Abstracts
The MN strain of HIV-1 is known to be more prevalent in Brazil, the BRU strain is more prevalent in Europe, and the NDK strain in Africa. It has been suggested in the literature to include different strains in the same vaccine against HIV-1. To contribute to the studies for the development of a universal vaccine, the occurrence of antibodies (Ab) against three HIV-1 strains (MN, BRU and NDK) was determined in serum samples from 85 HIV-1-positive patients, adult volunteers seen at the University Hospital of the Faculty of Medicine of Ribeirão Preto-USP. One-hundred tissue culture infective unit (TCIU) of the viruses reacted with serial dilutions of the sera (2x) and with MT4 cells added at a final concentration of 0.3 × 106 cells/ml, and a cytopathic effect was observed on the 7th and 11th days of incubation. Titres of less than 1/50 were considered to be negative. In 129 tests, the sera were negative for one of the three strains: 40 for MN, 29 for BRU and 60 for NDK. There was a predominance of strains MN and BRU, most of them presenting titres from 1/50 to 1/200. Titres for NDK were detected in 25 sera. We conclude that there seems to be a predominance of strains MN and BRU among the individuals from the region tested; however, the detection of sera with positive NKD titres indicates the need for further studies of this strain in other populations and regions of Brazil
AIDS; HIV-1 MN; HIV-1 BRU; HIV-1 NDK; Neutralizing antibodies
A cepa MN do HIV-1 é conhecida por ser mais prevalente no Brasil, a cepa BRU é mais prevalente na Europa e a cepa NDK na África. Tem sido sugerido na literatura que diferentes cepas devam ser incluídas em uma mesma vacina contra o HIV-1. Com o intuito de se contribuir para o desenvolvimento de uma vacina universal, a ocorrência de anticorpos (Ac) contra três cepas do HIV-1 (MN, BRU, NDK) foram determinadas em amostras de soros de 85 pacientes voluntários, adultos, soropositivos para o HIV-1, atendidos em um Hospital Universitário. Cem unidades infectantes para cultura de tecido (TCIU) dos vírus reagiram com diluições seriadas de soro (2x) e células MT4 adicionadas a uma concentração final de 0,3 × 106 células/ml, e o efeito citopático foi observado no 7- e 11- dias de incubação. Títulos menores de 1/50 foram considerados negativos. Em 129 testes realizados, encontrou-se negatividade para MN em 40 soros, para BRU em 29 e em 60 para NDK. Houve uma predominância das cepas BRU e MN, a maioria nos títulos de 1/50 a 1/200. Títulos para NDK foram detectados em 25 soros. Conclui-se haver uma predominância das cepas BRU e MN nos indivíduos testados, da região; entretanto a detecção de soros positivos para NDK alerta para a necessidade de estudos desta cepa em outras populações e regiões do Brasil
NEUTRALIZING ANTIBODIES IN BRAZILIAN SERA AGAINST THREE STRAINS OF HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 (HIV-1)
Alcyone Artioli MACHADO(1 (1 Alcyone Artioli Machado was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Proc. no. 201275/91-0 NV) ) Faculty of Medicine of Ribeirão Preto, SP, Brazil. (2 ) Inserm U322 Unité de Researches sur les Retrovirus et Maladies Associeés, Marseille, France. Correspondence to: Alcyone Artioli Machado, PhD. Faculty of Medicine of Ribeirão Preto, USP, Departamento de Clínica Médica. Av. Bandeirantes, 3900, Campus Universitário, 14048-900 Ribeirão Preto, SP, Brasil. ), Ivan HIRSCH(2 (1 Alcyone Artioli Machado was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Proc. no. 201275/91-0 NV) ) Faculty of Medicine of Ribeirão Preto, SP, Brazil. (2 ) Inserm U322 Unité de Researches sur les Retrovirus et Maladies Associeés, Marseille, France. Correspondence to: Alcyone Artioli Machado, PhD. Faculty of Medicine of Ribeirão Preto, USP, Departamento de Clínica Médica. Av. Bandeirantes, 3900, Campus Universitário, 14048-900 Ribeirão Preto, SP, Brasil. ), José Fernando de Castro FIGUEIREDO (1 (1 Alcyone Artioli Machado was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Proc. no. 201275/91-0 NV) ) Faculty of Medicine of Ribeirão Preto, SP, Brazil. (2 ) Inserm U322 Unité de Researches sur les Retrovirus et Maladies Associeés, Marseille, France. Correspondence to: Alcyone Artioli Machado, PhD. Faculty of Medicine of Ribeirão Preto, USP, Departamento de Clínica Médica. Av. Bandeirantes, 3900, Campus Universitário, 14048-900 Ribeirão Preto, SP, Brasil. ), Roberto MARTINEZ(1 (1 Alcyone Artioli Machado was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Proc. no. 201275/91-0 NV) ) Faculty of Medicine of Ribeirão Preto, SP, Brazil. (2 ) Inserm U322 Unité de Researches sur les Retrovirus et Maladies Associeés, Marseille, France. Correspondence to: Alcyone Artioli Machado, PhD. Faculty of Medicine of Ribeirão Preto, USP, Departamento de Clínica Médica. Av. Bandeirantes, 3900, Campus Universitário, 14048-900 Ribeirão Preto, SP, Brasil. ) & Jean Claude CHERMANN(2 (1 Alcyone Artioli Machado was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Proc. no. 201275/91-0 NV) ) Faculty of Medicine of Ribeirão Preto, SP, Brazil. (2 ) Inserm U322 Unité de Researches sur les Retrovirus et Maladies Associeés, Marseille, France. Correspondence to: Alcyone Artioli Machado, PhD. Faculty of Medicine of Ribeirão Preto, USP, Departamento de Clínica Médica. Av. Bandeirantes, 3900, Campus Universitário, 14048-900 Ribeirão Preto, SP, Brasil. )
SUMMARY
The MN strain of HIV-1 is known to be more prevalent in Brazil, the BRU strain is more prevalent in Europe, and the NDK strain in Africa. It has been suggested in the literature to include different strains in the same vaccine against HIV-1. To contribute to the studies for the development of a universal vaccine, the occurrence of antibodies (Ab) against three HIV-1 strains (MN, BRU and NDK) was determined in serum samples from 85 HIV-1-positive patients, adult volunteers seen at the University Hospital of the Faculty of Medicine of Ribeirão Preto-USP. One-hundred tissue culture infective unit (TCIU) of the viruses reacted with serial dilutions of the sera (2x) and with MT4 cells added at a final concentration of 0.3 × 106 cells/ml, and a cytopathic effect was observed on the 7th and 11th days of incubation. Titres of less than 1/50 were considered to be negative. In 129 tests, the sera were negative for one of the three strains: 40 for MN, 29 for BRU and 60 for NDK. There was a predominance of strains MN and BRU, most of them presenting titres from 1/50 to 1/200. Titres for NDK were detected in 25 sera. We conclude that there seems to be a predominance of strains MN and BRU among the individuals from the region tested; however, the detection of sera with positive NKD titres indicates the need for further studies of this strain in other populations and regions of Brazil.
KEYWORDS: AIDS; HIV-1 MN; HIV-1 BRU; HIV-1 NDK; Neutralizing antibodies.
INTRODUCTION
Human immunodeficiency virus (HIV) is the etiological agent of acquired immunodeficiency syndrome (AIDS)3, 11. The HIV family is characterized by a large genome and biological variability, generally in the glycoprotein of the envelope, gp120, which varies from isolate to isolate or in isolates from the same patient during different stages of the disease. The rate of variability in amino acid sequence among HIV isolates is 30%1.
Neutralizing antibodies (Ab) for HIV-1 are directed against linear or discontinuous epitopes of gp120. Those that arise early in infected humans and that are easily produced in animals by immunization are primarily directed against neutralizing determinants in the V3 loop of gp1202.
The development of an effective vaccine against HIV-1 depends on the determination of the exact role of Ab in patients with AIDS and on the study of the variability of HIV-1 isolates. Investigators testing different HIV-1 strains on Brazilian sera and in sera from other countries (Zaire, Zimbabwe, United States, Haiti) have demonstrated a high prevalence of Ab against HIV-1MN (subtype B) in Brazil4. The HIV-1BRU/LAV strain (subtype B) has been more often detected in Europe5 and strain HIV-1 NDK (subtype D) has been detected in African countries10 and is not prevalent in Brazil.
It has been recently suggested that it would be desirable to include neutralizing properties representative of different HIV-1 strains in a single vaccine8. Thus, the objective of the present investigation was to contribute to the studies on the development of a universal vaccine against HIV-1 by determining the occurrence of Ab against three HIV-1 strains (MN, BRU and NDK) in sera from patients living in a same region of Brazil.
PATIENTS AND METHODS
Serum samples were obtained from 85 adult volunteers (69 men and 16 women) anti- HIV-1 positive on the occasion of their first visit at the University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo.
