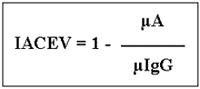Abstracts
Toxocariasis is caused by infection of man by Toxocara canis and Toxocara cati larvae, the common roundworm of dogs and cats. Because larvae are difficult to detect in tissues, diagnosis is mostly based on serology. Non specific reactions are observed mainly due to cross-reactivity with Ascaris sp antigens. This investigation aimed at developing and evaluating an indirect antibody competition ELISA (IACE) employing a specific rabbit IgG anti-Toxocara canis excretory-secretory antigens as the competition antibody, in order to improve indirect ELISA specificity performed for toxocariasis diagnosis. For that, the rabbit IgG was previously absorbed by Ascaris suum adult antigens. Sensitivity and specificity of IACE were first evaluated in 28 serum samples of mice experimentally infected with T. canis embryonated eggs. Adopting cut-off value established in this population before infection, sensitivity and specificity were 100% after 20 days post-inoculation. For human population IACE was evaluated using sera from 440 patients with clinical signs of toxocariasis and the cut-off value was established with 60 serum samples from apparently healthy individuals. Using as reference test the indirect ELISA performed by Adolfo Lutz Institute, sensitivity was 60.2%, specificity was 98% and concordance was 77.3%. Repeatability of IACE was evaluated by the inter-reactions variation coefficient (2.4%).
Toxocariasis; Visceral larva migrans; Toxocara canis; Indirect antibody competition ELISA; Immunodiagnosis
A toxocaríase, uma emergente zoonose, é uma síndrome clínica decorrente da infecção humana por larvas de Toxocara canis e Toxocara cati, parasitas intestinais de cães e gatos, respectivamente. A dificuldade de detecção das larvas nos tecidos e a inespecificidade dos sinais clínicos tornam os testes sorológicos os meios diagnósticos mais adequados. Os antígenos excretores-secretores de T. canis empregados nos testes sorológicos, embora tenham contribuído para melhorar a especificidade destes, apresentam reações cruzadas com diversos parasitas, particularmente com Ascaris sp. Com o intuito de aumentar a especificidade do diagnóstico sorológico da toxocaríase que rotineiramente é feito através do método de ELISA indireto, no presente trabalho desenvolveu-se um método de ELISA indireto de competição (EIC), utilizando IgG anti-TES produzida em coelhos e absorvida com um polímero de extrato antigênico de adultos de A. suum. A avaliação da sensibilidade e especificidade do EIC proposto foi inicialmente feita em camundongos experimentalmente infectados com T. canis. Adotando-se ponto de corte estabelecido nesta população previamente à infecção, os valores de sensibilidade e especificidade foram de 100% a partir de 20 dias pós-inoculação. Para a população humana, adotou-se um ponto de corte estabelecido a partir de 60 amostras de soro de indivíduos saudáveis e sem suspeita clínica de LMV. A avaliação do EIC foi realizada em amostras de soro de pacientes com suspeita clínica da doença e tomando como referência a classificação previamente feita pelo ELISA indireto realizado pelo Instituto Adolfo Lutz-SP. O EIC apresentou valores de sensibilidade relativa de 60,2 %, especificidade relativa de 98% e concordância de 77,3%. Na avaliação da repetibilidade do EIC obteve-se coeficiente de variação inter-reações de 2,4%.
