Abstracts
We provide morphological and morphometric descriptions of the developmental stages of Parauchenipterus galeatus, from the floodplain of the Upper Paraná River. Specimens were obtained by induced spawning. The species has large adhesive eggs with a double membrane. The incubation period is long, 65 hours at 27°C. The larvae are well developed at hatching, with relatively rapid larval development. Analysis of the morphometric data showed that the body parts of P. galeatus grow proportionately.
Morphological development; Parauchenipterus galeatus; larvae and juveniles; floodplain; Paraná River
Este trabalho teve por objetivo descrever morfológica e morfometricamente os estágios de desenvolvimento de Parauchenipterus galeatus capturados na planície de inundação do alto rio Paraná. O material utlizado foi obtido através de desova induzida. A espécie apresentou ovos grandes, adesivos e com membrana dupla. Período de incubação longo (65 horas a 27°C). As larvas são bem desenvolvidas no momento da eclosão, apresentando desenvolvimento larval relativamente rápido. A análise dos dados morfométricos revela que P. galeatus apresenta crescimento proporcional das partes do corpo.
descrição morfológica; Parauchenipterus galeatus; larvas e juvenis; planície de inundação; rio Paraná
MORPHOLOGICAL DESCRIPTION OF THE DEVELOPMENTAL STAGES OF Parauchenipterus galeatus (LINNAEUS, 1766) (SILURIFORMES, AUCHENIPTERIDAE) ON THE FLOODPLAIN OF THE UPPER PARANÁ RIVER
SANCHES, P. V.,1 NAKATANI, K.2 and BIALETZKI, A.1
1Departamento de Biologia/Universidade Estadual de Maringá, Av. Colombo,
5790, Bloco G-90, CEP 87020-900, Maringá, PR, Brazil
2Departamento de Biologia/Nupélia/Universidade Estadual de Maringá, Av. Colombo,
5790, Bloco G-90, CEP 87020-900, Maringá, PR, Brazil
Correspondence to: Paulo Vanderlei Sanches, Departamento de Biologia/Universidade Estadual de Maringá, Av.
Colombo, 5790, Bloco G-90, CEP 87020-900, Maringá, PR, Brazil
Received June 02, 1998 Accepted November 19, 1998 Distributed September 10, 1999
(With 4 figures)
ABSTRACT
We provide morphological and morphometric descriptions of the developmental stages of Parauchenipterus galeatus, from the floodplain of the Upper Paraná River. Specimens were obtained by induced spawning. The species has large adhesive eggs with a double membrane. The incubation period is long, 65 hours at 27°C. The larvae are well developed at hatching, with relatively rapid larval development. Analysis of the morphometric data showed that the body parts of P. galeatus grow proportionately.
Key words: Morphological development, Parauchenipterus galeatus, larvae and juveniles, floodplain, Paraná River.
RESUMO
Descrição morfológica dos estágios de desenvolvimento de Parauchenipterus galeatus (Linnaeus, 1766) (Siluriformes, Auchenipteridae) na planície de inundação do alto rio Paraná
Este trabalho teve por objetivo descrever morfológica e morfometricamente os estágios de desenvolvimento de Parauchenipterus galeatus capturados na planície de inundação do alto rio Paraná. O material utlizado foi obtido através de desova induzida. A espécie apresentou ovos grandes, adesivos e com membrana dupla. Período de incubação longo (65 horas a 27°C). As larvas são bem desenvolvidas no momento da eclosão, apresentando desenvolvimento larval relativamente rápido. A análise dos dados morfométricos revela que P. galeatus apresenta crescimento proporcional das partes do corpo.
Palavras-chave: descrição morfológica, Parauchenipterus galeatus, larvas e juvenis, planície de inundação, rio Paraná.
INTRODUCTION
The study of the initial phases of the life cycle is fundamentally important, as much for the taxonomy as for the ecology of a species. In addition to allowing proper identification, this study leads to better understanding of the relationships between organisms and their environment. The knowledge of biological parameters gained from such studies has provided a foundation for icthyology as well as fisheries biology.
Studies on the distribution and abundance of the ichthyoplankton have yielded important information regarding spawning locales and seasons (Nakatani, 1994), as well as improved understanding of reproductive dynamics.
Morphometric studies (studies of form relative to size) of the eggs and larvae of fishes are an extremely valuable tool, mainly in taxonomic studies. The great morphological similarity among different taxonomic groups has been the main obstacle to identification of larvae, mainly larvae collected in the natural environment. Analysis of morphometric measurements allows us to compare the different developmental stages within and between species, and together with other characteristics, permits correct identification of the larvae.
