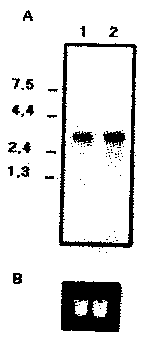Abstracts
In the search for Leishmania recombinant antigens that can be used as a vaccine against American Cutaneous Leishmaniasis, we identified a Leishmania (Leishmania) amazonensis recombinant protein of 33 kD (Larp33) which is recognized by antibodies and peripheral blood leukocytes (PBL) from subjects vaccinated with Leishvacin ®, Larp33 was expressed in Escherichia coli after cloning of a 2,2 kb Sau3A digested genomic fragment of L. (L.) amazonensis into the pDS56-6 His vector. Immunoblotting analysis indicated that Larp33 corresponds to an approximately 40-kD native protein expressed in promastigotes of L.(L.) amazonensis and L. (Viannia) braziliensis. Northern blots of total RNA also demonstrated that the gene coding for this protein is expressed in promastigotes of the major lineages of Leishmania causing American Cutaneous Leishmaniasis. Larp33 induced partial protection in susceptible mouse strains (BALB/c and C57BL/10) against L. (L.) amazonensis after vaccination using Bacille Calmette-Guerin (BCG) as adjuvant. In vitro stimulation of splenocytes from BALB/c protected mice with Larp33 elicited the secretion of IL-2 and IFN-<FONT FACE="Symbol">g</font>, suggesting that a Th1 cell-mediated protective response is associated with the resistance observed in these mice. As revealed by its immunogenic and antigenic properties, this novel recombinant antigen is a suitable candidate to compose a vaccine against cutaneous leishmaniasis
Leishmania; Vaccination; Recombinant Antigens; Cytokines
A resposta imune induzida por uma proteína recombinante de Leishmania (Leishmania) amazonensis de 33 kD (Larp33) foi avaliada em linfócitos de indivíduos vacinados com a Leishvacin® e em camundongos através de vacinação. Larp33 foi expressa em Escherichia coli após clonagem de um fragmento genômico de L. (L.) amazonensis de 2,2 kb no vetor pDS56-6His. Larp33 foi reconhecida por anticorpos IgG presentes no soro de indivíduos vacinados com Leishvacin® e induziu proliferação em linfócitos desses indivíduos em níveis comparáveis ao antígeno total de Leishmania. A análise por imunoblot indicou que Larp33 corresponde a uma proteína de aproximadamente 40 kD expressa em promastigotas de L. (L.) amazonensis e L. (Viannia) braziliensis. Hibridização com sonda de DNA correspondente a parte do fragmento clonado, também demonstrou que o gene codificador desta proteína é expresso em promastigotas destas duas espécies. Larp33 em associação com BCG (Bacille Calmette-Guerin) foi capaz de induzir 75% de proteção em camundongos C75BL/10 e BALB/c, suceptíveis à infecção por L. (L.) amazonensis. Linfócitos dos camundongos protegidos produziram IL-2 e IFN-<FONT FACE="Symbol">g</font> em resposta a Larp33. Nossos resultados indicam que Larp33 é imunogênica para linfócitos de indivíduos vacinados com Leishvacin ® e protetora para camundongos contra a infecção por L. (L.) amazonensis
Immune responses induced by a Leishmania (Leishmania) amazonensis recombinant antigen in mice and lymphocytes from vaccinated subjects
Ana Paula FERNANDES (1), Elizabeth Cortez HERRERA (1), Wilson MAYRINK (1), Ricardo T. GAZZINELLI (2), Wen Yu LIU (3), Carlos Alberto da COSTA (1), Carlos Alberto Pereira TAVARES (2), Maria Norma MELO (1), Marilene Susan Marques MICHALICK (1), Reiner GENTZ (4) & Evaldo NASCIMENTO (1)
Summary
In the search for Leishmania recombinant antigens that can be used as a vaccine against American Cutaneous Leishmaniasis, we identified a Leishmania (Leishmania) amazonensis recombinant protein of 33 kD (Larp33) which is recognized by antibodies and peripheral blood leukocytes (PBL) from subjects vaccinated with Leishvacin ®, Larp33 was expressed in Escherichia coli after cloning of a 2,2 kb Sau3A digested genomic fragment of L. (L.) amazonensis into the pDS56-6 His vector. Immunoblotting analysis indicated that Larp33 corresponds to an approximately 40-kD native protein expressed in promastigotes of L.(L.) amazonensis and L. (Viannia) braziliensis. Northern blots of total RNA also demonstrated that the gene coding for this protein is expressed in promastigotes of the major lineages of Leishmania causing American Cutaneous Leishmaniasis. Larp33 induced partial protection in susceptible mouse strains (BALB/c and C57BL/10) against L. (L.) amazonensis after vaccination using Bacille Calmette-Guerin (BCG) as adjuvant. In vitro stimulation of splenocytes from BALB/c protected mice with Larp33 elicited the secretion of IL-2 and IFN-g, suggesting that a Th1 cell-mediated protective response is associated with the resistance observed in these mice. As revealed by its immunogenic and antigenic properties, this novel recombinant antigen is a suitable candidate to compose a vaccine against cutaneous leishmaniasis.