The mean age of the study population was 27.7 years. Sixty-four patients were white and 21 nonwhite, and they belonged to the following exposure groups: 52 users of illicit intravenous drugs, 14 homosexuals, 11 promiscuous heterosexuals, 2 recipients of contaminated blood transfusions, 2 bisexuals, 9 indeterminate subjects, and 5 partners of individuals with HIV and/or AIDS (some patients presented more than one risk factor).
Patients were assigned to different stages of the disease according to the criteria of the Centers for Disease Control (CDC, 1986)5, as follows: group I = 1, group II = 9, group III = 7, group IVa = 9, group IVb = 1, group IVc = 59, group IVd = 2, group IVe = 2 (more than one category for the same individual).
The patients were tested for the presence of HIV-1 antibodies by an immunoenzymatic assay (Abbott recombinant HIV-1/2-IEA), following manufacturer instructions.
All samples were decomplemented by heating, aliquoted and stored at -20°C until the time of the tests.
Stocks of the viruses used, strain HIV-1MN, reference prototype HIV-1BRU and the Zairian strain HIV-1NDK were prepared from infected CEM cells and aliquots were stored
at -80°C until the time for use.
The virus neutralization assay was essentially carried out as described earlier9, with some adaptations. Briefly, 100 tissue culture infective units (TCIU) of the viruses were allowed to react with a two-fold serum dilution at a final volume of 200 µl RPMI medium supplemented with 10% fetal calf serum for 1 hour at 37°C. MT4 cells were then added at a final concentration of 0.3 × 106 cells/ml and development of the cytopathic effect was determined 7 and 11 days later. In each assay, one standardized human HIV-1 serum of known neutralizing titre and negative human sera were added, as well as a cell control and a virus dilution control. Serum samples were analysed in duplicate. The neutralization titre was calculated as the geometric mean (GMT) from two parallels of the last serum dilution inhibiting development of the cytopathic effect (CPE). A titre lower than 50 was arbitrarily considered to be negative.
RESULTS AND DISCUSSION
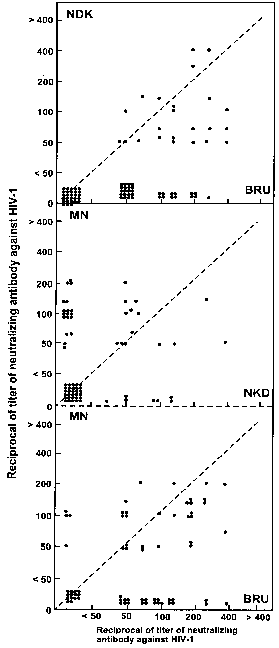
Table 1 presents the reciprocal of Ab titres against HIV-1MN, HIV-1BRU and HIV-1NDK in the 85 sera tested. It can be seen that a total of 129 tests were negative for one of the three strains, 40 of them for MN, 29 for BRU and 60 for NDK. There was a predominance of strains reactive to HIV-1MN and HIV-1BRU, with most individuals presenting Ab titres from 1/50 to 1/200.
Reciprocal of neutralizing antibody titres against HIV-1MN, HIV-1BRU, and HIV-1NDK in 85 sera from patients seen at the University Hospital, Faculty of Medicine of Ribeirão Preto, SP, Brazil
Although literature data have revealed that the NDK variant is predominant in African countries, we detected 25 sera that presented some Ab titre against this strain, with two sera presenting titres ³ 1/400 although they also reacted against BRU at lower titres.
Serum distribution with respect to the three different strains can be seen in Fig. 1, showing predominance of the MN and BRU variants. The predominance of sera with high Ab titres against strain HIV-1MN in the present population agrees with findings reported by others4.
Fig 1. - Correlation between the reciprocals of neutralizing antibody titres against three HIV-1 strains (MN, BRU, NDK) in 85 sera from patients seen at the University Hospital, Faculty of Medicine of Ribeirão Preto, USP, Ribeirão Preto, SP, Brazil.
The detection of sera with Ab titres against strain HIV-1NDK in the sample studied is a warning about the fact that this strain may be prevalent also in other regions of Brazil. In a previous study, among 86 Brazilian sera, we found 1% seropositive for anti HIV-1 NDK and 28% seropositive for both HIV-1 NDK and HIV-1 BRU6. In the same way as CARROW et al.4 demonstrated the existence of strain MN in African regions, we detected sera reacting against NDK in our region, and we agree with these authors that more studies for the determination of the true extent of world variation in HIV-1 strains may be of great importance for the development of a vaccine against this virus.
