Abstracts
OBJECTIVE: To study the susceptibility status of Aedes aegypti to the organophosphate insecticide temephos. METHODS: Samples of Ae. aegypti larvae were obtained, using ovitraps, from eight cities of the Federal District, central Brazil, in 2000 and 2001. Larvae were submitted to the diagnostic dose of 0.012 mg/l temephos, as recommended by standard World Health Organization methodology. Field populations were tested in parallel with reference strains Rockefeller and DIVAL, from the Environmental Surveillance Directory (DIVAL) insectary. The concentration and purity of temephos solutions were verified by gas chromatography. Correlation calculations were performed using StatView - SAS Institute Inc., version 5. Student's t test was used for detecting differences in susceptibility, with significance levels of alpha=0.05. RESULTS: In 2000, Ae. aegypti larvae populations from Taguatinga, Guará, and Núcleo Bandeirante showed resistance to temephos, with mortality ranging from 54.1 to 63.4%. The populations from Gama, Planaltina, and Sobradinho showed altered levels of susceptibility (mortality ranging from 83.6 to 92.8%). The population from Ceilândia was the only susceptible one, with 98% mortality. In 2001, all populations tested were resistant (44.4 to 66.4% mortality). No significant correlation was found between the susceptibility of populations and the distance between the cities of origin, or the amount of insecticide applied in the years preceding the study. CONCLUSIONS: Ae. aegypti susceptibility to temephos is changing in the Federal District. It is essential to continue monitoring the resistance of this vector to insecticides in order to ensure the efficiency of programs aimed at vector control and at the protection of human health.
Aedes; Temephos; Insecticide resistance; Vector control; Insect vectors
OBJETIVO: Estudar o padrão de suscetibilidade do Aedes aegypti ao inseticida organofosforado temefós. MÉTODOS: Amostras de larvas de Ae. aegypti foram obtidas com armadilhas para oviposição, em oito cidades do Distrito Federal, nos anos 2000 e 2001. As larvas foram submetidas à dose diagnóstica de 0,012 mg/l de temefós, segundo metodologia padronizada pela Organização Mundial da Saúde. As populações de campo foram testadas em paralelo com a cepa de referência Rockefeller e a cepa DIVAL, do insetário da Diretoria de Vigilância Ambiental. A concentração e a pureza das soluções de temefós foram analisadas por cromatografia gasosa. Os cálculos de correlação foram determinados pelo programa StatView - SAS Institute Inc., versão 5. Utilizou-se o teste t de Student para verificar diferenças de suscetibilidade, com níveis de significância, alfa=0,05. RESULTADOS: Em 2000, as populações de larvas de Ae. aegypti nas cidades de Taguatinga, Guará e Núcleo Bandeirante apresentaram-se resistentes ao temefós, com mortalidade de larvas entre 54,1 e 63,4%. As populações do Gama, Planaltina e Sobradinho apresentaram alterações nos níveis de suscetibilidade (mortalidade de 83,6 a 92,8%). A população de Ceilândia foi a única suscetível, com 98% de mortalidade. Em 2001, todas as populações testadas mostraram-se resistentes (44,4 a 66,4% de mortalidade). Nenhuma correlação significativa foi encontrada entre a suscetibilidade das populações e a distância entre essas cidades ou a quantidade de inseticida aplicado nos anos anteriores ao estudo. CONCLUSÕES: Os níveis de suscetibilidade do Ae. aegypti ao temefós vêm se alterando no Distrito Federal. É essencial a continuidade de programas de monitoramento da resistência desse vetor aos inseticidas para se garantir a eficiência dos programas de controle e a proteção da saúde humana.
Aedes; Temefós; Resistência a inseticidas; Controle de vetores; Insetos vetores
ORIGINAL ARTICLES
Susceptibility of Aedes aegypti larvae to the insecticide temephos in the Federal District, Brazil
Maria do Socorro Laurentino de CarvalhoI; Eloísa Dutra CaldasII; Nicolas DegallierIII; Paulo de Tarso Ribeiro VilarinhosI; Luís César Kenupp Rodrigues de SouzaIV; Maria Amélia Cavalcanti YoshizawaI; Monique Britto KnoxI; Cristiane de OliveiraI
IVigilância Ambiental do Distrito Federal. Brasília, DF, Brasil
IIUniversidade de Brasília. Brasília, DF, Brasil
IIIInstitut de Recherche pour le Développement. Paris, França
IVLaboratório Central de Saúde Pública do Distrito Federal. Brasília, DF, Brasil
Correspondence Correspondence to Maria do Socorro Laurentino de Carvalho Diretoria de Vigilância Ambiental Estrada Contorno do Bosque Lote 4. SAIN 70620-000 Brasília, DF, Brasil E-mail: mslcarvalho@abordo.com.br
ABSTRACT
OBJECTIVE: To study the susceptibility status of Aedes aegypti to the organophosphate insecticide temephos.