Keywords: Leishmania; Vaccination; Recombinant Antigens; Cytokines
Introduction
American cutaneous leishmaniasis (ACL) is an endemic disease caused by at least 12 species of the genus Leishmania. Besides the high incidence and the size of the population exposed to the risk of infection, difficulties to control the disease are aggravated by the diverse clinical, ecological and epidemiological features of this zoonosis 4, 16, 24. At present, there are no prophylactic drugs capable of preventing the disease and chemotherapy is frequently not effective 31. There is, therefore, a great interest in the development of safe vaccines as a mean of controlling the disease.
The nature of the host immune response to different Leishmania infections is one of the main factors controlling the final outcome of the disease 28, 29, 46. In mice, susceptibility and resistance to Leishmania major have been correlated with the selective stimulation of the different CD4+T cell subsets, Th1 and Th2, and the type of cytokines that they produce. In general, a protective role is associated with cells of the Th1 subset that secrete interleukin-2 (IL-2) and interferon-g (IFN-g) 8, 46, 47, whereas the expansion of cells of the Th2 subset, which produce IL-4 and IL-10, exacerbates disease 14, 25, 43. Similar CD4+T cell subsets have also been found in humans. Cytokine mRNA levels in human ACL lesions seem to correlate with the profiles observed in murine models and also with the spectrum of clinical manifestations of the disease. A Th1 profile is detected in patients with healing lesions, a mixed Th1-Th2 profile is observed in patients with active cutaneous or mucocutaneous leishmaniasis and a predominant Th2 response occurs in patients with the diffuse form of disease 10, 38. CD8+T cells are also able to synthesize IFN-g but, although some evidence for a protective role played by these cells has been already accumulated, they have been far less characterized 13, 17, 35, 36. Other cytokines, such as IL-12 2,50 and the tumor necrosis factor a (TNF-a) 49, 51, are also crucial to the establishment of a protective response in experimental leishmaniasis, whereas an increased expression of transforming growth factor b (TGF-b) is associated with susceptibility 6.
In agreement with these findings, adoptive or vaccine induced protection against leishmaniasis is largely dependent on cell mediated immunity, Th 1 lymphocytes and IFN-g 8, 33, 34, 46. Different antigen preparations, including defined and recombinant antigens, have been demonstrated to induce this type of beneficial response in experimental models 9, 12, 27, 33, 34, 44, 52 and also in lymphocytes from patients with clinical Leishmania infections 9, 40, 41, 48.
In this study, we have used a novel L. (L.) amazonensis recombinant antigen (Larp33) to vaccinate susceptible mice against Leishmania (L.) amazonensis infection. Larp33 was identified after screening recombinant polypeptides with sera and lymphocytes from subjects vaccinated with a killed promastigote vaccine 33, Leishvacin®, which is now commercially produced by bioBrás (Bioquímica do Brasil S.A.). Taken together, our results demonstrate that Larp33 is simultaneously immunogenic to humans and protective to mice.
Material and methods
Parasite stocks. For infection experiments, metacyclic promastigotes of L. (L.) amazonensis (IFLA/BR/67/PH8) were obtained by isolation from hamsters lesions and inoculation in NNN/Lit bifasic medium. Cultures were maintained for 12 days at 23º C. Cultures where further maintained in LIT medium11 at 23º C to obtain logarithmic phase promastigotes of L (L.) amazonensis (IFLA/BR/67/PH8) and L. (V.) braziliensis (MHOM/BR/75/M2903) used in the experiments of immunoblotting and nucleic acid isolation.
Preparation of Soluble Leishmania Antigens (SLA). For ELISA and lymphocyte proliferation assays, as well as mice vaccination, stationary phase promastigotes were pelleted by centrifugation (1200 g, 10 min, at 4º C) and washed tree times in PBS. Washed promastigotes were ressuspended in PBS and disrupted by sonification as described44. For immunoloblotting, 1 x 108 promastigotes were ressuspended in Tris-HCl 0.1 M (pH 7.0), 10% glycerol, 10% b mercaptoethanol and 2% SDS, boiled for 5 min26. and loaded in 12% polyacrylamide-SDS gels (SDS-PAGE). Protein concentrations were determined as previously described 30.
Nucleic acids isolation and Northern Blotting. Total DNA from 1 x 108 promastigotes of L. (L.) amazonensis was isolated by standard techniques involving lysis by 0.5% SDS, proteinase K (125 µg/ml) treatment followed by repeated phenol/chloroform extractions and finally, ethanol precipitation. Total RNA was extracted from 1 x 109 logarithmic promastigotes with TRIZOL (Gibco-BRL, Gaithersburg, MD) according manufacturers instructions. Northern hybridization analysis was carried out as previously described 42 using 10 µg ot total Leishmania RNA/lane on formaldehyde 1% agarose gels. Gels were transferred to nylon membranes and hybridizided with a probe comprising a 625 bp PstI and Sal I restriction fragment of the coding sequence of the Larp33 and labeled with [a-32P]-dCTP by the random priming method 19.
Construction of genomic library and expression of recombinant proteins.L.(L.) amazonensis genomic DNA was partially digested with Sau3A and separated by agarose gel electrophoresis. Fragments between 2 to 4 kb were excited from the gel, electro-eluted and cloned into the Bam HI site of the expression vector pDS56-6His. After transformation of Escherichia coli M-15 cells, individual transformants were grown in 500 µl of LB medium, and the expression of recombinant proteins was induced by IPTG (isopropylthio-b-D-galactoside) at 2mM. After incubation at 37º C for 4 hours under agitation, cells were pelleted by centrifugation and ressuspended in 100 µl of buffer containing 2.3% SDS, 20% glycerol, 5% b-mercaptoethanol and 0.5% Bromophenol Blue. Samples were boiled for 5 min. and loaded in 10% polyacrylamide gel in the presence of SDS. The identification of transformants expressing recombinant proteins was carried out by comparing the band profiles of crude lysates of induced and non-induced E. coli cultures. Recombinant proteins were purified from crude bacterial lysates by a nickel-chelate cromatography. Protein concentrations were determined as described 30.