We conclude that strains reactive to MN and BRU seem to predominate among the individuals from the region tested. However, the detection of sera with titres for NDK is a warning for the need for further studies searching for other antigenicaly diverse strains in other populations and regions of Brazil.
RESUMO
Anticorpos neutralizantes contra três cepas do vírus da imunodeficiência humana tipo 1 (HIV-1) em soros de brasileiros.
A cepa MN do HIV-1 é conhecida por ser mais prevalente no Brasil, a cepa BRU é mais prevalente na Europa e a cepa NDK na África. Tem sido sugerido na literatura que diferentes cepas devam ser incluídas em uma mesma vacina contra o HIV-1. Com o intuito de se contribuir para o desenvolvimento de uma vacina universal, a ocorrência de anticorpos (Ac) contra três cepas do HIV-1 (MN, BRU, NDK) foram determinadas em amostras de soros de 85 pacientes voluntários, adultos, soropositivos para o HIV-1, atendidos em um Hospital Universitário. Cem unidades infectantes para cultura de tecido (TCIU) dos vírus reagiram com diluições seriadas de soro (2x) e células MT4 adicionadas a uma concentração final de 0,3 × 106 células/ml, e o efeito citopático foi observado no 7- e 11- dias de incubação. Títulos menores de 1/50 foram considerados negativos. Em 129 testes realizados, encontrou-se negatividade para MN em 40 soros, para BRU em 29 e em 60 para NDK. Houve uma predominância das cepas BRU e MN, a maioria nos títulos de 1/50 a 1/200. Títulos para NDK foram detectados em 25 soros. Conclui-se haver uma predominância das cepas BRU e MN nos indivíduos testados, da região; entretanto a detecção de soros positivos para NDK alerta para a necessidade de estudos desta cepa em outras populações e regiões do Brasil.
Recebido para publicação em 20/06/1997
Aceito para publicação em 17/12/1997
- 1. ALBERT, J.; ABRAHANSSOM, B.; NAGY, K. et al. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS, 4: 107-112, 1990.
- 2. ALIZON, M.; WAIN-HOBSON, S.; MONTAGNIER, L. & SONIGO, P. Genetic variability of African AIDS viruses. Cell, 46: 63-84, 1986.
- 3. BARRÉ-SINOUSI, F.; CHERMANN, J. C.; REY, F. et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science, 220: 868-871, 1983.
- 4. CARROW, E. W.; VUJCIC, L. K.; GLASS, W. L. et al. High prevalence of antibodies to the gp120 V3 region principal neutralizing determinant of HIV-1MN in sera from Africa and the Americas. Aids Res. hum. Retroviruses, 7: 831-838, 1991.
- 5. CENTERS FOR DISEASE CONTROL Classification system for human Y-lymphotropic virus type III/lymphadenopathy associated virus infection. MMWR, 35: 344, 1986.
- 6. ELLRODT, A.; BARRÉ-SINOUSSI, F.; LE BRAS, P. et al. Isolation of a new human T-lymphotropic retrovirus (LAV) from a married couple of Zairians, one with Aids, the other with the prodromes. Lancet, 1: 1383-1385, 1984.
- 7. MACHADO, A. A.; BAKPABUA, M.; SALAUN, D. et al. Neutralizing antibodies against highly cytopathic Zairian human immunodeficiency type-1 virus (HIV-1) NDK are present in sera outside Africa. Vaccine, 13: 321-325, 1995.
- 8. NEURATH, A. R. & STRICK, N. Confronting the hypervariability of an immunodominant epitope eliciting virus neutralizing antibodies from the envelope glycoprotein of the human immunodeficiency virus type 1 (HIV-1). Molec. Immunol., 27: 539-549, 1990.
- 9. REY, F.; BARRÉ-SINOUSSI, F.; SCHMIDTMAYEROVA, H. & CHERMANN, J. C. Detection and titration of neutralizing antibodies to HIV using an inhibition of the cytopathic effect of the virus on MT4 cells. J. virol. Meth., 16: 239-249, 1987.
- 10. SPIRE, B.; SPIRE, J.; ZACHAR, V. et al. Nucleotide sequence of HIV-1 NDK: a highly cytopathic strain of the human immunodeficiency virus. Gene, 81: 275-284, 1989.
- 11. WONG-STAAL, F. & GALLO, R. C. Hyman T-lymphotropic retroviruses. Nature (Lond.), 317: 395-405, 1985.
Publication Dates
-
Publication in this collection
09 Oct 1998 -
Date of issue
Nov 1997
History
-
Accepted
17 Dec 1997 -
Received
20 June 1997