METHODS: Samples of Ae. aegypti larvae were obtained, using ovitraps, from eight cities of the Federal District, central Brazil, in 2000 and 2001. Larvae were submitted to the diagnostic dose of 0.012 mg/l temephos, as recommended by standard World Health Organization methodology. Field populations were tested in parallel with reference strains Rockefeller and DIVAL, from the Environmental Surveillance Directory (DIVAL) insectary. The concentration and purity of temephos solutions were verified by gas chromatography. Correlation calculations were performed using StatView - SAS Institute Inc., version 5. Student's t test was used for detecting differences in susceptibility, with significance levels of a=0.05.
RESULTS: In 2000, Ae. aegypti larvae populations from Taguatinga, Guará, and Núcleo Bandeirante showed resistance to temephos, with mortality ranging from 54.1 to 63.4%. The populations from Gama, Planaltina, and Sobradinho showed altered levels of susceptibility (mortality ranging from 83.6 to 92.8%). The population from Ceilândia was the only susceptible one, with 98% mortality. In 2001, all populations tested were resistant (44.4 to 66.4% mortality). No significant correlation was found between the susceptibility of populations and the distance between the cities of origin, or the amount of insecticide applied in the years preceding the study.
CONCLUSIONS: Ae. aegypti susceptibility to temephos is changing in the Federal District. It is essential to continue monitoring the resistance of this vector to insecticides in order to ensure the efficiency of programs aimed at vector control and at the protection of human health.
Keywords: Aedes. Temephos. Insecticide resistance. Vector control. Insect vectors.
INTRODUCTION
Mosquito-transmitted diseases are responsible for high rates of morbidity and mortality in Brazil. Dengue incidence rates in the last years show an increasing trend. In 2002, 672,371 cases of classical dengue and 2,090 cases of hemorrhagic dengue, of which 96 ended in death, were reported countrywide.5 Brazil has the largest area of occurrence and transmission of yellow fever in the world, which favors the occurrence of epidemics, and increases the risk of urbanization of this disease.6 The vectors of urban yellow fever and dengue are mosquitoes of the Aedes genus, the most important species of which is the Aedes (Stegomyia) aegypti (Linné, 1762).
The first foci of Ae. aegypti in the Federal District, central Brazil, were identified in 1985 and 1986. However, the mosquito established itself in the region only in 1994.17 The first autochthonous dengue cases appeared in 1997, when five cases were notified. Since then, dengue incidence has been on the rise. In 2002, 6,698 cases of the disease were notified, of which 1,463 were autochthonous.4,5
The main strategy for controlling Ae. aegypti in Brazil has been the intensive use of insecticides. In the Federal District, the consumption of the organophosphate insecticide temephos, used in the control of larvae, has been increasing progressively in the last years. Internal reports of the Environmental Surveillance Directory (DIVAL, unpublished) show that, of the 33,833 kg of temephos used between 1997 and 2001, approximately 60% were used in the last year alone.
The frequent use of insecticides may lead to the development of mosquitoes resistant to these compounds, hampering control and favoring the transmission of diseases. The number of cases of insecticide resistance is increasing in certain Asian, Caribbean, and Central and South American countries.14 In Brazil, studies of the resistance of Ae. aegypti to insecticides have been conducted in the states of São Paulo, Goiás, and Mato Grosso do Sul.10,11
In the present study we evaluate the resistance of Ae. aegypti populations in the Federal District to the organophosphate insecticide temephos.
METHODS
Samples of mosquito populations were obtained from eggs collected in ovitraps.9 Eggs were collected in randomly selected residences in the urban area of eight Federal District cities (Figure), in 2000 and 2001. Ovitraps were placed in the surroundings of the residence, in shaded places, and close to other mosquito breeding sites, such as plant vases, remaining for eight to 10 days.
