Abstracts
Three GST fusion recombinant antigen of Treponema pallidum, described as GST-rTp47, GST-rTp17 and GST-rTp15 were analyzed by Western blotting techniques. We have tested 53 serum samples: 25 from patients at different clinical stages of syphilis, all of them presenting anti-treponemal antibody, 25 from healthy blood donors and three from patients with sexually transmitted disease (STD) other than syphilis. Almost all samples from patients with syphilis presented a strong reactivity with GST-rTp17 antigen. Some samples were non-reactive or showed a weak reaction with GST-rTp47 and/or GST-rTp15, and apparently there was no correlation with the stage of disease. There was no seropositivity among blood donors. No sample reacted with purified GST. We concluded that due to their specificity these recombinant antigens can be used as GST fusion protein for development of syphilis diagnostic assays.
Recombinant antigen; Treponema pallidum; Western blotting; Syphilis
Os antígenos recombinantes de Treponema pallidum GST-rTp47, GST-rTp17 e GST-rTp15, produzidos em fusão com glutationa S-transferase (GST) em E. coli, foram analisados quanto ao potencial diagnóstico da sífilis pela técnica de Western blotting. Foram testadas 53 amostras, sendo 25 de pacientes em diferentes estágios clínicos da sífilis, com resultados positivos no teste treponêmico clássico; 25 amostras procedentes de doadores de banco de sangue, com sorologia negativa e 3 de pacientes com doença sexualmente transmissível não relacionado à sífilis. Todas as amostras de pacientes com sífilis apresentaram alta reatividade com o antígeno GST-rTp17. Quanto aos antígenos GST-rTp47 e GST-Tp15 verificou-se uma variação na presença ou na intensidade da reação em diferentes amostras de pacientes com sífilis, sem mostrar correlação com o estágio da doença. Nenhuma reatividade contra quaisquer desses antígenos foi observada com as amostras do grupo controle. Nenhuma das amostras testadas apresentaram reatividade com a GST purificada. A especificidade verificada na análise destes antígenos recombinantes, indicam a sua utilidade na padronização de novos testes para o imunodiagnóstico da sífilis.
ANALYSIS OF Treponema pallidum RECOMBINANT ANTIGENS FOR DIAGNOSIS OF SYPHILIS BY WESTERN BLOTTING TECHNIQUE
Neuza Satomi SATO(1, 2 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. ), Mário H.HIRATA(3 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. ), Rosário D.C. HIRATA(3 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. ),Lia Carmen M.S. ZERBINI(1 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. ), Edilene P.R. SILVEIRA(1 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. ), Carmem Silvia de MELO(1 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. ) & Mirthes UEDA(4 (1 ) Section of Serology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. (2 ) Post graduate student for PhD degree in Clinical Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (3 ) Dept. of Clinical and Toxicological Analysis, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil. (4 ) Service of Microbiology and Immunology, Division of Medical Biology, Institute Adolfo Lutz, São Paulo, SP, Brazil. )
SUMMARY
Three GST fusion recombinant antigen of Treponema pallidum, described as GST-rTp47, GST-rTp17 and GST-rTp15 were analyzed by Western blotting techniques. We have tested 53 serum samples: 25 from patients at different clinical stages of syphilis, all of them presenting anti-treponemal antibody, 25 from healthy blood donors and three from patients with sexually transmitted disease (STD) other than syphilis. Almost all samples from patients with syphilis presented a strong reactivity with GST-rTp17 antigen. Some samples were non-reactive or showed a weak reaction with GST-rTp47 and/or GST-rTp15, and apparently there was no correlation with the stage of disease. There was no seropositivity among blood donors. No sample reacted with purified GST. We concluded that due to their specificity these recombinant antigens can be used as GST fusion protein for development of syphilis diagnostic assays.
KEYWORDS: Recombinant antigen; Treponema pallidum; Western blotting; Syphilis
INTRODUCTION
Serological assays have been the fundamental procedures for syphilis screening and diagnosis. Comercially available tests such as Treponema pallidum hemagglutination (TPHA), indirect immunofluorescence (FTA-Abs), and enzyme immunoassay (EIA) have been used as confirmatory treponemal assay. Recently, Western blotting technique (WB) employing whole cell lisate of Treponema pallidum has been developed2,4,8,10,11,12,13,16,17, which has been suggested to be used as an alternative assay in place of FTA-Abs or MHA-TP2,8.
