by the volunteer and the ultrasound was no longer performed. Six hours after the ingestion, only one volunteer had gastric residue after ingesting solid food (Figs. 1 and 2, respectively).
The volunteers considered for the calculation at each time point are presented as n (%). Gastric volume (mL) and the ratio between gastric volume to volunteers' weight (mL.kg-1) for each evaluation time point. Data are presented as mean ± standard deviation.
The mean ± standard deviation values for time of complete gastric emptying were 2.5 ± 0.7 h for the drink and 4.5 ± 0.9 for the solid meal, respectively (p < 0.001).
Gastric volume (mL) after the ingestion of coconut water, 400 mL, according to ultrasonographic evaluation in 17 health volunteers.
Gastric volume (mL) after the ingestion of a standard meat sandwich according to ultrasonographic evaluation in 17 health volunteers.
Discussion
Gastric ultrasound has been considered an easy and fast way of measuring gastric volume and emptying.2222 Haruma K, Kusunoki H, Manabe N, et al. Real-time assessment of gastroduodenal motility by ultrasonography. Digestion. 2008;77(Suppl 1):48-51. With these latter purposes, it has been compared to other methods such as breathing test,2323 Aoki S, Haruma K, Kusunoki H, et al. Evaluation of gastric emptying measured with the 13C-octanoic acid breath test in patients with functional dyspepsia: comparison with ultrasonography. Scand J Gastroenterol. 2002;37:662-6. scintigraphy2424 Benini L, Sembenini C, Heading RC, et al. Simultaneous measurement of gastric emptying of a solid meal by ultrasound and by scintigraphy. Am J Gastroenterol. 1999;94:2861-5. and the direct measurement of gastric content by aspiration2121 Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357-63. and has showed a good correlation. Furthermore, the individual learning curve is short, and 24 and 33 are the suggested average number of cases needed to achieve 90% and 95% accuracy, respectively.2525 Arzola C, Carvalho JC, Cubillos J, et al. Anesthesiologists' learning curves for bedside qualitative ultrasound assessment of gastric content: a cohort study. Can J Anaesth. 2013;60:771-9.
In the present study with healthy subjects, according to a sonographic evaluation, we firstly observed that the stomach was completely empty after a 10 h period of fasting. We understand that this information is not new and is in accordance with what has been observed in healthy subjects fasting for a similar period of time.2626 Bisinotto FM, Pansani PL, Silveira LA, et al. Qualitative and quantitative ultrasound assessment of gastric content. Rev Assoc Med Bras (1992). 2017;63:134-41. On the other hand, despite the belief that preoperative fasting reduces the risk of pulmonary aspiration, evidence is inconclusive2727 Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003. CD004423. or even contrary to this fact, when fasting is considered an independent risk factor.2828 Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56-62.
Ten minutes after drinking 400 mL of a carbohydrate-composed drink, the sonographic determination of the gastric content resulted in a mean volume lower than the ingested volume. Considering that ultrasound provides an accurate measure of the gastric content and volume, the difference between the ingested volume and the gastric volume may be attributed to a very fast initial gastric emptying of this kind of drink.
Another important observation is that 2 h after the ingestion of the liquid, 10 subjects (59%) had their stomach empty. On the other hand, 2 subjects (12%) at 3 h after drinking still had some liquid in their stomachs as detected by the ultrasound. Even though the mean time for complete gastric emptying after coconut water ingestion was two and a half hours; that after 2 h only 41% of the subjects still had liquid in their stomachs; and that the "risk stomach" (gastric volume >0.8 mL.kg−1) was not seen neither 2 nor 3 h after the ingestion, a very conservative and safe analyses would recommend that preoperative fasting for a carbohydrate-composed drink, or specifically coconut water, thinking of reduced risk of pulmonary aspiration, should be 4 h.