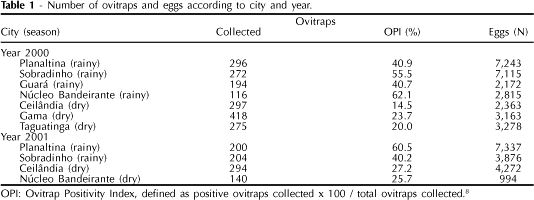
In 2000, ovitraps were installed in the cities of Planaltina, Sobradinho, Guará, Núcleo Bandeirante, Candangolândia, Gama, Ceilândia, and Taguatinga. This procedure was repeated in 2001 in these cities, with the exception of Gama and Taguatinga. Due to the close proximity of Núcleo Bandeirante and Candangolândia, and to the small number of eggs collected in the latter, the populations of these two cities were grouped into a single strain - the Núcleo Bandeirante strain (Table 1). In order to quantify the number of residences with ovitraps, the criterion established by the National Health Foundation (Funasa) - a minimum of 50 ovitraps for cities with up to 20,000 residences and a maximum of 300 for cities with over 500,000 residences - was adopted. Due to the low rates of mosquito infestation, approaching 1% in the dry season, two ovitraps were placed in each residence. In addition, during the dry season (May-September) the number of residences with ovitraps was increased by 20% so that a more representative sample of the mosquitoes could be obtained. In the city of Gama, where another investigation of Ae. aegypti oviposition was taking place (unpublished data), the number of traps was greater than in the other cities.
The paddles collected from the ovitraps were placed in Styrofoam boxes for three days for egg incubation. Eggs were counted using a stereoscopic microscope. Positive paddles were placed in polyethylene trays containing three liters of chlorine-free water and 1% alfalfa infusion, so as to facilitate the eclosion of eggs. The larvae generated were fed with autoclaved dog food. Adult mosquitoes (F0 generation) were fed with 10% glucose and with guinea pig blood, and were identified individually as to their species using a stereoscopic microscope. Mosquitoes identified as Ae. aegypti were used for breeding F1 and F2 generations. In the tests carried out with the F0 generations from Planaltina, Ceilândia, Sobradinho, and Gama in 2000, there was no need for species identification, since there was no record of the presence of Ae. albopictus in these areas until 2000. The F2 generation was used in 2000 because the number of larvae obtained in the F1 generation was insufficient for performing susceptibility tests.
Temephos solution (3 mg/l) were provided by Funasa or prepared from the 98% temephos standard solution (Prodelim Química). Solution concentration and purity were verified by gas chromatography using a flame photometric detector and a mass spectrometer detector (GC/FPD, Finnegan 9001; GC/Ion Trap Varian Saturn 2000). Calculated concentrations did not differ significantly from the theoretical concentration (3 mg/l). Tests performed on a same population using different temephos solutions did not show statistically significant differences in terms of mortality rates (p>0,05). Solutions were stored at -5ºC.
Susceptibility tests were carried out according to the methodology proposed by the World Health Organization.15 Each test included eight repetitions for exposed larvae and four for control larvae. 224 ml distilled water was placed into 400 ml plastic cups along with 1.0 ml of 3 mg/l temephos solution (exposed larvae) or 1.0 ml ethanol, PA grade (control larvae). 25 larvae between the third and the beginning of the fourth stage of development were placed into each cup. Final temephos concentration in the system was the diagnostic dose of 0.012 mg/l, which is capable of killing, with high probability, all susceptible Ae. aegypti larvae in a population unexposed to insecticide-driven selective pressure and in which resistant individuals are rare.14 Mortality was verified after 24h. Moribund larvae (presenting tremors, rigidity, or inability to reach the water surface when touched) were considered as dead.15 All surviving larvae were discarded.