Parauchenipterus galeatus, the object of this study, is popularly known in the region as the cangati. It is widely distributed in South America and is common in the Amazon, Orinoco, Paraguay, São Francisco, and Paraná basins (Fowler, 1950; Mees, 1974; Nomura, 1984; Britski et al., 1988). Nevertheless, P. galeatus has only recently arrived in the sub-basin of the Upper Paraná River. Until about 1982, Sete Quedas Falls near Guaíra in the State of Paraná functioned as a natural barrier separating two icthyofaunistic provinces that differed markedly in number of species (Bonetto, 1986). Upon formation of the reservoir for the Itaipu Hydroelectric Power Plant, this barrier was submerged, allowing access of P. galeatus and other species to this new environment.
Parauchenipterus galeatus differs from most other species in certain peculiarities of its mode of reproduction. Like other members of the family Auchenipteridae, P. galeatus has internal fertilization and marked sexual dimorphism, represented in males by a modification in the form of an intromittent organ in the first ray of the anal fin. Females have a saclike structure in the oviduct where spermatozoa are stored after copulation, and fertilization occurs only at the moment of spawning (Chacon & Mendes-Filho, 1972).
In spite of its wide distribution, few investigators have studied this species, and only Chacon (1975) has treated its initial developmental stages. The objective of the present study was to describe morphometrically and meristically the initial developmental stages of larvae and juvenile P. galeatus from the floodplain of the Upper Paraná River.
MATERIALS AND METHODS
The material used to describe the embryonic and larval stages of P. galeatus was obtained by induced spawning carried out at the Advance Research Base of the Center for Research in Limnology, Ichthyology, and Aquaculture (Nupélia), at Porto Rico, Paraná.
Fertilized females caught on the floodplain of the Upper Paraná River were treated with hypophyseal hormone.
For analysis and characterization of the different stages, collections were made at 4 hour intervals during the embryo stage, and at 8 hour intervals during the larval stage. Illustrations were made with the aid of a stereomicroscope fitted with a camera lucida.
The different stages of larval development were characterized according to the degree of flexion of the terminal region of the notochord and development of the fins, following the terminology proposed by Ahlstrom & Ball (1954) and Kendall et al. (1984), into yolk-sac, preflexion, flexion, and postflexion larval stages, and the juvenile stage.
Morphometric data for the eggs were obtained for external diameter (measurement of the diameter of the outer membrane), internal diameter (the diameter beginning at the inner membrane), diameter of the yolk sac (diameter of the yolk), perivitelline space (between the inner membrane and the yolk sac), and intermembrane space (the space between the outer and inner membranes), using a stereomicroscope fitted with an ocular micrometer (Fig. 1).
Outline of morphometrical measurements and counts in A) eggs, B) larvae and C) juveniles of Parauchenipterus galeatus.
The morphometry of larvae and juveniles was measured for 193 individuals, 4.0 to 18.6 mm standard length, according to Ahlstrom & Moser (1976). We measured standard length (SL), head length (HL), body depth (BD), predorsal distance (PDL), preanal distance (PAL), head depth (HD), snout length (SL), and eye diameter (ED) (Fig.1).
For analyses of body relationships, the morphometric data were related to standard length and head length during development. The relationships for body depth, head length, and eye diameter followed the categories proposed by Leis & Trnski (1989).
Morphometric development was analyzed by simple regression applied to the log-transformed data, which were fitted to the equation y = axb to obtain the values of b, which indicates the type of relationship among the variables (Peres-Neto, 1995). We also obtained meristic data such as the number of myomeres and fin rays (Fig. 1).
RESULTS AND DISCUSSION
Embryonic period
After hydration, the eggs of this species are large and have a double membrane; the outer membrane is adhesive. The eggs had a mean external diameter of 3.20 mm, internal diameter 2.67 mm, yolk diameter 1.6 mm, perivitelline space 0.47 mm, and intermembrane space 0.33 mm. In this stage the eggs are well developed and organized in an animal pole, represented by the blastodisc, and a vegetative pole (Fig. 2A). Approximately 4 hours after spawning, the eggs are in an advanced stage of division of the blastodisc, and the vegetative pole appears granular (Fig. 2B).
Stages of embryonic development: A) recently spawned; B) 4 hours; C) 16 hours; D) 38 hours after spawning.
