Abstracts
The clinical and public health importance of indeterminate results in HIV-1/2 testing is still difficult to evaluate in volunteer blood donors. At Fundação Hemominas, HIV-1/2 ELISA is used as the screening test and, if reactive, is followed by Western blot (WB). We have evaluated 84 blood donors who had repeatedly reactive ELISA tests for HIV-1/2, but indeterminate WB results. Sixteen of the 84 donors (19.0%) had history of sexually transmitted diseases; 18/84 (21.4%) informed receiving or paying for sex; 3/84 (3.6%) had homosexual contact; 2/26 women (7.6%) had past history of multiple illegal abortions and 3/84 (3.6%) had been previously transfused. Four out of 62 donors (6.5%) had positive anti-nuclear factor (Hep2), with titles up to 1:640. Parasitological examination of the stool revealed eggs of S. mansoni in 4/62 (6.4%) donors and other parasites in 8/62 (12.9%). Five (5.9%) of the subjects presented overt seroconversion for HIV-1/2, 43/84 (51.2%) had negative results on the last visit, while 36/84 (42.9%) remained WB indeterminate. Although some conditions could be found associated with the HIV-1/2 indeterminate WB results and many donors had past of risky behavior, the significance of the majority of the results remains to be determined.
HIV-1/2; Indeterminate Western blot; Blood donor; Risk behaviors
A importância clínica e de saúde pública de resultados indeterminados em exames para HIV-1/2 é ainda difícil de avaliar em doadores de sangue voluntários. Na Fundação Hemominas é utilizado um teste de triagem (ELISA) que, se reativo, é seguido pelo Western blot (WB). Avaliamos nesse estudo 84 doadores que apresentavam ELISA repetidamente reativo, mas WB indeterminado. Dos 84 indivíduos, 16 (19,0%) tinham história de doenças sexualmente transmissíveis; 18/84 (21,4%) informaram ter recebido ou pago por sexo; 3/84 (3,6%) informaram contato homossexual; 2/26 mulheres (7,6%) tinham história pregressa de múltiplos abortos ilegais e 3/84 (3,6%) tinham sido transfundidos. Quatro de 62 doadores (6,5%) tinham fator anti-nuclear (Hep2) positivo, com títulos de até 1:640. Exame parasitológico revelou a presença de ovos de S. mansoni nas fezes de 4/62 (6,4%) e outros parasitas em 8/62 (12,9%). Cinco indivíduos (5,9%) apresentaram franca conversão para HIV-1/2 no WB; 43/84 (51,2%) tinham resultados negativos na última visita, enquanto que 36/84 (42,9%) permaneceram com o WB indeterminado. Concluímos que, embora pudéssemos encontrar algumas condições associadas ao resultado indeterminado para HIV-1/2 no WB e muitos doadores com história pregressa de comportamento de risco, o significado da maioria dos resultados ainda necessita elucidação.
HIV-1/2 indeterminate Western blot results: follow-up of asymptomatic blood donors in Belo Horizonte, Minas Gerais, Brazil
A.B.F. CARNEIRO-PROIETTI(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , I.W. CUNHA(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , M.M. SOUZA(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , D.R. OLIVEIRA(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , N.M. MESQUITA(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , C.A. ANDRADE(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , B.C. CATALAN-SOARES(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br , M.V.C. LIMA-MARTINS(1) (1) Fundação Hemominas, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone: 5531-2733377 Fax: 5531-2262002 E-mail: arraia@gold.com.br & F.A. PROIETTI(2) (2) Departamento de Medicina Preventiva e Social, Escola de Medicina, Universidade Federal de Minas Gerais, Brasil. Phone: 5531-2240911
SUMMARY
The clinical and public health importance of indeterminate results in HIV-1/2 testing is still difficult to evaluate in volunteer blood donors. At Fundação Hemominas, HIV-1/2 ELISA is used as the screening test and, if reactive, is followed by Western blot (WB). We have evaluated 84 blood donors who had repeatedly reactive ELISA tests for HIV-1/2, but indeterminate WB results. Sixteen of the 84 donors (19.0%) had history of sexually transmitted diseases; 18/84 (21.4%) informed receiving or paying for sex; 3/84 (3.6%) had homosexual contact; 2/26 women (7.6%) had past history of multiple illegal abortions and 3/84 (3.6%) had been previously transfused. Four out of 62 donors (6.5%) had positive anti-nuclear factor (Hep2), with titles up to 1:640. Parasitological examination of the stool revealed eggs of S. mansoni in 4/62 (6.4%) donors and other parasites in 8/62 (12.9%). Five (5.9%) of the subjects presented overt seroconversion for HIV-1/2, 43/84 (51.2%) had negative results on the last visit, while 36/84 (42.9%) remained WB indeterminate. Although some conditions could be found associated with the HIV-1/2 indeterminate WB results and many donors had past of risky behavior, the significance of the majority of the results remains to be determined.