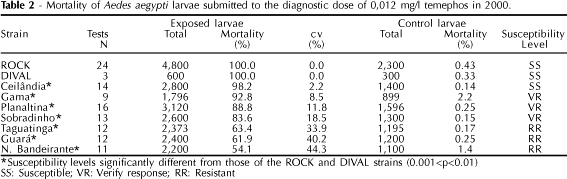
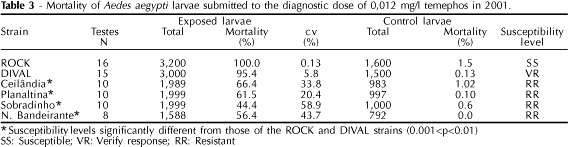
Parallel tests were carried out with a susceptible strain of Rockefeller Ae. aegypti (ROCK), provided by the Center for Medical Agricultural and Veterinary Entomology - U.S. Department of Agriculture at Gainesville, FL, and with the DIVAL strain, generated from mosquitoes from the Federal District and maintained in the DIVAL insectary since 1997. When mortality rates were equal to or higher than 98%, the population was considered as susceptible (SS); mortalities between 80 and 98% were regarded as requiring further verification of the response (VR), and when mortality rates were equal to or below 80%, the population was considered as resistant (RR).12 Mortality percentages were corrected using Abbot's formula when mortality among controls was between five and 20%. Tests were not considered when control mortality was above 20% or when the number of pupae reached 10%.15
Excel-MS 1998 software was used for statistical calculations and significance tests. Correlation tests were performed using StatView - SAS Institute Inc., version 5 software. Student's t test was used to verify significant susceptibility differences between populations. Significance levels of a=0.005 and p>a were considered for accepting the null hypothesis (H0), that is, no significant difference between the two samples. H0 was rejected for values below this threshold, that is, there is a significant difference between the two samples.
RESULTS
Table 1 shows the number of ovitraps collected, the ovitrap positivity index (OPI), and the number of eggs collected in each city in the two years of the study. All around, 1,867 of the 1,941 (96.2%) ovitraps installed in 2000, and 837 of the 842 (99.4%) installed in 2001 were recovered. Some of the ovitraps installed were removed by the human population or were without water. OPIs were higher in the rainy season than in the dry season in both years. OPIs were higher in 2001 than in 2000 in Planaltina (rainy season) and Ceilândia (dry season).
Tables 2 and 3 show Ae. aegypti larvae mortality according to the city of collection, for 2000 and 2001, respectively. In 2000, the mortality rates of all populations tested were significantly different from those of susceptible strains ROCK and DIVAL (0.001<p<0.01) (Table 2). The populations from Taguatinga, Guará, and Núcleo Bandeirante showed mortality rates below 80%, and were therefore resistant to temephos. The populations from Gama, Sobradinho, and Planaltina had altered susceptibility patterns, and were included in the 'verification of response' category (83.6 to 92.8% mortality). The population from Ceilândia was the only one susceptible to temephos (>98%). In all cities, the coefficient of variation (cv) between mortality tests in exposed larvae increased along with decreases in percentage mortality in each population, showing a high genetic variability among the individuals of resistant populations.
In 2001 (Table 3), the susceptibility levels of the field populations tested were different from those of the ROCK and DIVAL strains (0.01<p<0.05), and these populations were considered as resistant (mortality <80%). Percent mortality in most tested strains was below those of the previous year (Table 2), with the exception of Núcleo Bandeirante, where mortality was virtually unaltered between both years (p>0.05). Ceilândia showed a change in susceptibility status, from susceptible in 2000 (98.2%) to resistant (66.4%) in 2001 (p<0.001).
The smaller number of ovitraps installed in Planaltina and Sobradinho in 2001 if compared to 2000 (Table 1) did not compromise sampling, since the distribution of traps was homogeneous in both years. The mortality rates for Planaltina and Sobradinho decreased significantly in 2001 (p<0.001), when they were classified as resistant. These results are consistent with those observed in 2000, when these populations were already showing altered responses (Table 2).
The DIVAL strain showed a different response in 2001 when compared with the ROCK strain (Table 3). Such reduction was not detected in 2000, probably due to the small number of tests conducted that year (three tests, compared with 15 tests in 2001).
The distances between the cities studied ranged from 5 to 70 km (Figure). In order to evaluate a possible relationship between the distance between two cities and the significance levels obtained in terms of susceptibility, linear regression tests were performed. The regression coefficient obtained (R2=-0.101; p=0.16) shows the lack of a significant correlation between the distance between cities and the levels of susceptibility of their respective mosquito populations.