Due to inability to cultivate this bacterium in vitro for most research and diagnosis purposes the specific antigens are obtained by means of in vivo propagation, where treponemes are inoculated into rabbit testes. The difficulties in purifying of specific polypeptides because of the complexity of Treponema pallidum antigenic structures14, in addition to contamination with rabbit testicular tissue components have resulted in poorly defined native antigens; and also this tissue contamination has been responsible for inducing nonspecific results. In these cases, an absorption of samples with non-pathogenic treponeme extract or filtrate has been performed to improve the specificity of treponemal assays, removing genus-specific antibodies.
Recombinant DNA technology and recent advances in cloning techniques have allowed the production and characterization of specific protein in unlimited quantities, providing a reliable and consistent antigen source. Non-cultivable microorganism such as T. pallidum has been particularly attractive candidate for studying by means of recombinant techniques. The ability to use specific recombinant antigen will improve the sensitivity, specificity, and reproducibility of serological tests for syphilis. The major antigens of Treponema pallidum identified in studies on humoral ontogeny in experimental syphilis7, and by means of WB2,4,8,10,11,12,16,17 analysis with human serum have presented molecular weight of 47kDa, 17 kDa, and 15 kDa. All three antigens have been characterized as membrane lipoprotein1,3,15,21.
The aim of present study was to analyze the reactivity of three GST fusion recombinant antigen of Treponema pallidum, previously produced in E. coli: GST-rTp47, GST-rTp17 and GST-rTp15; by means of Western blotting technique with serum samples from patients with syphilis.
MATERIAL AND METHODS
Serum samples: A total of 53 serum samples devided into three groups were analyzed. Group 1: composed by 25 serum samples from patients at different clinical stage of syphilis (4 in secondary syphilis, 12 in recent latent phase, and 9 in late latent syphilis).
These samples were constituent of a control sera panel of Department of Serology - Institute Adolfo Lutz, São Paulo-SP, obtained from patients who referred to Department of Sanitary Dermatology of School of Public Health - University of São Paulo (USP), São Paulo - Brazil. Blood samples were drawn from patients attending the outpatient STD clinic, who have had syphilis diagnosis according to clinical, epidemiological and laboratory data. All of serum samples from these patients presented seropositivity for syphilis by means of VDRL and RPR, and on treponemal assays - TPHA, FTA-abs, ELISA-TP.
Group 2: constituted by 25 normal donor serum sample was collected from healthy blood donors who referred to Blood Center of São Paulo - General Hospital of School of Medicine - USP. All donors were routinely screened for syphilis serodiagnosis, and all of them presented negative results.
Group 3: Three serum samples from patients of Department of Sanitary Dermatology of School of Public Health - USP with clinical diagnosis of Sexually Transmitted Disease (STD) other than syphilis, who were seronegative for syphilis.
After blood clotting serum samples were separated by centrifuging, and aliquots were stored at -20oC.
Recombinant antigen of Treponema pallidum: Frozen Nichol's strain of Treponema pallidum was kindly provided by Dr. Zila R. Belém, São Paulo-SP.
Recombinant antigens of Treponema pallidum were produced in E. coli as a fusion protein with a 26 kDa glutathione S-transferase (GST), and purified individually by affinity chromatography using Glutathione Sepharose 4B19. The predict molecular weight were 72.0 kDa, 40.8 kDa, and 40.3 kDa for GST-rTp47, GST-rTp17, and GST-rTp15 recombinant protein, respectively. (These procedures were carried out for PhD thesis essay of one of the authors of present paper (SATO, NS - 1998).
Sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (WB): An equimolar mixture of three purified recombinant fusion; antigens and GST were boiled for one minute in sample buffer (50 mM Tris pH 6.8, 1% SDS, 1% b-mercaptoethanol, 10% glycerol, 0.1% bromophenol blue), and SDS-PAGE was performed employing 4.8% stacking gel and 12% separating gel in Laemmli discontinuous buffer9. Vertical mini gels were run at 100 V (stacking gel) and 180 V (separation gel). Gels were stained for 10 minutes at room temperature with 0.5% Coomassie Brilliant Blue (CBB) and destained with methanol: acetic acid : H2O (10:10:80) solution. For WB performance, separated proteins were electrotransferred to nitrocellulose membrane (pore size 0.2 µm) for 60 minutes at 2 mA/cm2 by semi-dry transfer system (Biorad, Hercules, CA, USA), and using the buffer as described by Towbin et al.20. After identifying the transferred proteins by staining with 0.5% Ponceau solution, the membrane were cut into 3mm - width strips. Nonspecific binding sites were blocked with sample buffer (0.15M PBS pH 7.3, 5% skim milk, 0.1% Tween 20) for 15 minutes, and the strips were washed once with washing buffer (0.15M PBS pH 7.3 0.2% Tween 20). All steps of assay were performed by shaking at room temperature. After that, human serum sample diluted at 1:100 or anti-GST [goat serum anti-GST (Pharmacia, Uppsala, Sweden), used as control to identify the GST bands] diluted to 1:2,000 in sample buffer were added to strips, and incubated for 15-18 hours. After washing three times for 10 minutes each, the strips were incubated for 60 minutes with second antibody conjugated to horseradish peroxidase (HRP). Goat anti-human IgG-HRP (Sigma, St. Louis, USA) at 1:1000 dilution or rabbit anti-goat IgG -HRP (Sigma, St. Louis, USA) at 1:2,000 dilution in sample buffer were employed as second antibody. Strips were washed as before, and the color was developed by adding 0.6 mg/ml 4-chloro-1-naphthol (Sigma, St. Louis, USA) in 0.15M PBS pH 7.3 with 0.02% H2O2. Final reaction was stopped by washing the strips with distilled H2O.
RESULTS AND DISCUSSION
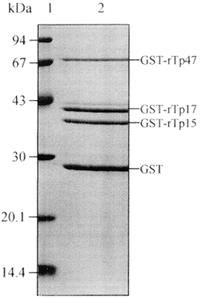
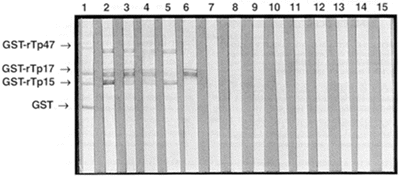
SDS-PAGE analysis of recombinant antigens of molecular weight of 72.0 kDa, 40.8 kDa and 40.3 kDa, and GST of 26 kDa (Figure 1) demonstrate that there is no degeneration of them, indicating that these fusion proteins are stable molecules. GST-rTp17 (40.8 kDa) exhibits a duplet band on SDS-PAGE, and a third lower band of low intensity revealed by some samples on WB (Figure 2) seems to be hallmark of expression of this definite antigen. Reaction with anti-GST (1) on WB (Figure 2) display the positions of GST-rTp and of GST.
- SDS-PAGE, recombinant antigens of Treponema pallidum GST-rTp47, GST-rTp17, GST-rTp15, and purified GST (2). At left, standard protein markers (molecular mass in kDa).
- Profile of immunoreactivity of recombinant Treponema pallidum antigens. Western blotting technique with anti-GST serum (1), serum from patients with syphilis (2-6), serum from seronegative blood donors (7-12), and serum from patients with STD other than syphilis (13-15).
Almost all of samples from patients suffering from syphilis presented reactivity with rTp17 antigen, and in a higher intensity in relation to the others antigens. Some samples demonstrated low or lack of reactivity against 47 kDa and/or 15 kDa antigens. This result agrees with previous data obtained from the analysis on WB employing native antigen, where some samples presented reactivity to 17 kDa fraction only18.
Major reactivity to 17 kDa antigen was also reported by Fujimura et al.5 in comparing the reactivity against different recombinant antigen of Treponema pallidum employing separated ELISA for every antigen. Similar remarks were made by GERBER et al.6 when immunereactivity of Treponema pallidum recombinant antigens with sera from patients with secondary syphilis was analyzed by means of WB.
Based on the samples analyzed in the present study, there was no possibility to observe a correlation between prevalence or intensity of reactivity and clinical stage of disease. Possibly, 47 kDa and 15 kDa proteins epitopes might be important for primary syphilis detection, of which samples were not available in this study. Some authors report a great concern on 47 kDa antigen as active syphilis marker, which is confirmed by the presence of specific IgM antibody performing WB with samples from patients with congenital syphilis 10,16,17.