Lymphocyte proliferation assays. Peripheral blood leukocytes of seven subjects vaccinated with Leishvacin ® according the protocol described by NASCIMENTO et al., (1990), which had a Leishmania skin test positive and of four-non-vaccinated control subjects were used to access immunogenicity of the recombinant proteins to humans. Proliferation assays were perfomed as previously described37, except that cultures were stimulated with 20 µg/ml (concentration determined by previous titration) of either Leishmania soluble antigens (SLA) or recombinant proteins. Five micrograms per mililiter of PHA was used as mitogen. Proliferation was measured by the incorportion of 0.2 µCi of [3H] thymidine during the final 18 hours of culture. Results are expressed as Lymphocyte Stimulation Index (LSIs), which represent the ratios of the average of [3H] thymidine incorporation of triplicate cultures in the presence versus the absence of antigen or mitogen.
Rabbit antisera. Antiserum against Larp33 and native proteins were raised by four subcutaneous immunizations of a rabbit with 100 µg/ml of protein in Freunds complete adjuvant at 15 days intervals. The booster immunization of 50 µg/ml of protein was administered in saline. Aliquots of antisera were stored at -20ºC until used. To obtain an antiserum against the native protein a 40 kD protein fraction was isolated from Leishvacin® by SDS-PAGE an electroelution (CARDOSO et al., manuscript in preparation) and used to immunize a rabbit as described above.
Antibody measurements. Enzyme-linked immunosorbent assays (ELISA) were used to quantitate antibody levels in sera of subjects vaccinated with Leishvacin®, and to access cross-reactivity between native and recombinant proteins. The assays were carried out as previously described 37, except that microtiter wells were coated with either 10 µg/ml of recombinant protein or 50 µg/ml of SLA in carbonate buffer (pH 9.6). To quantitate human antibody levels, plates were incubated with serial dilutions of human sera followed by incubation with diluted anti-human IgG (Cappel, USA) conjugated with peroxidase. To detect reactivity between proteins, plates were incubated with rabbit sera raised against a Leishvacin® 40 kD protein fraction or against Larp33 as a control, followed by incubation with an anti-rabbit IgG conjugated with peroxidase (Sigma, St. Louis, MO). The significance of differences between groups was determined by Students paired t test 18.
Immunoblotting analysis. Western blots were performed with crude promastigote antigen preparations resolved by 12% SDS-PAGE and electrophoretically transferred to nitrocellulose paper (Sigma, USA). Proteins were detect by incubating blots with rabbit antisera raised against Larp33 followed by incubation with 1:5000 peroxidase-conjugated anti-rabbit immunoglobulin G and 3,3 - diaminobenzidine and H2O2 (Vectastain, Inc., USA).
Vaccination of mice. Female C57BL/10 and BALB/c mice 8 to 12 weeks old, eight per group, were vaccinated subcutaneously in the base of the tail and foot-pad, respectively, with three doses of 30 µg of Larp33, or SLA at 15 days intervals. Live BCG (Fundação Ataulpho de Paiva, RJ, Brazil), was used only in the first dose, and 30 µg of PPD (Purified Protein Derivative Fundação Ataulpho de Paiva, RJ, Brazil) were used in subsequent injections as adjuvants. Control groups received only BCG or PBS. BALB/c animals were challenged with 104 in the hind footpad and C57BL/10 with 105 late-log-phase promastigotes at the base of tail 7 days after the last immunization. All groups of mice were evaluated for lesion development for up to 6 months.
Cytokine assays. Spleen cells were collected from 4 BALB/c mice of each vaccinated group, as well as control mice, at 4 months after parasite challenge for IL-2 and IFN-g measurements. Cells were prepared as previously described20 and cytokine measurements were performed on supernatants of cultures stimulated with either Larp33 (20 µg/ml), SLA (20 µg/ml), PPD (40 µg/ml) or ConA (5 µg/ml). IL-2 was measured on supernatants collected at 24 hours by a CTLL cell proliferation assay and IFN-g by a two site ELISA with culture supernatants collected at 48 hours, as previously described 20, 21. Results are presented as average of cytokine concentrations determined for 4 animals in each group.
Results
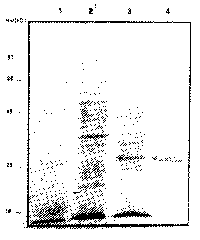
Expression of Leishmania (L.) amazonensis recombinant proteins in the pDS56-6His system. In this work we report the use of the pDS56-6His expression system to express L. (L.) amazonensis genomic fragments in Escherichia coli cells. DNA fragments cloned into pDS56-6His are expressed as recombinant proteins containing a poly-histidine metal binding peptide fused to their amino terminal portion, which allows the subsequent purification of the recombinant proteins by means of a nickel-chelate containing column22. As it has been previously described for other parasite proteins22, the use of the pDS56-6His system allowed the improvement of the expression yield and purification of high levels of recombinant proteins. The L. (L.) amazonensis recombinant proteins expressed and purified by this strategy were further evaluated for their immunogenic properties using sera and lymphocytes from subjects vaccinated with Leishvacin ®. Among the recombinant proteins screened, we identified a 33-kD protein (Larp33), which reacted with sera and lymphocytes from subjects vaccinated with Leishvacin®. Figure 1 shows a coomassie-blue stained 10% polyacrylamide gel of bacterial lysates of the non-induced cultures (lane 2), IPTG induced cultures (lane 3) and the purified Larp33 (Lane 4).