The presence of a double membrane is not exclusive to this species. According to Sato (personal communication), most of the Siluriformes have this characteristic, though its function is poorly understood.
The larger egg diameter may be related to the mode of reproduction of this species, which has internal fertilization. According to Vazzoler (1996), P. galeatus has low fecundity and an oocyte mean diameter of 1.62 mm. Other species with a similar mode of reproduction, such as Ageneiosus ucayalensis and Ageneiosus valenciennesi, have even larger oocytes, 1.85 mm and 1.74 mm respectively.
The diameter of the oocyte appears to be related to reproductive strategy, in this as well as other species inhabiting the Upper Paraná River floodplain. Suzuki (1992) confirmed this trend, showing that species having oocytes with diameters greater than 1.50 mm have some form of strategy for protection of the eggs.Hoplias malabaricus (traíra) and Serrasalmus marginatus (piranha), for example, build nests where they deposit their eggs, and have oocytes with diameters greater than 2.00 mm (2.45 and 2.03 mm respectively). Another fact supporting this trend is that species with complete spawning and no parental care of the young, such as Hypophytalmus edentatus (mapará) and Plagioscion squamosissimus (curvina), have oocytes with diameters of 0.75 and 0.51 mm respectively.
Egg size and fecundity tend to be inversely proportional in different species (Blaxter, 1988). According to Blaxter, the influence of initial egg size on survival and development has important ecological implications, i.e. large eggs will give rise to larger larvae with large yolk sacs. Although the capacity for locomotion of such larvae may be reduced, the possession of large yolk sacs prolongs the period of endogenous feeding, so that the individual is more developed when it first begins to feed exogenously.
After about 16 hours of incubation, the embryonic axis is defined. The head and tail regions are differentiated, with formation of somites and the optic vesicle (Fig. 2C). After 24 hours the tail of the embryo is completely free, the head is differentiated, the auditory vesicle (which will cover the otoliths) is developing, and the eyes are becoming pigmented.
Pigmentation appears after about 38 hours of development, when the embryo has several chromatophores on the yolk sac, body, and head. In this stage the mouth becomes differentiated, and formation of the maxillary barbels and the opercular opening begins. The embryonic fins (finfold), myomeres, and notochord are evident (Fig. 2D).
Near hatching, the embryo is well developed, with the mouth and intestine formed, the maxillary barbels developing, pigmented eyes, and chromatophores scattered over the body and yolk sac.
Hatching occurs after about 64 hours of incubation,at a water temperature of 27-28°C, when the larvae are well developed. This is a long incubation period, since for species such as Prochilodus scrofa (curimba), incubation lasts for about 22 hours (Cavicchioli & Leonhardt, 1993), and 18-19 hours in Pseudoplatystoma corruscans (pintado) at water temperatures of 25-26°C (Cardoso et al., 1995).
This long incubation period may be related to the greater diameter of the oocyte. Sargent et al. (1987) observed that larger eggs develop more slowly. Nevertheless, slow development, which might be considered disadvantageous for the species since the eggs run a greater risk of predation than the larvae, is compensated by the more advanced development of the larva at hatching.
Larval period
Yolk Sac Larval stage
This stage extends from hatching to the beginning of absorption of the yolk sac. In most cases this period is characterized by extensive morphological and physiological development, during which all the structures necessary for vision, food capture and processing, and gills, among others, are formed (Ahlstrom & Ball, 1954). Nevertheless for this species, because of the advanced development of the larvae at hatching, this stage is relatively short since the larvae hatch with most of these structures already formed.
Soon after hatching, the larvae have a large yolk sac, the mouth and intestine formed, the nares differentiated, and the maxillary and mentonian barbels developing. The anterior part of the eyes is pigmented, and chromatophores are scattered over the body, head, and yolk sac. The terminal region of the notochord is slightly flexed, however formation of the hypural bones is not observed (Fig. 3A).
Development stages of Parauchenipterus galeatus: A) yolk sac larval stage (4.67 mm SL); B) preflexion stage (6.00 mm SL); C) beginning of flexion stage (7.20 mm SL); D) final flexion stage (7.80 mm SL); E) beginning of postflexion stage (8.70 mm SL); F) final postflexion stage (11.25 mm SL); G) juvenile (16.50 mm SL)
The larvae hatched at standard lengths from 4.2 to 5.3 mm (x = 4.9 ± 0.7), the head small to moderate in size (15.2% to 21.4%), the eye small (12.2% to 25%), and the body moderate (26.1% to 28.9%). The total number of myomeres varied from 36 to 41 (12 to 14 preanal and 24 to 26 postanal).