TOXOCARIASIS: SEROLOGICAL DIAGNOSIS BY INDIRECT ANTIBODY COMPETITION ELISA
Cáris Maroni NUNES (1 (1 ) Department of Production and Animal Health, Veterinary Medicine, São Paulo State University-UNESP, Brasil. (2 ) Serology Section, Adolfo Lutz Institute of São Paulo, SP - Brasil. (3 ) Department of Veterinary Preventive Medicine and Animal Health, Faculty of Veterinary Medicine, University of São Paulo, Brasil. ), Regina Nardini TUNDISI (2 (1 ) Department of Production and Animal Health, Veterinary Medicine, São Paulo State University-UNESP, Brasil. (2 ) Serology Section, Adolfo Lutz Institute of São Paulo, SP - Brasil. (3 ) Department of Veterinary Preventive Medicine and Animal Health, Faculty of Veterinary Medicine, University of São Paulo, Brasil. ), Marcos Bryan HEINEMANN (3 (1 ) Department of Production and Animal Health, Veterinary Medicine, São Paulo State University-UNESP, Brasil. (2 ) Serology Section, Adolfo Lutz Institute of São Paulo, SP - Brasil. (3 ) Department of Veterinary Preventive Medicine and Animal Health, Faculty of Veterinary Medicine, University of São Paulo, Brasil. ), Saemi OGASSAWARA (3 (1 ) Department of Production and Animal Health, Veterinary Medicine, São Paulo State University-UNESP, Brasil. (2 ) Serology Section, Adolfo Lutz Institute of São Paulo, SP - Brasil. (3 ) Department of Veterinary Preventive Medicine and Animal Health, Faculty of Veterinary Medicine, University of São Paulo, Brasil. ) & Leonardo José RICHTZENHAIN (3 (1 ) Department of Production and Animal Health, Veterinary Medicine, São Paulo State University-UNESP, Brasil. (2 ) Serology Section, Adolfo Lutz Institute of São Paulo, SP - Brasil. (3 ) Department of Veterinary Preventive Medicine and Animal Health, Faculty of Veterinary Medicine, University of São Paulo, Brasil. )
SUMMARY
Toxocariasis is caused by infection of man by Toxocara canis and Toxocara cati larvae, the common roundworm of dogs and cats. Because larvae are difficult to detect in tissues, diagnosis is mostly based on serology. Non specific reactions are observed mainly due to cross-reactivity with Ascaris sp antigens.
This investigation aimed at developing and evaluating an indirect antibody competition ELISA (IACE) employing a specific rabbit IgG anti-Toxocara canis excretory-secretory antigens as the competition antibody, in order to improve indirect ELISA specificity performed for toxocariasis diagnosis. For that, the rabbit IgG was previously absorbed by Ascaris suum adult antigens.
Sensitivity and specificity of IACE were first evaluated in 28 serum samples of mice experimentally infected with T. canis embryonated eggs. Adopting cut-off value established in this population before infection, sensitivity and specificity were 100% after 20 days post-inoculation.
For human population IACE was evaluated using sera from 440 patients with clinical signs of toxocariasis and the cut-off value was established with 60 serum samples from apparently healthy individuals. Using as reference test the indirect ELISA performed by Adolfo Lutz Institute, sensitivity was 60.2%, specificity was 98% and concordance was 77.3%.
Repeatability of IACE was evaluated by the inter-reactions variation coefficient (2.4%).
KEYWORDS: Toxocariasis; Visceral larva migrans; Toxocara canis; Indirect antibody competition ELISA; Immunodiagnosis.
INTRODUCTION
Visceral larva migrans was first described by BEAVER et al.2 to define a clinical syndrome of man characterized by hepatomegaly, fever, chronic eosinophilia and hypergamaglobolulinemia3. A wide range of zoonotic helminths cancause visceral larva migrans but Toxocara canis and Toxocara cati, the common roundworms of dogs and cats are still the most frequently incriminated agents19.
Transmission to human beings occurs by ingestion of contaminated soil or eggs on hands and fomites. Direct contact with infected dogs and cats plays a secondary role in transmission because eggs need an extrinsic period to become infective. Neither worms nor eggs are eliminated in human feces and because larvae are difficult to detect in tissues, diagnosis is mostly based on serology7.
Since the studies made by de SAVIGNY18, the antigens mostly used for the immunodiagnostic tests derive from larvae cultivated in vitro and are referred to as Toxocara excretory-secretory (TES) antigens19.
Not all TES antigens are species or genus specific and serum samples from patients with ascariasis, filariasis, schistosomiasis and strongyloidiasis show reactivity with TES antigens1,4,11,14,15,16.
Evaluation of the true sensitivity and specificity of serology tests for toxocariasis in human populations is not possible because of the lack of parasitological methods to definitively diagnose the disease and to exclude infections in controls19.
Nevertheless, the introduction of enzyme-linked immunosorbent assay (ELISA) based upon TES antigens has resulted in greatly increased specificity14.