Fig 1. Expression and purification of Larp33 from E. coli. Coomasie blue stained SDS-PAGE gel of E. coli lisates of non-induced cultures (Lane 2), IPTG induced cultures (Lane 3) and Larp33 purified by niquel-column cromatography. Numbers represent molecular weight standarts in kD (Lane1).
Larp33 is recognized by PBL and sera antibodies from subjects vaccinated with Leishvacin ®. Human T-cell responses to recombinant proteins were assessed with PBL from seven subjects vaccinated with Leishvacin ® and four non-vaccinated control subjects. Figure 2A shows the results obtained with Larp33. Lymphocytes from all seven vaccinated subjects proliferated in response to Larp33. The LSI value of vaccinated subjects in response to Larp33 was significantly higher (P<0.05) than that of control subjects, however no statistically significant difference was found between LSIs of vaccinated subjects in response to the Larp33 and SLA. In addition, FACS analysis indicated that stimulation of lymphocytes from vaccinated subjects with Larp33 resulted in expansion of both CD4+ and CD8+ T lymphocytes (data not shown). The reactivity of antibodies present in sera of vaccinated subjects to the Larp33 was assayed by ELISA. No statistical difference was found on IgG (Figure 2B) antibody titers present in sera of vaccinated subjects in response to the Larp33 and SLA. In agreement with the T-cell proliferation results, sera from all seven vaccinated subjects reacted with Larp33. Significant differences (p<0.05) were detected by comparing antibody titers of vaccinated subjects with those of control subjects. The results clearly indicate that epitopes present in Larp 33 are recognized by sera of vaccinated subjects and are able to elicit strong proliferative responses on human T-cells. The data is also suggestive that the native form of this antigen is an important component of Leishvacin® and might be associated with the protective immunity previously observed in subjects vaccinated with Leishvacin® 3, 33, 37.
Fig. 2. The Larp33 is recognized by lymphocytes and antibodies from subjects vaccinated with Leishvacin ®. Average and SDs of LSIs in response to Larp33 or SLA (A) of seven subjects vaccinated with Leishvacin ® and four control non-vaccinated subjects. Phytohemaglutinin A (PHA) was used as positive control. Levels of IgG (B) determined by ELISA by using either L. (L.) amazonensis point represents the soluble antigens (SLA - ), Larp33 ( ) or control (SLA + Larp33 - ) as antigens. Each average and SD for antibody titres of seven vaccinated subjects and four non-vaccinated control subjects. OD, optical density (Absorbance).
Immunoblotting analysis. Immunoblots of promastigote lysates from L. (L.) amazonensis (Figure 3A) and L.(V.) braziliensis (Figure 3B) were performed with a polyclonal rabbit anti-serum raised against Larp33. A dominant protein of apparent mobility of 40 kD was detected on both Leishmania species, which was consistent with the result obtained in the northern blotting of total RNA of the two species. This strongly indicates that the recombinant and native forms of this antigen share common epitopes. Besides immunoblotting, the identity between the recombinant and native proteins were also assayed by ELISA using Larp33 as antigen and the polyclonal rabbit sera raised against the recombinant and the 40 kD L. (L.) amazonensis native protein. No significant differences (p < 0.001) were detected between the titers of the antisera raised against the native and recombinant proteins. Thus, Larp33 corresponds to a 40 kD Leishmania native protein and probably represents nearly the full-lenght native protein.
Fig. 3. Detection of thenative form of Larp33. (A) Promastigote lysate from L. (L.) amazonensis (lane 2) was Leishmania probed with a rabbit polyclonal serum raised against Larp33 (Lane 3) and (B) L. (V.) braziliensis promastigote lysate (lane 1) probed with the same serum (lane 2).
Northern blotting analysis. Hybridization of promastigote total RNA from both Leishmania species (Figure 4) with a probe comprising part of the coding region of Larp33 revealed the presence of a single band with estimated size of a 2.8 kb compatible with the size of the expressed protein.
Fig. 4. Northern Blotting analysis of total promastigote RNA hybridized with the coding region of Larp33. Total RNA was extracted from logarithmic phase promastigotes of L. (V.) braziliensis (Lane 1) and L. (L.) amazonensis (Lane 2) separated by agarose-formaldehyde gel electrophoresis, blotted and hybridized with radiolabelled probe. In Panel B, a portion of the RNA ethidium bromide stained gel showing the ribosomal RNA bands and that equivalent amounts of total RNA were loaded in the gel.
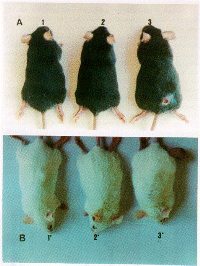
Larp 33 induces protection in susceptible mice. Groups of eight BALB/c and C57BL/10 mice were vaccinated subcutaneously with 30 µg of either Larp33 or SLA in association with BCG, and challenged with 104 and 105 L (L.) amazonensis infective promastigotes, respectively. The results obtained are summarized on Table I. Vaccination with SLA induced 50% of protection. Protection of 75% (6 in 8 mice) against disease was obtained in both susceptible strains by vaccination with Larp33. Lesion development was monitored for up 20 weeks and during all this period no signs of lesion were observed in the protected animals (Figure 5). These results were confirmed by examining for the presence of parasites at the site of inoculation by impression smears. In contrast, control mice that received only BCG or PBS developed progressive lesions, and metastasis to lynfonodes and other sites (Figure 5). The remaining 25% of animals which were not protected by vaccination with Larp33 had slow and progressive local swelling.