The analysis of the gastric emptying after the ingestion of the sandwich showed that, after 10 min, the sandwich weighting 145 g resulted in a gastric volume that was apparently higher than that related to the sandwich itself. This can be justified by an increase in gastric juice secretion prompted by the meal.2929 Richardson CT, Walsh JH, Hicks MI, et al. Studies on the mechanisms of food-stimulated gastric acid secretion in normal human subjects. J Clin Invest. 1976;58:623-31. After 4 and 5 h, 65% and 88% of the subjects had their stomachs empty. The mean time for complete gastric emptying was four and a half hours. On the other hand, the mean values of the gastric volume on the basis of the subjects' weights were 0.72 (4 h) and 0.82 (5 h), may not be completely safe regarding the possibility of pulmonary aspiration. Yet, 6 h after eating the sandwich, one volunteer had some content in his/her stomach. The analysis of the "risk stomach" would consider this content as low risk (0.65 mL.kg−1) for pulmonary aspiration. Nonetheless and again, a very conservative analysis would recommend that preoperative fasting for this kind of sandwich would be 7 h. This is shorter than the preoperative fasting time recommended by the current guidelines for a meal containing meat.
Some arguments have been presented in favor of a reduced preoperative fasting time: increased patients' satisfaction3030 Imbelloni LE, Pombo IA, Filho GB. Reduced fasting time improves comfort and satisfaction of elderly patients undergoing anesthesia for hip fracture. Rev Bras Anestesiol. 2015;65:117-23.,3131 Yildiz H, Gunal SE, Yilmaz G, et al. Oral carbohydrate supplementation reduces preoperative discomfort in laparoscopic cholecystectomy. J Invest Surg. 2013;26:89-95.; a preoperative ingestion of a carbohydrate-rich drink increases the gastric pH, does not increase gastric residue volumes3232 Yagci G, Can MF, Ozturk E, et al. Effects of preoperative carbohydrate loading on glucose metabolism and gastric contents in patients undergoing moderate surgery: a randomized, controlled trial. Nutrition. 2008;24:212-6.,3333 Schmidt AR, Buehler P, Seglias L, et al. Gastric pH and residual volume after 1 and 2 h fasting time for clear fluids in children. Br J Anaesth. 2015;114:477-82. and decreases insulin resistance preserving muscle metabolism and strength.3434 Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol. 2009;23:401-9. When fasting is associated with bowel preparation for colonic surgery, there is also a chance of intravascular volume depletion.3535 Holte K, Nielsen KG, Madsen JL, et al. Physiologic effects of bowel preparation. Dis Colon Rectum. 2004;47:1397-402. The protocols for enhanced recovery after surgery have suggested a reduced preoperative fasting period and carbohydrate and protein ingestion.3636 Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-8.,3737 Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS(R)) society recommendations. Clin Nutr. 2012;31:783-800.
Ultrasound can be used to quantitatively and qualitatively assess the gastric content in an accurate, fast and practical manner that does not harm the patient.3838 Cubillos J, Tse C, Chan VW, et al. Bedside ultrasound assessment of gastric content: an observational study. Can J Anaesth. 2012;59:416-23. In the present study, the radiologist was unaware of the food ingested by the volunteer and was capable of describing correctly the type of food ingested, i.e., liquid or solid. Based on this information, a trained anesthesiologist performing a bedside test could determine the best time to induce the anesthesia and the most suitable induction technique for the patient in question. In fact, Perlas and collaborators suggested an algorithm to stratify the risk of pulmonary aspiration based on sonographic gastric evaluation and the most appropriate anesthetic approach for an individual case.3939 Perlas A, Van de Putte P, Van Houwe P, et al. I-AIM framework for point-of-care gastric ultrasound. Br J Anaesth. 2016;116:7-11.
We chose coconut water to be studied because it is economical and has higher energy content, thus possibly providing comfort and satiety to the patients, similarly to other carbohydrate-rich drinks. As expected, in our study, a much faster gastric emptying occurred with the coconut water as compared to the meat sandwich. Nonetheless, thinking of the lowest chance of bronchoaspiration, 4 h were necessary to have all the subjects with the stomach completely empty. In a more liberal approach and also in accordance with the literature and the possible individual variation,4040 Nakamura M, Uchida K, Akahane M, et al. The effects on gastric emptying and carbohydrate loading of an oral nutritional supplement and an oral rehydration solution: a crossover study with magnetic resonance imaging. Anesth Analg. 2014;118:1268-73. 2 h after the ingestion of 400 mL of coconut water could be considered a low-risk fasting time regarding pulmonary aspiration.
Considering the meat sandwich that we chose to study, weighting 145 g and containing 355 kcal, according to current guidelines, a period of fasting of 8 h would be necessary to warrant gastric emptying and decrease the risk of bronchoaspiration. Nonetheless, the mean time for gastric emptying was four and a half hours but only after 7 h all the individuals have their stomach completely empty. Again, individual variation was seen, similarly to what happened with the coconut water. Four hours were enough for 65% of the individuals to have their stomach completely empty.