The greater degree of development of the larvae at hatching, because of the long incubation period, may be related to the diameter of the oocyte in this species. Balon (1986) observed that eggs with larger reserves give rise to more developed larvae, assuring the elimination of the larval stage, and resulting in the larvae having a phenotype similar to the adult by the time of first exogenous feeding.
Machado-Allison (1978) noted that in Loricariichthys typus the embryonic stage lasts about 96 hours, and its larval stage about 24 hours. In this respect P. galeatus shows intermediate characteristics, since although it hatches well developed it still possesses a larval stage.
The advanced degree of development may be an ecological advantage for the species, since the more developed larvae may be able to exploit more niches and be less susceptible to predation. Moreover, the pigmentation of the larvae at time of hatching favors camouflaging in the littoral vegetation, lessening their risk of being eaten.
Ecologically, these factors may compensate for the relatively low fecundity of this species, since they contribute to increased survival of the larvae, and more individuals reach the adult stage.
Preflexion stage
This stage normally extends from the beginning of absorption of the yolk sac to the beginning of flexion of the terminal region of the notochord, with formation of the hypural bones. In this stage the body varies from moderate to long (19.6% to 31.9%), the head from small to moderate (16.1% to 29.2%), and the eye from small to moderate (9.8% to 30.8%). The standard length varies between 5.5 mm and 6.8 mm (x = 6.26 ± 0.40). Myomeres are evident (36 to 41), with 11 to 15 preanal, and 25 to 29 postanal. The yolk sac and a large, sub-inferior mouth (maxilla larger than mandible) are present. The maxillary and lateral mentonian barbels are developing, and the median barbels are beginning to form. The intestine is formed, with the anal opening in the anterior third of the body. The notochord and swim bladder are evident. The pectoral fin is beginning to form and is covered by the operculum. Chromatophores are scattered over the entire body. The embryonic fin is present (Fig. 3B).
Flexion stage
This stage lasts from the beginning of flexion of the terminal region of the notochord until flexion is complete. The standard length varies from 6.0 mm to 7.8 mm (x = 6.51 ± 0.57). The body varies from long to moderate (15.3% to 28.6%), the head from small to moderate (19.9% to 29.6%), and the diameter of the eye is small (9.5% to 24.2%). The myomeres and the notochord are visible because of the transparency of the body; there are 36 to 41 myomeres, with 11 to 15 preanal and 25 to 29 postanal.
The yolk sac is present at the beginning of this stage. The swim bladder is visible. The mouth is large and sub-inferior, with the intestine opening at the anterior third of the body. The maxillary barbels reach to the last third of yolk sac, and the lateral mentonian barbels are well developed, reaching the end of the operculum. The median mentonian barbels are poorly developed. The pectoral fin is developing (Fig. 3C).
At the end of the flexion stage, the larva has the caudal fin with several segmented rays, and the pectoral fin differentiated and with rays forming (including the aculeum). The mouth is sub-inferior, the maxillary barbels reach the middle of the body, and the lateral mentonian barbels reach near to the end of the abdominal cavity. The median mentonian barbels are developing. The embryonic fin is present. The larva is strongly pigmented and the yolk sac is completely absorbed. The transition from endogenous to exogenous feeding occurs during this stage (Fig. 3D).
The transition between endogenous and exogenous feeding is a critical period for survival and development of the larvae. Studies under experimental conditions as well as in natural environments show that high mortality occurs when the larva passes through this phase. Mortality is influenced mainly by the quantity and quality of food, and by the size of the buccal opening in relation to the size of available prey (Blaxter, 1988).
Postflexion stage
This stage begins at the end of notochord flexion and continues until the hypural bones are completely formed. In this stage, the larvae have a standard length varying from 9.4 to 12.7 mm (x = 10.78 ± 1.16). The head is moderate to large (22.1% to 34.2%), body moderate (24.4% to 35.4%), and eye small (11.6% to 21.0%).
At the beginning of postflexion, the first rays of the anal and dorsal fin and the beginning of the pelvic bud are seen. The pectoral fin has the aculeum formed. The mouth is still sub-inferior, the mentonian barbels are developing, and the nares are evident. The myomeres are indistinct (Fig. 3E).