Fig. 5. Larp33 protects susceptible mice against L. (L.) amazonensis challenge infection. In Panel A shows C57BL10 mice vaccinated and protected by Larp33 (1) or SLA (2) and lesion development in mice that received BCG (3). In Panel B, the correspondent BALB/c mice are shown.
Table 1
Efficacy of vaccination of BALB/c and C57BL/10 mice with Larp33 against challenge
infection with infective promastigotes of L. (L.) amazonensis
(% of protection)
Groups of eight animals were vaccinated subcutaneously with three doses of 30 µg of Larp33, or SLA at 15 days intervals. BCG was used as adjuvant only in the first dose and PPD in the subsequent injections. Control mice received adjuvant or PBS alone. BALB/c mice were challenged with 104 and C57BL/10 with 105 infective promastigotes of L. (L.) amazonensis in the hind footpad and in the base of the tail, respectively. Results represent percentage of protection obtained after 20 weeks post infection.
Cytokine responses in protected BALB/c mice. In order to evaluate whether the protection observed in Larp33 vaccinated BALB/c mice could be related to a specific pattern of lymphokine production, the levels of murine IL-2 and IFN-g were determined for cells from mice vaccinated with either Larp 33, SLA or BCG alone. Cytokines were not detected in supernatants of spleen cells from vaccinated mice cultured in absence of antigen. However, increased levels of IL-2 (Figure 6A) and IFN-g (Figure 6B) were detected when spleen cells from Larp33 protected animals were stimulated in vitro with Larp33 or SLA. The same was observed with spleen cells from SLA protected mice. T-cells from these mice produced lower levels of IFN-g when stimulated with PPD. Low levels of IFN-g were also detected in cells from mice that received BCG alone when stimulated with either Larp33, SLA or PPD.
Fig 6. Cytokine profile of spleen cells from vaccinated BALB/c mice. Spleen cells from BALB/c mice vaccinated with Larp33 (black bars), with SLA (cross-hatched bars), BCG (open bars) and control group (hatched bars) were stimulated in vitro with ConA, Larp33, SLA and PPD, 4 months after challenge infection. Production of IL2 e IFN-g were measured in the supernatant of stimulated cultures as described in Material and Methods.
Discussion
As part of our strategy to produce a recombinant vaccine against American Cutaneous Leishmaniasis, we screened L. (L.) amazonensis recombinant proteins for their ability to react with sera and lymphocytes from subjects vaccinated with Leishvacin ®. Using this approach, we have identified a recombinant protein (Larp33) which is simultaneously immunogenic to humans and protective to mice. In addition no homology with other protein/gene sequences was found at GeneBank between the DNA sequences obtained for the genome fragment coding for Larp33 (FERNANDES et al., manuscript in preparation), indicating that Larp33 is a novel Leishmania antigen. As documented above, Larp33 elicited strong proliferative responses in PBL and was recognized by IgG antibodies in sera of subjects vaccinated with Leishvacin® which had a positive DTH reaction. Leishvacin ® has been tested in endemic areas and was able to prevent cutaneous leishmaniasis in vaccinated subjects, with higher efficiency in those subjects who showed skin test conversion after vaccination3. Analysis of the cellular immune responses induced by Leishvacin® indicated that CD8+ lymphocytes of vaccinated subjects are preferentially stimulated by L. (V.) brazilienses total antigens. In addition, it has been also demonstrated that IFN-g, but not IL4, is produced by lymphocytes from vaccinated subjects which had converted the skin test from negative to positive33. Preliminary analysis of the T cells from subjects vaccinated with Leishvacin® that proliferate in response to Larp33 suggest that CD8+ and CD4+ lymphocytes are both stimulated. Both T cell subsets have been demonstrated to participate of cure and resolution of cutaneous leishmaniases and to be important sources of IFN-g, the principal effector lymphokine in the activation of macrophages to a leishmanicidal state13, 47. These results indicate that Larp33 contains T-cell epitopes recognized by human lymphocytes, which is an important requirement for vaccine development against human cutaneous leishmaniasis.
As indicated by immunoblotting analysis of promastigote total lysates, Larp33 correspond to a 40 kD native protein expressed in promastigotes of L. (L.) amazonensis and also of L. (V.) braziliensis. The results obtained from northern blotting of total RNA also indicated that the gene coding for this protein is expressed in promastigotes of both species. In agreement, sera raised against a 40 kD protein fraction separated by SDS-PAGE and electroeluted from Leishvacin® reacted with Larp33. Previous results from this laboratory demonstrated that this 40 kD native protein is one of the major antigens of Leishvacin® 37. It stimulates T-cells from vaccinated subjects to proliferate and to produce high levels of IFN-g and also induces partial protection on susceptible mice against Leishmania challenge infections, after vaccination (CARDOSO et al., manuscript in preparation).