In order to determine the existence of a relationship between susceptibility and selective pressure, the total amount of temephos applied in each city in the one-year period preceding sample collection (available in the monthly Mosquito Control Activity reports of DIVAL and Funasa from 1999 and 2000) was analyzed. There was no record of the amount of insecticide applied in the different cities between January and August 1999 and April and September 2000. Generally speaking, the amount of insecticide applied in the one-year period preceding the 2001 sampling was twice that of the 2000 sampling (data not shown). The regression coefficient between the amount of insecticide applied in each city and percent mortality was R2=0.039, showing a weak, non significant correlation (p=0.56; a=0.05).
DISCUSSION
The higher Ae. aegypti oviposition rates in the rainy season observed in the present study were expected, and have been reported by other authors.5 During this period, the mosquito finds a greater abundance of water-filled deposits - its natural breeding site - and temperature and humidity conditions that favor its biological cycle.
The use of insecticides, associated with environmental management and educational measures should have been able to control mosquito infestations, leading to a reduction in oviposition rates in subsequent years in a same season; however, this did not occur in the period analyzed.
Strains resistant to a given chemical may appear as a result of genetic, operational, or biological factors. Genetic and biological factors are characteristic of different populations, and include the frequency and the dominant character of resistance genes, isolation, endogamy, and the population's reproductive potential.12 Operational factors are related to the use of insecticides, and may appear as a result of selective pressure or of failures in control operations.2
Resistance genes are rare, and appear after prolonged periods of selection, as individuals carrying susceptible alleles die.3 The degree of dominance of the resistance gene influences the growth of the population under selective pressure. When the resistance gene is recessive, growth is slower; when it is dominant, growth is faster.7
In 2000, populations with altered responses, showing a typical resistance profile, were detected in Taguatinga, Guará, and Núcleo Bandeirante (Table 2). The populations from Planaltina and Sobradinho, which showed an altered response in 2000, evolved to the resistance profile in 2001. Ceilândia, studied in the dry season, showed the greatest reduction in susceptibility in 2001, when the number of eggs collected was almost twice that of 2000.
The reduction in OPR observed in Sobradinho in 2001 may be a consequence of control measures adapted by the population and by the healthcare organ. On the other hand, a lower efficiency of these measures might have had an impact on the increase in OPR observed in Planaltina in 2000. In Núcleo Banderiante, the OPR and the number of eggs in the dry season (2001) was, approximately, 60% lower than in the rainy season (2000). This result was expected, since the rain favors the formation of breeding sites and the increase in mosquito populations.
The greater coefficient of variation in more resistant populations seem to indicate that these populations are heterogeneous in terms of resistance genes. By contrast, the ROCK strain, which is 100% susceptible, is a homogeneous population. The mosquitoes that gave origin to the DIVAL population were collected in 1997 from areas of the Federal District which had been exposed to insecticide application, and may have arrived at the insectary already with resistance genes. Campos & Andrade4 reported resistance or tolerance to temephos in insectary mosquitoes coming from environments never exposed to insecticide application.
Genetic factors may largely explain the differences in susceptibility between the tested populations. In addition, factors such as migration and amount of insecticide applied may also have had an impact on the number of surviving individuals and, consequently, on the evolution of resistance. The influence of migration takes place if susceptible mosquitoes from treated areas escape from treatment, enabling the permanence of susceptible alleles in population, or if there is the immigration of susceptible individuals to a population under selective pressure and where a process of resistance may be already underway.7 In the present study, however, no correlation between susceptibility levels and the amount of insecticide applied or the distance between cities was found. Rawlins13 also reported differences in the levels of temephos resistance in Ae. aegypti populations of nearby neighboring localities.
The increase in resistance is directly dependent on the selective pressure exerted by the insecticide. Since mosquito infestation levels are influenced by seasonality, the amount of temephos applied in each season varies, being greater in the rainy season, when infestation levels are higher. The weak correlation between susceptibility and the amount of insecticide applied found in the present study may, however, be due to the lack of available data regarding insecticide application in some of the months evaluated.
The results of the present study confirm the detection of Ae. aegypti populations with altered susceptibility to temephos reported in other surveys conducted in Brazil.10,11
The finding of temephos-resistant Ae. aegypti populations in the Federal District confirms the need for monitoring the susceptibility of these populations as one of the strategies for ensuring the efficacy of control programs for this vector in the area.