KEYWORDS: HIV-1/2; Indeterminate Western blot; Blood donor; Risk behaviors.
INTRODUCTION
Belo Horizonte, Minas Gerais State, is located in South-eastern Brazil and has a population of about 4 million inhabitants in its metropolitan area. In Brazil, 116,389 AIDS cases have been reported to the Ministry of Health between 1980 and 199717. According to the absolute number of cases reported, Minas Gerais is the third state in the country in number of AIDS cases. In 1983 the male to female ratio of HIV-1 infection was forty to one in Brazil. In 1997, this ratio went down to 3:1. In 1997, 2.9% of all cases reported to the Ministry of Health were attributed to exposure to blood and blood products, excluding intravenous drug users17.
Blood donation generates presently a number of test results in healthy people who otherwise would not be tested. The implementation in blood banks of new tests to recently discovered viruses leads to uncertainty on how to interpret the results, specially if they are indeterminate4. The screening of volunteer blood donors for antibodies to the human immunodeficiency viruses (HIV-1/2) is usually done with enzyme immunoassay tests, followed by a supplemental technique if repeatedly reactive3,16. The supplemental tests routinely used include Western blot (WB), immunofluorescence and particle agglutination assays. The WB is the most widely used supplemental test3. It usually reveals six to nine characteristic bands if antibodies to HIV-1 proteins of different molecular weights are present, and no bands if such antibodies are absent. New tests include proteins specific for HIV-2. Some donor blood samples present one or two bands characteristic of HIV-1, reacting for example to one or more of the HIV-1 core proteins (p17, p24 and p55) and therefore the result does not meet the definition of either a positive specimen (with the following bands present: p24, gp41, and either gp120 or gp160 bands) or a negative specimen (no bands present). These specimens are designated HIV indeterminate1,2,3.
The clinical and transfusional importance of these indeterminate WB results are poorly understood in populations generally considered at low-risk1,2,8,9 and, as a result, donor counseling becomes extremely complex. One study has also shown that a relatively high percentage of individuals with negative anti-HIV-1 antibody with commercial ELISA had an indeterminate WB result14.
To evaluate selected factors possibly related to the indeterminate WB results, we conducted a follow-up study of 84 blood donors with repeatedly indeterminate results. This study consisted of baseline medical examination and an interview eliciting high risk behaviors for HIV and history of blood transfusion, hospitalization and surgeries. The donors were followed with periodic clinical and laboratorial evaluation (one to six months intervals). We have also analyzed the band patterns, trying to relate them with the conditions detected.
MATERIAL AND METHODS
Population studied
At Fundação Hemominas, the Minas Gerais State Blood Center, potential donors are submitted to a medical examination to exclude individuals with conditions considered unsuitable for blood donation (e.g., fever, history of use of illegal intravenous drugs, etc.) and/or with hemoglobin levels below 12.0 g/dL and 13.0 g/dL for females and males, respectively. Donors considered fit for donation have their blood screened for several blood transmissible agents (T. palladium, T. cruzi, Hepatitis B and C virus, HIV-1/2, HTLV-I/II). Those with negative results in all serological tests have their blood considered eligible for processing and transfusion; otherwise, the blood is appropriately discarded.
From mid-1993 to January 1998, all individuals with indeterminate HIV-1/2 WB results were invited by letter to an interview with a social worker. Eighty-four agreed to participate in the study and were referred to a physician at the Research Service. After obtaining informed consent for this study, the physician applied a questionnaire to elicit past history of high risk behaviors and situations for transmission of HIV-1/2, performed physical examination and requested a series of complementary testing (detailed in the following section). Donors were followed-up while they remained with indeterminate results. In case of two consecutive HIV-1/2 negative results (ELISA plus WB), donors were not further tested. If they presented overt seroconversion during the study period, they were referred to the State AIDS Clinic.