For comparison, Tougas and collaborators evaluated gastric emptying by scintigraphy in healthy subjects and verified that, after ingesting a low-fat meal with a caloric value equal to 255 kcal, 4 h warranted gastric empty in more than 80% of the individuals.4141 Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456-62. Bolondi and collaborators studied gastric emptying in both asymptomatic and dyspeptic subjects after the ingestion of an 800 cal-Italian food, including pasta with tomato sauce and 300 mL of water. They verified complete gastric emptying in all healthy subjects and in all symptomatic subjects after approximately four and half hours and 8 h, respectively.1818 Bolondi L, Bortolotti M, Santi V, et al. Measurement of gastric emptying time by real- time ultrasonography. Gastroenterology. 1985;89:752-9.
Even though we found differences in the gastric emptying in the healthy subjects for both the drink and the solid food, we think that the crossover design of our study reduced the possibility of more individual variations, ensuring homogeneity of the sample and for the comparison between the two interventions. As expected, gastric emptying was much faster after ingestion of coconut water than the sandwich. Our results are in accordance with the literature but we think that the anesthesiologist who is planning his/her anesthesia should always consider the possibility of individual variation, even in healthy subjects. For this reason, ultrasound may be a powerful resource to help the anesthesiologist to make his/her best decision.
A limitation of our study is that we studied healthy volunteers not undergoing any surgical procedure. Thinking of preoperative fasting and the risk of bronchoaspiration, researchers should consider the study of patients who would be in this scenario as perioperative stress may influence physiological behavior.
Considering that a residual gastric volume equal to or higher than 0.8 mL.kg−1 is a "risk stomach" for pulmonary aspiration, we concluded that 2 h after the ingestion of 400 mL of coconut water would be safe and in accordance with current guidelines for preoperative fasting, even though the stomach may not be completely empty. Likewise, 6 h after the ingestion of a 355 kcal meat sandwich would be safe, considering the residual gastric volume, as a preoperative fasting time. This period of time is shorter than that suggested by current guidelines, for this kind of meal. Nonetheless, according to the clinical situation and patients' characteristics, individual variation may be a concern.
Studies in real clinical situations are necessary to validate this information and also enable the clinical application of ultrasound for evaluation of the gastric content immediately before the induction of anesthesia, which would help anesthesiologists decide whether to postpone the anesthetic induction and choose the most appropriate technique at the time.
References
-
1Hausel J, Nygren J, Lagerkranser M, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93:1344-50.
-
2Tosun B, Yava A, Acikel C. Evaluating the effects of preoperative fasting and fluid limitation. Int J Nurs Pract. 2015;21:156-65.
-
3Tsutsumi R, Kakuta N, Kadota T, et al. Effects of oral carbohydrate with amino acid solution on the metabolic status of patients in the preoperative period: a randomized, prospective clinical trial. J Anesth. 2016;30:842-9.
-
4Sada F, Krasniqi A, Hamza A, et al. A randomized trial of preoperative oral carbohydrates in abdominal surgery. BMC Anesthesiol. 2014;14:93.
-
5Pignaton W, Braz JR, Kusano PS, et al. Perioperative and anesthesia-related mortality: an 8-year observational survey from a tertiary teaching hospital. Medicine (Baltimore). 2016;95:e2208.
-
6International Surgical Outcomes Study G. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016;117:601-9.
-
7Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
-
8Bannister WK, Sattilaro AJ. Vomiting and aspiration during anesthesia. Anesthesiology. 1962;23:251-64.
-
9Roberts RB, Shirley MA. Reducing the risk of acid aspiration during cesarean section. Anesth Analg. 1974;53:859-68.
-
10Sutherland AD, Stock JG, Davies JM. Effects of preoperative fasting on morbidity and gastric contents in patients undergoing day-stay surgery. Br J Anaesth. 1986;58:876-8.
-
11Vieira AM, Rios RC, Brandão ACA, et al. Water ingestion and residual gastric content evaluation in pediatric patients undergoing elective surgeries. Rev Bras Anestesiol. 1997;47:283-7.
-
12Lewis P, Maltby JR, Sutherland LR. Unrestricted oral fluid until three hours preoperatively: effect on gastric fluid volume and pH. Can J Anaesth. 1990;37:S132.