In areas like Brazil where Ascaris infection is endemic, cross-reactions may be mostly removed by previously absorbing each serum sample with Ascaris suum antigens4. The use of A. suum antigens for both pure and applied work on toxocariasis is justified because of the close homology between A. suum and A. lumbricoides antigens13.
Few reports have been published in Brazil in order to characterize the different forms of toxocariasis but most of them apply serological diagnosis by ELISA using TES antigens and pre-absorption with Ascaris antigens5,9,10.
In the present study, in order to improve indirect ELISA specificity performed for toxocariasis diagnosis, we evaluated the specificity and sensitivity of an indirect antibody competition ELISA based upon TES antigen using sera from patients with clinical signs of toxocariasis and sera from mice experimentally infected with T. canis eggs. The IgG fraction of a rabbit hyperimmune serum against TES was employed as the competition antibody after absorption with A. suum extract immobilized in glutaraldehyde polymer.
MATERIALS AND METHODS
Toxocara canis excretory-secretory antigens (TES)
Female adults of T. canis were collected from feces of puppies treated with piperazine adipate (100mg/Kg). After collection worms were washed in saline and fertile eggs were obtained from the uteri of gravid female and left to embryonate for 30 days at 28°C in formalin solution. Secretory-excretory antigen was prepared by the method of de SAVIGNY18 with some modifications. Briefly, eggs were hatched mechanically to allow the migration of larvae in a Baerman apparatus and were maintained in Eagle's Minimal Essential Medium for no more than 5 months. Culture medium was collected every seven days, then concentrated by ultrafiltration (PM10, Amicon, Lexington, USA) and protein content was estimated by a commercial BCA method kit (Pierce-USA).
Ascaris suum adult stage extract
Female worms were collected from swine intestine and washed in saline. Adult extract was prepared as described by CAMARGO et al.4 and protein concentration was estimated by a commercial BCA method kit (Pierce-USA).
Extracts of other parasites
One ml of Strongyloides venezuelensis filariform larvae were collected from experimentally infected rats feces. After addition of 0.02M phenylmethyl sulphonyl fluoride (PMSF,Sigma) and 0.05M EDTA they were disrupted in an ice-bath using ultrasound (Heat Systems, Microson XL). The homogenate was gently stirred for 2h at 4°C and centrifuged at 1000 x g for 30 min. at 4°C. The supernatant was stored at -20°C.
Schistosoma mansoni adult stage of the BH strain were obtained from experimentally infected hamsters and prepared according to PINTO et al.17
Metacestode extract of the ORF strain of Taenia crassiceps (Cysticercus longicollis) was prepared as described by VAZ21.
Antiserum
Two rabbits were immunized by injecting intradermically 300µg of TES antigen in Freund's complete adjuvant in multiple sites on the back skin. Six weeks later rabbits were subcutaneously injected with 300µg of TES in Freund's incomplete adjuvant followed 1 month later by another subcutaneous injection of 300µg of TES. Blood was taken 3 weeks later by intracardiac puncture.
Absorption with Ascaris suum extract
Antiserum from immunized rabbits were precipitated by 40% ammonium sulfate according to HEBERT et al.8 IgG fraction was purified by DEAE-cellulose as described by CORTHIER et al.6 IgG fraction was then absorbed twice with A. suum (AS) extract polymerized with glutaraldehyde as described by TERNYNCK & AVRAMEAS20. This methodology was chosen in order to avoid the presence of AS soluble antigens. Briefly, 50 mg of AS extract and 400 mg of bovine serum albumin were polymerized with 2 ml of glutaraldehyde 2.5%. After 3 h the polymer was centrifuged at 6,000 x g for 15 min. at 4°C, washed 3 times with PBS and 0.2M glycine-HCl buffer, pH 2.8 was added to eliminate proteins that might not had been polymerized. For absorption 1 ml of rabbit specific IgG was added to the polymer and left at room temperature for 3 h. The polymer was removed by centrifugation at 30,000 x g for 20 min. at 4°C.
Experimental infection
CH3 Rockfeller mice were infected by stomach tube with 1,100 embryonated T. canis eggs. Mice were bled from retrorbital plexus on the day of infection and on days 10, 20, 30 and 43 to 48 when they were euthanasied to have their brain tissue examined for larvae presence.