Once susceptibility levels are mapped, and the impact of resistance on the residual effect of temephos is evaluated in the field, it will be possible to adopt different control strategies, which may be specific for each site. In addition, it will be important to conduct surveys in the area of isolation, and to characterize resistance, in order to determine the biochemical mechanisms and to verify whether the results obtained using the diagnostic test are compatible with the results in terms of the alteration of these mechanisms.
The confirmation of the pattern of loss of susceptibility in the Federal District may, for example, indicate the need for changes in chemical control strategies. It is essential, however, that this change be accompanied by integrated control actions, including environmental control, the use of biological insecticides, and the participation of the community.
ACKNOWLEDGEMENTS
To the team of the entomology laboratory of the Federal District Environmental Surveillance Directory for their assistance with the installation of ovitraps and with the performance of susceptibility tests.
REFERENCES
Received on 25/3/2003. Reviewed on 9/3/2004. Approved on 10/5/2004.
*Based on the masters dissertation presented at the Faculdade de Ciências da Saúde, Universidade de Brasília, 2002. Study supported by OPAS/Funasa, with resources from the Programa de Erradicação do Aedes aegypti (PEAa).
- 1 Boletim Epidemiológico. Fundação Nacional da Saúde. Brasília (DF); 2001;1.
- 2. Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use time-mortality determinations in glass bottles. J Am Mosquito Control Assoc 1998;14:59-164.
- 3. Brown AWA. Insecticide resistence in mosquitoes: a pragmatic review. J Am Mosq Control Assoc 1986;2:23-140.
- 4. Campos J, Andrade CFS. Suscetibilidade larval de duas populações de Aedes aegypti a inseticidas químicos. Rev Saúde Pública 2001;35:232-6.
- 5. Dégallier N, Vilarinhos, PTR, Dusi RM. Aspectos eco-epidemiológicos da dengue e do Aedes aegypti no Distrito Federal, Brasil. Rev Saúde Distrito Federal 1998;9:67-71.
-
6[FUNASA] Fundação Nacional de Saúde. Programa Nacional de Controle da Dengue — PNCD. Brasília (DF); 2002. p. 51.
- 7. Georghiou GP, Taylor CE. Genetic and biological influences in the evolution of insecticide resistance. J Econ Entomol 1977;70:319-23.
- 8. Gomes AC. Medidas dos níveis de infestação urbana para Aedes (Stegomyia) aegypti e Aedes (Stegpmyia) albopictus em Programa de Vigilância Entomológica. IESUS 1998;7:49-57.
- 9. Jakob WL, Bevier GA. Application of ovitraps in the U.S. Aedes aegypti eradication program. Mosq News 1969;29:55-62.
- 10. Macoris MLG, Andrighetti MTM, Takaku L, Glasser CM, Garbeloto VC, Cirino VCB. Alteração da resposta de susceptibilidade de Aedes aegypti a inseticidas organofosforados em municípios do Estado de São Paulo, Brasil. Rev Saúde Pública 1999;33:521-2.
- 11. Macoris MLG, Camargo MF, Silva IG, Takaku L, Andrighetti MT. Modificação da susceptibilidade de Aedes (Stegomyia) aegypti ao temefós. Rev Patol Trop 1995;24:31-40.
-
12Organizacion Mundial de la Salud. Resistencia de vectores y reservorios de enfermidades a los plaguicidas. Informe del Comité de Expertos de la OMS en Insectcidas. Ginebra; 1976. (OMS - Serie de Informes Tecnicos, 585).
- 13. Rawlins S. Spatial distribution of insecticide resistance in Caribbean populations of Aedes aegypti and its significance. Rev Panam Salud Publica 1998;4:243-51.
-
14World Health Organization (WHO). Vector resistance to pesticides: fifteenth report of the WHO Expert Committee on Vector Biology and Control. Geneva; 1992. p. 61 ( WHO Technical Report Series, 818 ).
-
15World Health Organization (WHO). Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides: report of the WHO Expert Committee on Resistance of Vectors and Reservoirs of Diseases to Pesticides. Geneva; 1981.
- 16. Yoshizawa MAC. Diversidade e freqüência das espécies de culicídeos em criadouros no Distrito Federal. Rio de Janeiro: Fundação Oswaldo Cruz; 1995.
Publication Dates
-
Publication in this collection
18 Oct 2004 -
Date of issue
Oct 2004
History
-
Accepted
10 May 2004 -
Received
25 Mar 2003 -
Reviewed
09 Mar 2004