Leishmania (Leishmania) amazonensis, one of the ethiological agents of ACL, has a broad geographical distribution 23 and has been associated with the complete spectrum of clinical manifestations of this disease 5, ranging from single localized lesions to the diffuse cutaneous form, which is characterized by a chronic infection associated with the impairment of the host cell -mediated immune response16. We have also investigated the potential of Larp33 to induce protective responses in BALB/c and C57BL/10 mice against L. (L.) amazonensis. Subcutaneous immunization with Larp33 induced 75% of protection on these two susceptible mouse strains. The L. (L.) amazonensis infection in BALB/c mice is accompanied by progressive increase in lesion size, metastasis and visceralization of the parasites7. Associated with this disease pattern, a Th-2 type response is observed with the production of increased levels of IL-41. Similarly to the L. (L.) major infection in BALB/c 14, treatment of BALB/c mice with anti-IL4 monoclonal antibodies abrogates its susceptibility to L. (L.) amazonensis, with a corresponding increase in the IFN-g production 1. Thus, in BALB/c mice a consistent correlation has been found between the production of IFN-g and either resolution or resistance to infection. Accordingly, in vitro stimulation with Larp33 or SLA induced spleen cells from Larp33 protected BALB/c mice to produce high levels of IL-2 and IFN-g. This amplicates that vaccination with Larp 33 is able to influence the course of the natural infection in these susceptible mice, maybe through the induction of a Th1 protective response. In this study, the cytokine profile associated with the protective response induced by Larp33 in C57BL/10 mice was not determined. This would be of interest, since the mechanisms underlining the susceptibility phenotype of this mice strain are not completely understood. The course of L. (L.) amazonensis infection in C57BL/10 and the associated cytokine profile is quite distinct from BALB/c. In C57BL/10 mice the lack of a Th1 response rather than the induction of a Th2 response might be the determinant factor of its susceptibility 1. This lack of response, apparently, is not due to a genetic defect of these mice, since they are able to heal from L. (L.) major infections producing IFN-g.
In summary, we have demonstrated that Larp33 fulfills important requirements to a potential vaccine candidate molecule. It is expressed in the two major lineages of Leishmania causing ACL, and is immunogenic to vaccinated subjects and protective to mice. In addition to the results presented here, current work in our laboratory also indicates that Larp33 is a novel leishmania recombinant antigen.
Abreviations:
ACL, American Cutaneous Leishmaniasis; Larp33, Leishmania (Leishmania) amazonensis recombinant protein of 33 kD; Leishvacin®, is a killed promastigote vaccine commercialized by BioBrás (Brazil); PPD, Purified protein Derivate; SLA Soluble Leishmania Antigens; LSIs, Lymphocyte stimulation index.
Resumo
Resposta Imune induzida por uma proteína recombinante de Leishmania (Leishmania) amazonensis de 33 kD (Larp33) em camundongos e em linfócitos de indivíduos vacinados
A resposta imune induzida por uma proteína recombinante de Leishmania (Leishmania) amazonensis de 33 kD (Larp33) foi avaliada em linfócitos de indivíduos vacinados com a Leishvacin® e em camundongos através de vacinação. Larp33 foi expressa em Escherichia coli após clonagem de um fragmento genômico de L. (L.) amazonensis de 2,2 kb no vetor pDS56-6His. Larp33 foi reconhecida por anticorpos IgG presentes no soro de indivíduos vacinados com Leishvacin® e induziu proliferação em linfócitos desses indivíduos em níveis comparáveis ao antígeno total de Leishmania. A análise por imunoblot indicou que Larp33 corresponde a uma proteína de aproximadamente 40 kD expressa em promastigotas de L. (L.) amazonensis e L. (Viannia) braziliensis. Hibridização com sonda de DNA correspondente a parte do fragmento clonado, também demonstrou que o gene codificador desta proteína é expresso em promastigotas destas duas espécies. Larp33 em associação com BCG (Bacille Calmette-Guerin) foi capaz de induzir 75% de proteção em camundongos C75BL/10 e BALB/c, suceptíveis à infecção por L. (L.) amazonensis. Linfócitos dos camundongos protegidos produziram IL-2 e IFN-g em resposta a Larp33. Nossos resultados indicam que Larp33 é imunogênica para linfócitos de indivíduos vacinados com Leishvacin ® e protetora para camundongos contra a infecção por L. (L.) amazonensis.
Acknowledgements
We are grateful to Dr. Elizabeth Spangler and Dr. Maria de Lourdes Petrillo Peixoto from the Dept. of Microbiology, UFMG, Brazil for helpful discussions. We also thank Dr. Darrel T. Liu from FDA (NIH, Bethesda, MD), Dr. Robert W. Mac Master from the British Columbia Universitity, Vancouver, Canada and Dr. Maurício Rezende from the Dept. of Microbiology, UFMG, Brazil for scientific support. APF received Ph.D. Fellowships from the Brazilian Council for Scientific Development (CNPq) and from Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG).
Recebido para publicação em 25/02/1997
Aceito para publicação em 24/04/1997
- 1 AFONSO, L.C.C. & SCOTT, P. - Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis Infect. Immun., 61: 2952-2959, 1993.
- 2. AFONSO, L.C.C.; SCHARTON, T.M.; VIEIRA, L.Q. et al. - The adjuvant effect of interleukin-12 in a vaccine against Leishmania major Science, 263: 235-237, 1994.
- 3. ANTUNES, C.M.F.; MAYRINK, W.; MAGALHĂES, P.A. et al. - Controlled field trials of a vaccine against New World cutaneous Leishmaniasis. Int. J. Epidem., 15: 572-580, 1986.