Neither blood donors serum samples nor samples from patients with STD other than syphilis presented positivity on WB (Figure 2), indicating highly specific reactivity of these tested recombinant antigens.
Analysis on WB technique denotes that none of studied samples (from patients with syphilis, from patients with STD other than syphilis, and from blood donors) presents reactivity to GST, indicating that the reaction to treponemal portion of fusion protein is specific. Although treponemal portion (rTp) might be purified after cleavage of fusion protein (GST-rTp) by treatment with thrombin, this step could be excluded from the protocol considering the specificity observed in present study.
In addition to this the data from this investigation show that these recombinant antigens can be employed, even in fusion with GST, for enzyme immunoassay (EIA) standardization and also for improvement of assay for detection of different antibody isotypes anti-Treponema pallidum.
RESUMO
Análise de antígenos recombinantes de Treponema pallidum no diagnóstico da sífilis utilizando a técnica de Western Blotting
Os antígenos recombinantes de Treponema pallidum GST-rTp47, GST-rTp17 e GST-rTp15, produzidos em fusão com glutationa S-transferase (GST) em E. coli, foram analisados quanto ao potencial diagnóstico da sífilis pela técnica de Western blotting. Foram testadas 53 amostras, sendo 25 de pacientes em diferentes estágios clínicos da sífilis, com resultados positivos no teste treponêmico clássico; 25 amostras procedentes de doadores de banco de sangue, com sorologia negativa e 3 de pacientes com doença sexualmente transmissível não relacionado à sífilis. Todas as amostras de pacientes com sífilis apresentaram alta reatividade com o antígeno GST-rTp17. Quanto aos antígenos GST-rTp47 e GST-Tp15 verificou-se uma variação na presença ou na intensidade da reação em diferentes amostras de pacientes com sífilis, sem mostrar correlação com o estágio da doença. Nenhuma reatividade contra quaisquer desses antígenos foi observada com as amostras do grupo controle. Nenhuma das amostras testadas apresentaram reatividade com a GST purificada. A especificidade verificada na análise destes antígenos recombinantes, indicam a sua utilidade na padronização de novos testes para o imunodiagnóstico da sífilis.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Luiz Jorge Fagundes, from Center of Health Geraldo de Paula Souza, School of Public Health - USP, São Paulo-SP for providing serum samples from patients of clinical diagnosis of syphilis; to Dr. Amadeo Saez Alquézar, from Blood Center of São Paulo, São Paulo-SP for providing seronegative samples from blood donors; and Prof. Kimitsuna Watanabe, Dr. Tsutomu Suzuki, Dr. Takuya Ueda from Dept. of Chemistry and Biotechnology, Graduate School of Engineering - University of Tokyo for their help and support with characterization of recombinant DNA and protein of T. pallidum.
REFERENCES
1. AKINS, D.R.; PURCELL, B.K.; MITRA, M.M.; NORGARD, M.V. & RADOLF, J.D. - Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect. Immun., 61: 1202-1210, 1993.
2. BYRNE, R.E; LASKA, S.; BELL, M. et al. - Evaluation of a Treponema pallidum Western Immunoblot assay as a confirmatory test for syphilis. J. clin. Microbiol., 30: 115-122, 1992.
3. CHAMBERLAIN, N.R.; BRANDT, M.E., ERWUIN, A.L.; RADOLF, J.D. & NORGARD, M.V. - Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect. Immun., 57: 2872-2877, 1989.
4. DOBSON, S.R.M.; TABER, L.H. & BAUGHN, R.E. - Recognition of Treponema pallidum antigen by IgM and IgG antibodies in congenitally infected newborns and their mothers. J. infect. Dis., 157: 903-910, 1988.
5. FUJIMORI, K.; ISE, N.; UENO, E. et al. - Reactivity of recombinat Treponema pallidum (r-Tp) antigens with anti-Tp antibodies in human syphilitic sera evaluated by ELISA. J. clin. Lab. Anal., 11: 315-322, 1997.
6. GERBER, A.; KRELL, S. & MORENS, J. - Recombinant Treponema pallidum antigens in syphilis serology. Immunobiology, 196: 535-549, 1996.