Human sera
Sixty serum samples from healthy individuals aged from 20 to 35 and 440 serum samples from patients with at least one of the common features of toxocariasis (eosinophilia, hepatomegaly and/or pulmonary symptoms, hypergammaglobulinemia, geophagia) were evaluated. Of these, 241 samples were positive by indirect ELISA performed at Adolfo Lutz Institute (IEAL) as described by CAMARGO et al.4 and 199 samples were negative. IEAL requires pre-absorption of each serum sample with A. suum extract.
Mouse sera
Serum samples of twenty-eight mice were collected before and 10, 20, 30 and 43 to 48 days after experimental infection with T. canis eggs. Sera from 2 mice not infected with T. canis eggs were used as control.
Indirect ELISA
The assay was performed to evaluated rabbit IgG reactivity before and after absorption with A. suum extract employing different parasites antigens. Procedures were as described below in the absence of competition antibody. Antigens concentration were 1.2 and 0.6µg/ml for extracts TES and AS; 1.2 and 3 µg/ml for extracts of S. venezuelensis, S. mansoni and C. longicollis.
Indirect antibody competition ELISA (IACE)
Maxisorp® microplates (Nunc, Denmark) were employed and all immunoreagents were assayed in 100 µl volumes. Between all steps of the reaction microplates were washed 3 x 5 min. with PBS-T (0.01M phosphate-buffer saline, pH 7.2 containing 0.05% Tween 80). For microplate coating TES antigen was diluted in 0.05M carbonate-bicarbonate buffer pH 9.6 and incubated overnight at 4°C. Testing sera, specific rabbit anti-TES IgG fraction used as competition antibody as well as conjugate (goat IgG anti rabbit IgG labelled to peroxidase, Sigma A-4914) were diluted in PBS-T containing 5% skimmed milk and incubated for 1h at 37°C. Five minutes after the addition of the substrate (orthophenylene diamine 0.04% in 0.1M citrate-phosphate buffer pH 5.0 and 0.03% H2O2), the enzymatic reaction was stopped with 50 µl of 1N HCl. Absorbance was read at 492 nm using an ELISA reader (Multiskan Plus® - Labsystems, Finland). Data were expressed as IACE values (IACEV), as follows:
where µA represents the mean absorbance of the duplicate of the test sample and µIgG represents the mean absorbance of the quadruplicate of the rabbit IgG
A checkerboard titration employing sera from healthy people and from patients with clinical signs of toxocariasis was performed to determine the best antigen protein concentration for coating the microplates (0.6 and 1.2 µg/ml) and the best dilution of sera (1:20, 1:40 and 1:80), rabbit IgG (1:1250, 1:2500 and 1:5000) and conjugate (1:1250, 1:2500 and 1:5000).
Cut-off value was calculated by the arithmetic mean of the absorbance of the 60 serum samples from the healthy population plus 2 standard deviation.
Repeatability of IACE was measured by repetition of a serum sample from a patient with clinical signs of toxocariasis in 25 plates.
RESULTS
Figure 1 shows results of rabbit anti-TES IgG reactivity by indirect ELISA before and after absorption with A. suum extract (AS) employing different parasite antigens. Cross-reactivity with AS extract can be seen which decreases after absorption but reactivity to TES antigens still remains. Figure 1 also shows absence of cross-reactivity between specific IgG and extracts of S. mansoni or C. longicollis but certain degree of cross-reactivity with S. venezuelensis extract can be seen which is absent after absorption.
- Reactivity of rabbit anti-TES IgG by indirect ELISA, before and after absorption with polymer of Ascaris suum extract employing different parasite antigens.
TES = Toxocara canis excretory-secretory antigens; AS = Ascaris suum adult extract; St = Strongyloides venezuelensis extract; Sm = Schistossoma mansoni extract; MTc = Taenia crassiceps metacestoide form extract
All mice experimentally infected showed live larvae in brain tissue after 43 to 48 days of infection while negative control mice did not.
After checkerboard titration the best results of indirect antibody competition ELISA (IACE) were obtained by employing TES antigen at 0.6 µg/ml, sera diluted at 1:40, rabbit IgG at 1:2500 and conjugate at 1:2500.