- 4. ASHFORD, R.W.; DESJEUX, P. & RAADT, P. - Estimation of population at risk of infection and number of cases of Leishmaniasis. Parasit. today, 8: 104-106, 1992.
- 5. BARRAL, A.; PEDRAL-SAMPAIO, D.; GRIMALDI, JR., G. et al. - Leishmaniasis in Bahia, Brazil evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Amer. J. trop. Med. Hyg, 44: 536-546, 1991.
- 6. BARRAL-NETO, M.; BARRAL, A.; BROWNELL, C.E. et al. - Transforming Growth Factor-b in leishmanial infection: a parasite escape mechanism. Science, 257: 545-548, 1992.
- 7. BARRAL-NETO, M.; A.;CARDOSO, M.S.A. & BARRAL, A. - Different disease patterns in two inbred mouse strains infected with a clone of Leishmania mexicana amazonensis Acta trop. (Basel), 44: 5-11, 1987.
- 8. BRETSCHER, P.A.; WEI, G.; MENON, J.N. & BIELEFELDT, H. - Establishment of stable, cell mediated immunity that makes "susceptible" mice resistant to Leishmania major Science, 257: 539-542, 1992.
- 9. BURNS, J.M.; SCOTT, J.M.; CARVALHO, E.M. et al - Characterization of a membrane antigen of Leishmania amazonensis that stimulates human immune responses. J. Immunol., 146: 742-748, 1991.
- 10. CÁCERES-DITTMAR, G.; TAPIA, F.J.; SANCHES, M.A. et al. - Determination of the cytokine profile in American Cutaneous Leishmaniasis using the polymerase chain reaction. Clin. exp. Immunol., 91: 500-505, 1993.
- 11. CAMARGO, E.P. - Growth differentiation in Trypanosoma cruzi: origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. trop. S. Paulo, 6: 93-100, 1964.
- 12. CHAMPSI, J. & MCMAHON-PRATT, D. - Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect. Immun., 52: 3272-3279, 1988.
- 13. CHAN, M.M. - T cell response in murine Leishmania mexicana amazonensis infection: production of interferon-g by CD8+T cells. Europ. J. Immunol., 23: 1181-1184, 1993.
- 14. CHATELAIN, R.; VARKILA, K. & COFFMAN, R.L. - IL-4 induces a Th2 response in Leishmania major-infected mice. J. Immunol., 148: 1182-1187, 1992.
- 15. CONNEL, N.D.; MEDINA-ACOSTA, E.; MCMASTER, W.R.; BLOOM, B.R. & RUSSEL, D.G. - Effective immunization against cutaneous leishmaniasis with recombinant Bacille Callmette-Guérin expressing the Leishmania surface proteinase gp63. Proc. nat. Acad. Sci. (Wash.) 90: 11473-11477, 1993.
- 16. CONVIT, J.; ULRICH, M. & FERNÁNDEZ, C.T. - The clinical and immunological spectrum of American Cutaneous Leishmaniasis. Trans. roy. Soc. trop. Med. Hyg., 87: 444-448, 1993.
- 17. DA-CRUZ, A.; CONCEIÇĂO-SILVA, F.; BERTHO, A.L. & COUTINHO, S.G. - Leishmania-reactive CD4+ and CD8+ T cells associated with the cure of human cutaneous leishmaniasis. Infect. Immun., 62: 2614-2618, 1994.
- 18. DIXON, W. & MASSEY Jr., F.J. - The normal distribution. In: ____________ Introduction to statistical analysis. Tokyo, McGraw-Hill Book, 1969. p. 56.
- 19. FEINBERG, A.P. & VOLGELSTEIN, B. - A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Analyt. Biochem., 137: 266-270, 1984.
- 20. GAZZINELLI, R.T.; HAKIM, F.T.; HIENY, S.; SHEARER, G.M. & SHER, A. - Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-g production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol., 146: 286-292, 1991.
- 21. GAZZINELLI, R.T.; XU, Y.; HIENY, S.; CHEEVER, A. & SHER, A - Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii J. Immunol., 149: 175-182,1992.
- 22. GENTZ, R.; CERTA, U.; TAKACS, B. et al. - Major surface antigen p190 of Plasmodium falciparum: detection of common epitopes present in a variety of plasmodia isolates. Europ. J. Mol. Biol., 7: 225-231, 1988.
- 23. GRIMALDI, G.J.; TESH, R.; & MCMAHON-PRATT, D. - A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Amer. J. trop. Med. Hyg., 41: 695-725, 1989.
- 24. GRIMALDI, G.J., & TESH, R.B. - Leishmaniasis of the New World: current concepts and implications for future research. Clin. Microbiol. Rev., 6: 230-250, 1993.
- 25. HEINZEL, F.P.; SADICK, M.D.; MUTHA, S.S. et al. - Reciprocal expression of interferon gamma or interleukin-4 during the resolution or progression of murine leishmaniasis. J. exp. Med., 169: 59-72, 1989.
- 26. HUNTER, K.W.; COOK, C.L. & HAYUNGA, E.G. - Leishmanial differentiation in vitro: induction of heat shock proteins. Biochem. biophys. Res. Commun., 125: 755-759, 1984.
- 27. JARDIM, A.; ALEXANDER, J.; TEH, H.S.; OU, D.W. & OLAFSON, R.W. - Immunoprotective Leishmania major synthetic T cell epitopes. J. exp. Med., 172: 645-648, 1990.