Medical evaluation and laboratory testing
Medical examination was performed in all individuals to assess general health. In the epidemiological interview, emphasis was placed on a variety of risky behaviors for HIV-1/2 transmission, on autoimmune diseases, previous surgeries, hospitalizations and use of medications. HIV-1/2 testing consisted of ELISA (Abbott recombinant, USA), done in triplicate, followed by WB (Cambridge Biotech, USA), if repeatedly reactive. Both were done according to the manufacturers instructions. Five samples with persistently indeterminate WB results were submitted to HIV-2 immunoassay test (Karpas Cell Test, Cambridge, UK), in which HIV-2 infected cells fixed in slides are used as antigen to detect HIV antibodies in serum11. Each individual was asked to have a number of blood assays performed, which included hematological tests (complete blood cell counts and blood sedimentation) plus markers of other infectious diseases (hepatitis B and C, syphilis, Chagas disease, HTLV-I/II). Anti-nuclear test (FAN-Hep2) was done in 62 of the studied individuals and in 30 seronegative healthy controls. Routine urine examination and search of parasites in the stools were also performed.
RESULTS
During 1997, 143,869 individuals presented to Fundação Hemominas for donation. Of these, 82,286 (57.2%) were considered eligible for donation. The reasons more frequent for not donating were: elevated blood pressure, use of medication, common cold and high risk behavior for sexually transmissible diseases. Most of the donors were males (82.8%), between 20 and 34 years of age (64.5%) and single (50.7%). The prevalence for antibodies to HIV-1 in the donated blood at Fundação Hemominas is approximately 2/10,000 donations (ELISA confirmed by WB).
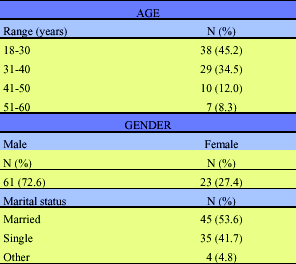
Eighty-four donors with indeterminate WB results were enrolled in the study, and followed-up over time according to the pre-established protocol. Of the 84 donors, 61 (72.6%) were males and 23 (27.4%) were females (Table 1). The mean age was 33.2 ± 5.2 SD. Most of them were married (n = 45; 53.6%). Seventy-five (89.3%) were from the Belo Horizonte metropolitan area and nine (10.7%) from other cities in Minas Gerais State. Forty-nine (58.3%) had only elementary schooling or below. All of them denied high risk behaviors for sexually transmitted diseases (STDs) when first presenting for donation. After enrollment in the study, twenty-two donors (26.2%) reported factors associated with high risk for HIV-1/2 infection, sometimes more than one for a single individual (Table 2). These factors were: paying or receiving money for sex (n = 18; 21.4%), STDs (n = 16; 19.0%), multiple abortion (n = 2; 2.4%) and transfusion (n = 4; 4.8%). Two (2.4%) reported use of intravenous drugs, two (2.4%) were bisexual, and one (1.2%) had recent heterosexual contact with an HIV-1 seropositive individual.
Age, gender and marital status of 84 blood donors with HIV-1/2 indeterminate WB results
Risk behavior in blood donors with HIV indeterminate WB results
1 Some donors reported multiple risk behaviors; total number of donors at risk = 22.
2 Intravenous drug user.
The physical exam and the remaining laboratory results, including tests for blood transmissible diseases (hepatitis B and C, Chagas disease, syphilis and HTLV-I/II) were negative in all except three donors. Two of these three had positive anti-HBc (hepatitis B virus core antigen). The third donor showed positive syphilis tests (VDRL and FTA-Abs) and HBsAg (hepatitis B surface antigen).
Four donors (6.5%), two females and two males, showed laboratory results suggestive of auto-immune conditions. Anti-nuclear antibodies (Hep-2) were present in two donors with titles of 1:40 and in one with 1:640. In the control group (n = 31), composed of seronegative asymptomatic donors, only one (3.2%) had the Hep 2 test positive at the dilution 1:80. All others were negative.
Thirty-six of the 84 donors (42.9%) remained with indeterminate WB results during the follow-up period, which was up to five years long. Of these, five were non-reactive for HIV-2. Forty-three (51.2%) became negative in the WB, although five of them remained with a profile of low reactivity and one was persistently reactive in the ELISA. During the follow-up period, five (5.9%) of the donors presented overt seroconversion for HIV-1 with appearance of all diagnostic bands in the WB.
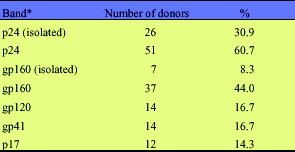
The WB band more commonly present was p24 (gag), isolated (n = 26; 30.9%) or in association with other bands of gag and/or env and/or pol (n = 51; 60.7%). The next more common were env bands, with gp160 isolated (n = 7; 8.3%) or present in combination with other bands in 37 (44.0%) individuals (Table 3). The band pattern was not stable and could be changed in the same individual over time, i.e., it could change for example from gag to env and vice-versa.