Sensitivity and specificity of IACE were first evaluated in 28 serum sample of mice experimentally infected with T. canis eggs. Adopting cut-off value as arithmetic mean of absorbance of mice sera before experimental infection plus 2 standard deviation, sensitivity and specificity of IACE was 100% after 20 days post-inoculation (Table 1). On the 10th day post-infection 19 out of 28 mice showed positive titers to TES antigens.
For human population IACE was evaluated using sera from 440 patients with clinical signs of toxocariasis and the cut-off value was established with 60 serum samples from apparently healthy individuals. Figure 2 shows the results of ICE applied to human serum samples compared with indirect ELISA results performed at Adolfo Lutz Institute (IEAL) according to CAMARGO et al.4 .
Indirect antibody competition ELISA values (IACEV) of 241 positive and 199 negative human serum samples to visceral larva migrans by indirect ELISA performed at Adolfo Lutz Institute-SP (IEAL).
Using IEAL as the reference test sensitivity was 60.2%, specificity was 98% and concordance was 77.3%.
Repeatability of ICE was evaluated by the inter-assay variation coefficient which was 2.4%.
DISCUSSION
Although some authors references have not related cross-reactivity of TES antigens to other parasites antigens this cross-reactivity has been shown by different means and authors1,4,14,16. A wide variety of helminths like Ancylostoma, Strongyloides, Schistosoma and Taenia can show immunological cross-reactivity with Toxocara but the commonest is cross-reactivity between Ascaris and Toxocara13,14.
A major problem of using patients sera to study cross-reactivity between Toxocara and Ascaris is the uncertain infection histories so we preferred to evaluate the reactivity of specific rabbit IgG raised against TES antigens with other parasite extracts by indirect ELISA. Before absorption, this IgG showed cross-reactivity mostly to Ascaris suum extract (AS) although certain degree of cross-reactivity with S. venezuelensis extract could be seen which is absent after absorption (Figure 1), as observed before by KENNEDY et al.13 Cross-reactivity with AS extract decreases after absorption but reactivity to TES antigens still remains, showing the existence of cross-reactivity and necessity of absorption.
Difficulties on obtaining human sera from patients truly positive to toxocariasis lead us to perform a experimental infection of mice with T. canis infective eggs. Infection was confirmed by live larvae observed in brain tissue after 43 to 48 days of infection in all mice experimentally infected. Although in natural condition paratenic hosts are exposed to small doses of infective T. canis eggs, we used high doses of infective eggs to assure humoral immune response. IgG anti-TES antigens could be detected by indirect antibody competition ELISA (IACE) as early as 10th day post inoculation in 19 of 28 mice but detection of IgG anti-TES in all experimentally infected mice was possible only by the 20th day. KAYES et al.12 have showed that antibody level peaks at 14 days post inoculation and remain more or less constant thereafter. We did not evaluate persistence of anti-Toxocara antibodies but it remained for at least 48 days post-infection.
Indirect ELISA for VLM performed at Adolfo Lutz Institute (IEAL) according to CAMARGO et al.4 requires pre-absorption of each serum sample with A. suum extract and had a sensitivity of 95.4% and specificity of 89.3%4. In our study, although we had to immunize rabbits to obtain specific IgG and we still had to absorb it with A. suum extract, it has the advantage that absorption is performed in a polymer that will not let remainder soluble antigens interfere in the test besides the fact that it will be done only for 2 times, not for each serum sample. Lack of relative sensitivity of IACE (60.2%) here presented compared with IEAL, can be explained by the fact that IACE is based on a more specific competition between antibodies from sera of patients and rabbit anti-TES IgG. Another reason to explain the lack of relative sensitivity of IACE could be insufficient absorption of antibodies from patients with AS extract while performing IEAL.
Data of specificity and sensitivity of diagnostic tests performed by most authors are based on serum samples from patients with toxocariasis signs and from patients with other parasitic infection1,4,11,15,16. In this matter our study gives different results because we tested the reactivity of some parasite extracts to the specific rabbit IgG anti-TES besides the fact that it was performed with experimentally infected mice sera truly positive to VLM.
IACE here presented has not yet been performed with sera of patients with covert or ocular toxocariasis but we think this new test will improve the diagnostic capacity of laboratories for the classical form of toxocariasis because its specificity is higher than the indirect ELISA so far performed in São Paulo State-Brazil. Therefore, cross-reactivity with Ascaris should not misdiagnose toxocariasis.