- 28. LIEW, F.Y. - Cell-mediated immunity in experimental cutaneous leishmaniasis. Parasit. today, 2: 264-266, 1986.
- 29. LOCKSLEY, R.M. & LOUIS, J.A. - Immunology of leishmaniasis. Curr. Oppin. Immunol., 4: 413-416, 1992.
- 30. LOWRY, O.H.; ROSEMBROUGH, N.J.; FARR, A.L. & RANDALL, R.J. - Protein measurement with folin phenol reagent. J. biol. Chem., 193: 265-275, 1951.
- 31. MARSDEN, P.D. - Pentavalent antimonyal: old drug for new disease. Rev. Soc. bras. Med. trop., 18: 187-188, 1985.
- 32. MAYRINK, W.; DA COSTA, C.A.; MAGALHĂES, P.A. et al. - A field trial of a vaccine against American Dermal leishmaniasis. Trans. roy. Soc. trop. Med. Hyg., 73: 385-387, 1979.
- 33. MENDONÇA, S.C.E.; DE LUCA, P.M.; MAYRINK, W. et al. - Characterization of human T lymphocyte-mediated immune response induced by a vaccine against American tegumentary leishmaniasis. Amer. J. trop. Med. Hyg., 53: 195-201, 1995.
- 34. MOGNEAU, E.; ALTARE, F.; WAKIL, A.E. et al. - Expression cloning of a protective Leishmania antigen. Science, 268: 563-567, 1995.
- 35. MULLER, I.; PEDRAZINI, T.; KROPF, P.; LOUIS, J. & MILLON, G. - Establishment of resistance of L. major infection in susceptible BALB/c mice requires parasite-specific CD8+T cells. Int Immunol., 3: 587-597, 1991.
- 36. MULLER, I.; KROPF, P. & LOUIS, J.A. - Gamma interferon response in secondary Leishmania major infection: role of CD8+T cells. Infect. Immun., 61: 3730-3738, 1993.
- 37. NASCIMENTO, E.; MAYRINK, W.; DA COSTA, C.A. et al. - Vaccination of humans against cutaneous leishmaniasis: cellular and humoral immune responses. Infect. Immun., 58: 2198-2203, 1990.
- 38. PIRMEZ, C.; YAMAMURA, M.; UEYMURA, K. et al. - Cytokine patterns in the pathogenesis of human leishmaniasis. J. clin. Invest., 91: 1390-1395, 1993.
- 39. RUSSELL, D. & ALEXANDER, J. - Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J. Immunol., 140: 1274-1279, 1988.
- 40. RUSSO, D.M.; JARDIM, A.; CARVALHO, E.M. et al. - Mapping T-cell epitopes in Leishmania gp63. J. Immunol., 150: 932-939, 1993.
- 41. RUSSO, D.M.; BURNS Jr., J.M.; CARVALHO, E.M. et al. - Human T cell responses to gp63, a surface antigen of Leishmania J. Immunol. ,147: 3575-3580, 1991.
- 42. SAMBROOK, J.; FRITSH, E.F. & MANIATIS, T. - Molecular cloning: a laboratory manual 2 ed. New York, Cold Spring Harbor, 1989.
- 43. SADICK, M.D.; HEINZEL, F.P.; HOLADAY, B.J. et al. - Cure of murine leishmaniasis with anti-interleukin-4 monoclonal antibody. Evidence for a T-cell dependent, interferon mechanism J. exp. Med., 171: 115-127, 1990.
- 44. SCOTT, P.; PEARCE, E.; NATOVITZ, P. & SHER, A. - Vaccination against cutaneous leishmaniasis in a murine model. II. Immunologic properties of protective and non-protective subfractions of a soluble promastigote extract. J. Immunol., 139: 3118-3125, 1987.
- 45. SCOTT, P.; NATOVITZ, P.; COFFMAN, R.L. ; PEARCE, E.; & SHER, A. - Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. exp. Med., 168: 1675, 1684, 1988.
- 46. SCOTT, P. - The role of TH1 and TH2 cells in experiemntal cutaneous leishmaniasis. Exp. Parasit., 68: 369-372, 1989.
- 47. SCOTT, P. - IFN-g modulates the arly development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol., 147: 3149-3155, 1991.
- 48. SKEIKY, Y.A.W.; GUDERIAN, J.A.; BENSON, D.R. et al. - A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile ant do produce interleukin 12: J. exp. Med., 181: 1527-1537, 1995.
- 49. STENGER, S.; THÜRING, H.; RÖLLINGHOFF, M. & BOGDAN, C. - Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major J. exp. Med., 180: 783-793, 1994.
- 50. SYPEK, J.P.; CHUNG, C.L.; MAYOR, S.E.H. et al. - Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. exp. Med., 177: 1797-1802, 1993.
- 51. TITUS, R.G.; SHERRY, B. & CERAMI, A. - Tumor necrosis factor plays a protective role in experimental murine leishmaniasis. J. exp. Med., 170: 2079-2104, 1989.
- 52. YANG, D.M.; FAIRWEATHER, N.; BUTTON, L.L. et al. - Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmania surface protein (gp63) preferencially induces T helper 1 cells and protective immunity against leishmaniasis. J. Immunol., 145: 2281-2285, 1990.
Publication Dates
-
Publication in this collection
16 June 1999 -
Date of issue
Mar 1997
History
-
Accepted
24 Apr 1997 -
Received
05 Feb 1996