In the final stage of postflexion, the larva has a pair of siphon-shaped nares on the anterior part of the head. The maxillary barbels reach the midlength of the body, and the mentonian barbels are well developed.
The myomeres are indistinct. Vestiges of the embryonic fin are observed. The fins are completely formed, with the rays in the final stage of development; the pectoral and dorsal fins have i + 4 and i + 6 rays.
The anal fin varied from 18 to 26 rays, and the pelvic fin from 4 to 6. Pigmentation is distributed over the entire body and fins, and chromatophores are present on the embryonic fin. In this phase, the mouth shifts from sub-inferior to terminal (Fig. 3F).
Juvenile stage
This period lasts from the formation of all the fin rays to the first reproduction of the individual. At approximately 16.0 mm standard length, the individual has all the fin rays formed and assumes the definitive adult form. The duration of the larval period, according to Chacon (1975), is 480 hours (20 days), which is rapid relative to that of other species such as P. scrofa. Cavicchioli & Leonhardt (1993) reported that this species terminates its postflexion stage at 696 hours (29 days). This short larval phase results from the greater degree of development at hatching; therefore, metamorphosis in this species is not accentuated. According to Machado-Allison (1978), Pseudohemiodon laticeps does not appear to have a larval period, since the larvae hatch with adult characteristics, pigmented, and with advanced bone development.
Body depth is moderate (28% to 33.6%), the head moderate to large (28% to 35.5%), and the eye small (11.8% to 17.2%). The formerly terminal mouth comes to be prognathous, siphon-shaped nares are evident, the maxillary barbels reach the body midlength, and the lateral pair of mentonian barbels is more developed than the median pair, reaching approximately to the base of the pelvic fin (Fig. 3G). The pectoral fin with number of rays varying from i + 5 to i + 7, and aculeum serrate. Dorsal fin with i + 4 to i + 7, pelvic fin with 6 rays, and anal fin from 25 to 26 rays. Similar results were obtained by Britzki et al. (1988) for the same species.
The measured body relationships, considering all stages together, indicate a low degree of variation in body measurements, mainly in the initial developmental stages. The single exception is the head depth, which appears to be practically stable as the individual grows (Fig. 4). The slight variation in these values may be related to the fact that the species does not undergo a very accentuated metamorphosis, mainly in the more advanced developmental stages.
Fig.4 Body relations between: A) head lenght; B) body deepth; C) predorsal lenght; D) preanal lenght in relation to standard length; E) head deepth; F) eye diameter in relation to head length for larvae and juveniles of Parauchenipterus galeatus.G) snout length;
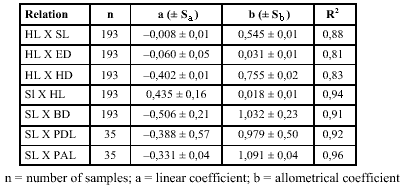
The values for b obtained in the regression analysis (Table 1) show that the species has a positive allometry (b > 1) for body depth and preanal distance, that is these measurements increase proportionately more than the standard length. In relation to head length, the species has negative allometry (b < 1) for snout length, eye diameter, and head depth, showing a greater growth of the head in relation to these measurements. It is worth emphasizing that the values quite close to 1 in the measurements of body depth, preanal distance, and predorsal distance indicate that in this species, growth in relation to standard length is nearly isometric.
Values for potential equation (y = axb) and r2 (determination coefficient) for logarithm data of morphometrical variables in relation to head and standard lengths for larvae and juveniles of Parauchenipterus galeatus.
Acknowledgements The authors are grateful to the Center for Research in Limnology, Ichthyology, and Aquaculture (Nupélia) for facilities to carry out this work; to CAPES for awarding a study grant; to PADCT/CIAMB for financial support for the research project; and to the coordinators of the Postgraduate Course in Ecology of Continental Aquatic Environments, State University of Maringá, for their support during all stages of this work. Janet W. Reid translated the manuscript into English.
- AHLSTROM, E. H. & BALL O. P., 1954, Description of eggs and larvae of jack mackerel (Trachurus symmetricus) and distribution and abundance of larvae in 1950 and 1951. Fish. Bull., 56: 209-245.
- AHLSTROM, E. H. & MOSER, H. G., 1976, Eggs and larvae of fish and their role in systematic investigations and in fisheries. Rev. Trav. Inst. Pech. Marit., 40(3): 379-398.