Besides good specificity and repeatability of IACE one advantage of this system is that it can be used in experimental studies of different animal species since the conjugate will always be against the rabbit IgG used as competition antibody.
RESUMO
Diagnóstico sorológico da toxocaríase através do método de elisa indireto de competição
A toxocaríase, uma emergente zoonose, é uma síndrome clínica decorrente da infecção humana por larvas de Toxocara canis e Toxocara cati, parasitas intestinais de cães e gatos, respectivamente.
A dificuldade de detecção das larvas nos tecidos e a inespecificidade dos sinais clínicos tornam os testes sorológicos os meios diagnósticos mais adequados. Os antígenos excretores-secretores de T. canis empregados nos testes sorológicos, embora tenham contribuído para melhorar a especificidade destes, apresentam reações cruzadas com diversos parasitas, particularmente com Ascaris sp.
Com o intuito de aumentar a especificidade do diagnóstico sorológico da toxocaríase que rotineiramente é feito através do método de ELISA indireto, no presente trabalho desenvolveu-se um método de ELISA indireto de competição (EIC), utilizando IgG anti-TES produzida em coelhos e absorvida com um polímero de extrato antigênico de adultos de A. suum.
A avaliação da sensibilidade e especificidade do EIC proposto foi inicialmente feita em camundongos experimentalmente infectados com T. canis. Adotando-se ponto de corte estabelecido nesta população previamente à infecção, os valores de sensibilidade e especificidade foram de 100% a partir de 20 dias pós-inoculação.
Para a população humana, adotou-se um ponto de corte estabelecido a partir de 60 amostras de soro de indivíduos saudáveis e sem suspeita clínica de LMV. A avaliação do EIC foi realizada em amostras de soro de pacientes com suspeita clínica da doença e tomando como referência a classificação previamente feita pelo ELISA indireto realizado pelo Instituto Adolfo Lutz-SP. O EIC apresentou valores de sensibilidade relativa de 60,2 %, especificidade relativa de 98% e concordância de 77,3%.
Na avaliação da repetibilidade do EIC obteve-se coeficiente de variação inter-reações de 2,4%.
ACKNOWLEDGMENTS
Authors wish to thank the Serology Section of Adolfo Lutz Institute for providing human serum samples. We also thank Dr. Pedro Luis Pinto and Dr. Adelaide Vaz for providing antigens extracts and Dr. Peter Schantz and Marianna Wilson (CDC-USA) for providing a reference serum sample. This work was supported by FAPESP (processo n° 92/74927-3).
4. CAMARGO, E.D.; NAKAMURA, P.M.; VAZ, A.J. et al. - Standardization of dot-ELISA for the serological diagnosis of toxocariasis and comparison of the assay with ELISA. Rev. Inst. Med. trop. S. Paulo, 34: 55-60, 1992.
Correspondence to: Prof. Dra. Cáris Maroni Nunes. Curso de Medicina Veterinária-UNESP. Rua Clóvis Pestana 793. Jd. D. Amélia, 16050-680 Araçatuba, SP, Brasil. Fax: 55-18-622-2638. E-mail: caris@fmva.unesp.br
Received: 18 August 1998
Accepted: 05 February 1999
- 1. BACH-RIZZATTI, B.C. - Desenvolvimento de teste imunoenzimático, ELISA, para o diagnóstico da toxocaríase humana. Săo Paulo, 1984. (Dissertaçăo de Mestrado - Faculdade de Cięncias Farmacęuticas da Universidade de Săo Paulo).
- 2. BEAVER, P.C.; SNYDER, G.M.; CARRERA, J.H. et al. - Chronic eosinophilia due to visceral larva migrans. Pediatrics, 9: 7-19, 1952.
- 3. BEAVER, P.C. - Parasitological reviews: larva migrans. Exp. Parasit., 5: 587-621, 1956.
- 5. CHIEFFI, P.P.; UEDA, M.; CAMARGO, E.D. et al. - Visceral larva migrans: a seroepidemiological survey in five municipalities of Săo Paulo State, Brazil. Rev. Inst. Med. trop. S. Paulo, 32: 204-210, 1990.