-
13Sutherland AD, Maltby JR, Sale JP, et al. The effect of preoperative oral fluid and ranitidine on gastric fluid volume and pH. Can J Anaesth. 1987;34:117-21.
-
14Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology 2017;126:376-93.
-
15Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556-69.
-
16Perlas A, Davis L, Khan M, et al. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113:93-7.
-
17Bouvet L, Mazoit JX, Chassard D, et al. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;14:1086-92.
-
18Bolondi L, Bortolotti M, Santi V, et al. Measurement of gastric emptying time by real- time ultrasonography. Gastroenterology. 1985;89:752-9.
-
19Perlas A, Chan VW, Lupu CM, et al. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82-9.
-
20Arzola C, Perlas A, Siddiqui NT, et al. Bedside gastric ultrasonography in term pregnant women before elective cesarean delivery: a prospective cohort study. Anesth Analg. 2015;121:752-8.
-
21Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357-63.
-
22Haruma K, Kusunoki H, Manabe N, et al. Real-time assessment of gastroduodenal motility by ultrasonography. Digestion. 2008;77(Suppl 1):48-51.
-
23Aoki S, Haruma K, Kusunoki H, et al. Evaluation of gastric emptying measured with the 13C-octanoic acid breath test in patients with functional dyspepsia: comparison with ultrasonography. Scand J Gastroenterol. 2002;37:662-6.
-
24Benini L, Sembenini C, Heading RC, et al. Simultaneous measurement of gastric emptying of a solid meal by ultrasound and by scintigraphy. Am J Gastroenterol. 1999;94:2861-5.
-
25Arzola C, Carvalho JC, Cubillos J, et al. Anesthesiologists' learning curves for bedside qualitative ultrasound assessment of gastric content: a cohort study. Can J Anaesth. 2013;60:771-9.
-
26Bisinotto FM, Pansani PL, Silveira LA, et al. Qualitative and quantitative ultrasound assessment of gastric content. Rev Assoc Med Bras (1992). 2017;63:134-41.
-
27Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003. CD004423.
-
28Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56-62.
-
29Richardson CT, Walsh JH, Hicks MI, et al. Studies on the mechanisms of food-stimulated gastric acid secretion in normal human subjects. J Clin Invest. 1976;58:623-31.
-
30Imbelloni LE, Pombo IA, Filho GB. Reduced fasting time improves comfort and satisfaction of elderly patients undergoing anesthesia for hip fracture. Rev Bras Anestesiol. 2015;65:117-23.
-
31Yildiz H, Gunal SE, Yilmaz G, et al. Oral carbohydrate supplementation reduces preoperative discomfort in laparoscopic cholecystectomy. J Invest Surg. 2013;26:89-95.
-
32Yagci G, Can MF, Ozturk E, et al. Effects of preoperative carbohydrate loading on glucose metabolism and gastric contents in patients undergoing moderate surgery: a randomized, controlled trial. Nutrition. 2008;24:212-6.
-
33Schmidt AR, Buehler P, Seglias L, et al. Gastric pH and residual volume after 1 and 2 h fasting time for clear fluids in children. Br J Anaesth. 2015;114:477-82.
-
34Ljungqvist O. Modulating postoperative insulin resistance by preoperative carbohydrate loading. Best Pract Res Clin Anaesthesiol. 2009;23:401-9.
-
35Holte K, Nielsen KG, Madsen JL, et al. Physiologic effects of bowel preparation. Dis Colon Rectum. 2004;47:1397-402.
-
36Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-8.
-
37Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS(R)) society recommendations. Clin Nutr. 2012;31:783-800.
-
38Cubillos J, Tse C, Chan VW, et al. Bedside ultrasound assessment of gastric content: an observational study. Can J Anaesth. 2012;59:416-23.
-
39Perlas A, Van de Putte P, Van Houwe P, et al. I-AIM framework for point-of-care gastric ultrasound. Br J Anaesth. 2016;116:7-11.
-
40Nakamura M, Uchida K, Akahane M, et al. The effects on gastric emptying and carbohydrate loading of an oral nutritional supplement and an oral rehydration solution: a crossover study with magnetic resonance imaging. Anesth Analg. 2014;118:1268-73.
-
41Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456-62.
Publication Dates
-
Publication in this collection
Nov-Dec 2018
History
-
Received
11 Jan 2018 -
Accepted
15 June 2018 -
Published
20 July 2018