- BALON, E. K., 1986, Patterns in the reproductive styles in fishes. In: G. W. Poots & R. J. Wooton (eds.), Fish Reproduction: estrategies and tactics Academic Press, London, pp.35-54.
- BLAXTER, J. H. S., 1988, Pattern and variety in development. In: W. S. Hoar & D. J. Randall (eds.), Fish Physiology. Academic Press Inc., San Diego, vol. 11, pte. A, pp. 1-48: eggs and larvae.
- BONETTO, A. A., 1986, The Parana river system. In: B. R. Daves & K. F. Walker (eds.), The ecology of river system, Dr. Junk Publishers, Dordrecht, pp. 541-555.
- BRITSKI, H. A., SATO, Y. & ROSA, A. B. S., 1988, Manual de Identificação de Peixes da região de Três Marias: com chaves de identificação para os peixes da bacia do São Francisco 3a ed., CODEVASF, Brasília, 115p.
- CARDOSO, E. L., ALVES, M. S. D., FERREIRA, R. M. A. & GODINHO, H. P., 1995, Embryogenesis of neotropical freshwater Siluriforme Pseudoplatystoma coruscans. Aquat. Living Resour., 8: 343-346.
- CAVICCHIOLI, M. & LEONHARDT, J. H., 1993, Estudo do desenvolvimento morfológico de larvas de Prochilodus scrofa (Steindachner, 1891), obtidos de reprodução induzida. Revista UNIMAR, 15(suplemento): 109-124.
- CHACON, J. O., 1975, Embryonic and early larval of Cangati catfish Trachycorystes galeatus, Linnaeus, 1756, at the Anamari fish culture station, Maranguape, Ceará, Brazil. Rev. Bras. Biol., 35(4): 737-744.
- CHACON, J. O. & MENDES-FILHO, A., 1972, Estudo do aparelho genital de Cangatí, Tracchycorystes galeatus, LINNAEUS, 1766. Ciênc. Cult.,(24) 6: 531-536.
- FOWLER, H. W., 1950, Os peixes de água doce do Brasil. Arq. Zool. Est. S. Paulo, 6(2): 205-404.
- KENDALL, A. W. Jr., AHLSTROM, E. H. & MOSER, H. G., 1984, Early life history stages of fishes and their characters. Spec. Publi. Amer. Soc. Ichthyol. and Herp., 1: 11-22
- LEIS, J. M. & TRNSKI, T., 1989, The larval of Indo-Pacific shorefishes University of Hawaii press, Honolulu, 371p.
- MACHADO-ALLISON, A., 1978, Los peces de los llanos de Venezuela: Un ensayo sobre su história natural Universidad Central de Venezuela, Caracas, Consejo de desarrollo científico y humanistico, 140p.
- MEES, G. F., 1974, The Auchenipteridae and Pimelodidae of Suriname (Pisces, Nematognathi). Zoologische Verhand Elingen, 132, pp.1-256, Rijiksmuseum Natuurliske Historie, Lieden.
- NAKATANI, K., 1994, Estudo do ictioplâncton no reservatório de Itaipu (rio Paraná Brasil): levantamento das áreas de desova Tese de Doutorado. Universidade Federal do Paraná, Curitiba, 254p.
- NOMURA, H., 1984, Dicionário de peixes do Brasil Editerra, Brasília, 482p.
- PERES-NETO, P. R., 1995, Introdução a análises morfométricas. In: P. R. Peres-Neto, J. L. Valentin & F. A. S. Fernandez (eds.), Tópicos em tratamentos de dados biológicos Oecologia Brasiliensis, UFRJ, Rio de Janeiro, vol., 2, pp. 57–89.
- SARGENT, R. C., TAYLOR, P. D. & GROSS, M. R., 1987, Parental care and evolution of egg size in fishes. Am. Nat., 121(1): 32-46.
- SUZUKI, H. I., 1992, Variações na morfologia ovariana e no desenvolvimento do folículo de peixes teleósteos da bacia do rio Paraná no trecho entre a foz do rio Paranapanema e a do rio Iguaçu Dissertação de Mestrado, Universidade Federal do Paraná, Curitiba, 190p.
- VAZZOLER, A. E. A. M., 1996, Biologia da reprodução de peixes teleósteos: Teoria e prática EDUEM, Maringá, 169p.
Publication Dates
-
Publication in this collection
01 June 2001 -
Date of issue
Aug 1999
History
-
Accepted
19 Nov 1998 -
Received
02 June 1998