7. HANFF, P.A.; BISHOP, N.H.; MILLER, J.N. & LOVETT, M.A. - Humoral immune response in experimental syphilis to polypeptides of Treponema pallidum. J. Immunol., 131: 1973-1977, 1983.
8. HENSEL, U; WELLENSIEK, H.J. & BHAKDI, S. - Sodium dodecyl sulfate-polyacrylamide gel electrophoresis immunoblotting as a serological tool in diagnosis of syphilitic infections. J. clin. Microbiol., 21: 82-87, 1985.
9. LAMMLI, U.K. - Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond.), 227: 680-695, 1990.
10. LEWIS, L.L.; TABER, L.H & BAUGHN, R.E. - Evaluation of immunoglobulin M Western Blot analysis in the diagnosis of congenital syphilis. J. clin. Microbiol., 28: 296-302, 1990.
11. LUKEHART, S.; BAKER-ZANDER, S. & GUBISH, E. - Identification of Treponema pallidum antigens: comparisom with non-pathogenic treponeme. J. Immunol., 129: 833-838, 1982.
12. MEYER, M.P.; EDDY, T. & BAUGHN, R.E. - Analysis of Western blotting (Immunoblotting) technique in diagnosis of congenital syphilis. J. clin. Microbiol., 32: 629-633, 1994.
13. NORGARD, M.V. - Clinical and diagnostic issues of acquired and congenital syphilis encompassed in the current syphilis epidemic. Curr. Opin. infect. Dis., 6: 9-16, 1993.
14. NORRIS, S.J. & STELL, S. - Antigenic complexicity of Treponema pallidum: antigenicity and surface localization of major polypeptides. J. Immunol., 133: 2686-2692, 1984.
15. PURCELL, B.K.; CHAMBERLAIN, N.R.; GOLDBERG, M.S. et al. - Molecular cloning and characterization of the 15-kilodalton major immunogen of Treponema pallidum. Infect. Immun., 57: 3708-3714, 1989.
16. SANCHEZ, P.J.; McCRACKEN JR., G.H.; WENDEL, G.D. et al. - Molecular analysis of the fetal IgM response to Treponema pallidum antigens: implications for improved serodiagnosis of congenital syphilis. J. infect. Dis., 159: 508-517, 1989.
17. SANCHEZ, P.J.; WENDEL, G.D. & NORGARD, M.V. - IgM antibody to Treponema pallidum in cerebrospinal fluid of infants with congenital syphilis. Amer. J. Dis. Child., 146: 1171-1175, 1992.
18. SATO, N.S. & TAKEDA, A.K. - Antígeno recombinante 47 kDa de Treponema pallidum: caracterização imunológica e estudo comparativo com o antígeno nativo. Rev. bras. Anal. clín., 28: 8-11, 1996.
19. SMITH, D.B. & JOHNSON, K.S. - Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene, 67: 31-34, 1988.
20. TOWBIN, H.; STAEHELIN, T. & GORDON, J. - electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. nat. Acad. Sci. (Wash.), 76: 4350-4354, 1979.
21. WEIGEL, L.M.; BRANDT, M.E. & NORGARD, M.V. - Analysis of the N-terminal region of the 47-kilodalton integral membrane lipoprotein of Treponema pallidum. Infect. Immun., 60: 1568-1576, 1992.
Correspondence to: Neuza S. Sato. Av. Dr. Arnaldo 335, 10th floor, 01243-902 São Paulo, SP, Brazil. Tel.: 55-11-3061-0111, r. 2063/2064. Fax: 55-11-853-2197. E-mail: neuzasat@uol.com.br
Received: 8 October 1998
Accepted: 8 March 1999
- 9. LAMMLI, U.K. - Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond.), 227: 680-695, 1990.
- 12. MEYER, M.P.; EDDY, T. & BAUGHN, R.E. - Analysis of Western blotting (Immunoblotting) technique in diagnosis of congenital syphilis. J. clin. Microbiol., 32: 629-633, 1994.
Publication Dates
-
Publication in this collection
02 July 1999 -
Date of issue
Mar 1999
History
-
Accepted
08 Mar 1999 -
Received
08 Oct 1998