- 6. CORTHIER, G.; BOSCHETTI, E. & CHARLEY-POULAIN, J. - Improved method for the IgG purification from various animal species by ion exchange chromatography. J. immunol. Meth., 66: 75-79, 1984.
- 7. GLICKMAN, L.T. & SCHANTZ, P.M. - Epidemiology and pathogenesis of zoonotic toxocariasis. Epidem. Rev., 3: 230-250,1981.
- 8. HERBERT, G.A.; PELHAM, P.L. & PITTMAN, B. - Determination of the optimal ammonium sulphate concentration for the fractionation of rabbit, sheep, horse and goat antisera. Appl. Microbiol., 25: 26-36, 1973.
- 9. JACOB, M.C.A.; PASTORINO, A.C.; PERES, B.A. et al. - Clinical and laboratorial features of visceral toxocariasis in infancy. Rev. Inst. Med. trop. S. Paulo, 36: 19-26, 1994.
- 10. JACOB, M.C.A. - Análise evolutiva dos parâmetros clínico-laboratoriais da toxocaríase visceral na infância. Săo Paulo, 1995. (Tese de Doutoramento - Faculdade de Medicina da Universidade de Săo Paulo).
- 11. JACQUIER, P.; GOTTSTEIN, B.; STINGELIN, Y. & ECKERT, J. - Immunodiagnosis of toxocarosis in human: evaluation of a new enzyme-liked immunosorbent assay kit. J. clin. Microbiol., 29: 1831-1835, 1991.
- 12. KAYES, S.G.; OMHOLT, P.E.; GRIEVE, R.B. - Immune responses of CBA/J mice to graded infection with Toxocara canis Infect. Immun., 48: 697-703, 1985.
- 13. KENNEDY, M.W.; QURESHI, F.; HASWAELL-ELKINS, M. & ELKINS, D.B. - Homology and heterology between secreted antigens of the parasitic larval stages of Ascaris lumbricoides and Ascaris suum Clin. exp. Immunol., 67: 20-30, 1987.
- 14. LYNCH, N.R.; WILKES, L.K.; HODGEN, A.N. & TURNER, K.J. - Specificity of Toxocara ELISA in tropical population. Paras. Immunol., 10: 323-337,1988.
- 15. MAGNAVAL, J.F.; FABRE, R.; MAURIČRES, P. et al. - Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Paras. Res., 77: 697-702, 1991.
- 16. MAIZELS, R.M.; SAVIGNY, D.H. de & OGILVIE, B.M. - Characterization of surface and excretory-secretory antigens of Toxocara canis infective larvae. Paras. Immunol., 6: 23-37, 1984.
- 17. PINTO, P.L.S.; KANAMURA, H.Y.; SILVA. R.M. et al. - Dot-ELISA for detection of IgM and IgG antibodies to Schistosoma mansoni worm and eggs antigens, associated with egg excretion by patients. Rev. Inst. Med. trop. S. Paulo, 37: 109-115, 1995.
- 18. SAVIGNY, D.H de - In vitro maintenance of Toxocara canis larvae and a simple method for the production of Toxocara ES antigens for use in serodiagnostic tests for visceral larva migrans. J. Parasit., 61: 781-782, 1975.
- 19. SCHANTZ, P.M. - Toxocara larva migrans now. Amer. J. trop. Med. Hyg., 41(suppl. 3): 21-34, 1989.
- 20. TERNYNCK, T. & AVRAMEAS, S. - The cross-linking of proteins with glutaraldehyde and its use for the separation of immunoadsorbents. Immunochemistry, 6: 53-66, 1969.
- 21. VAZ, A.J. - Cysticercus longicollis: caracterizaçăo antigęnica e desenvolvimento de anticorpos em líquido cefalorraquidiano no imunodiagnóstico da neurocisticercose humana. Săo Paulo, 1993. (Tese de Doutoramento - Faculdade de Cięncias Farmacęuticas da Universidade de Săo Paulo).
Publication Dates
-
Publication in this collection
02 July 1999 -
Date of issue
Mar 1999
History
-
Accepted
05 Feb 1999 -
Received
18 Aug 1998