Western Blot: bands present in donors tests with indeterminate results
* Present in association with other bands, unless otherwise specified.
DISCUSSION
The long-term outcome and conditions associated with persons identified as having HIV-1 indeterminate WB after being repeatedly reactive by screening ELISA are not well characterized and may vary from one geographical region to the other. A vast number of conditions have been reported by different authors as being associated with indeterminate WB results. They include: systemic lupus erythematosus1,2,4, presence of rheumatoid factor and polyclonal gamopathy1,2, antibodies to DR-HLA5,16, cross-reactivity to core proteins of other retroviruses like Bovine Immunodeficiency Virus7,10,19, Mycobacterium leprae infection12, heat inactivation of serum samples6, in vitro hemolysis, elevated bilirrubin levels (Cambridge Biotech HIV WB kit insert) and tetanus vaccination1,2.
We have prospectively evaluated a group of blood donors with indeterminate WB patterns. Presumably, volunteer blood donors in a low prevalence area who report no risky behavior for HIV infection at the donor screening interview and who have repeatedly indeterminate results on WB at both initial and follow-up evaluations are not infected with HIV13,15. In some populations, even long-term follow-up of blood donors with HIV Western blot indeterminate results does not reveal evidence of HIV infection9. It has even been suggested that no further clinical and serological follow-up would be necessary in these cases and that it should be considered a mechanism to return these donors to the donor pool8. In our view, this should be regarded with caution. In our study, many donors reported risky behavior for HIV-1 only when their blood tested positive after donating and even then only after multiple interviews with different members of the team. Knowing that there was a problem with their blood test sometimes led to a voluntary disclosure of multiple risk behaviors. The reasons for the denial on the part of the donor at the initial and subsequent interview(s) may be multiple. One important motivation in our milieu is the individual donating to get a free HIV test, that otherwise would mean attending State subsidized STD clinics, long lines or be difficult to get free of charge. One of the main objectives of Fundação Hemominas is to get a substantial percentage of the voluntary blood donors to be what is called repetition donors, i.e., individuals who donate spontaneously two to four times a year. They are usually considered safer donors, once they are consistently negative. However, this concept has been recently reviewed (Dr. de Pinho AM, personal communication), because it was realized that individuals reporting high risk behaviors for sexually transmitted diseases (sex workers, homo or bisexual men reporting multiple sexual partners) donate repeatedly in order to obtain HIV test free of charge, even when they know the possible implications of HIV transmission associated with blood donation. Even physicians are still known to recommend blood donation as a fast, free and reliable means of knowing their HIV status.
The progression to a full pattern of HIV WB and ELISA positivity observed in five individuals was rapid (one to three months). One of them reported a recent heterosexual contact with an HIV positive individual, which was the motivation for donating blood, aiming to know the HIV serostatus. Five other donors who remained with indeterminate WB, had PCR results (data not shown) compatible with previous exposure to HIV-1, which has been designated viral WB indeterminate results18, as opposed to individuals in whom no apparent exposure to retroviruses has been observed, called non-viral cases. In the non-viral situation, we could detect four individuals with autoantibodies which could be interfering with the WB reaction, as reported previously1,2,4.
We have observed variation in the WB band patterns during the follow-up period in some donors, and the variation was not restricted to the original groups of bands corresponding to gag, pol and env products.
Even though indeterminate HIV-1 WB results could be due to cross-reaction with HIV-2, samples of individuals persistently indeterminate tested for HIV-2 with a highly sensitive and specific technique11 were not reactive. This was an expected result due to the paucity of reported cases of HIV-2 infection in Brazil.
The significance of the majority of the cases of donors with an HIV-positive ELISA and indeterminate WB results remains to be determined. Prolonged clinical and laboratorial follow-up of these individuals in reference centers, with addition of molecular tests may be necessary to answer these questions and to improve donor selection and notification strategies.
RESUMO
Resultados indeterminados no Western Blot para HIV-1/2: follow-up de doadores de sangue assintomáticos em Belo Horizonte, Minas Gerais, Brasil
A importância clínica e de saúde pública de resultados indeterminados em exames para HIV-1/2 é ainda difícil de avaliar em doadores de sangue voluntários. Na Fundação Hemominas é utilizado um teste de triagem (ELISA) que, se reativo, é seguido pelo Western blot (WB). Avaliamos nesse estudo 84 doadores que apresentavam ELISA repetidamente reativo, mas WB indeterminado. Dos 84 indivíduos, 16 (19,0%) tinham história de doenças sexualmente transmissíveis; 18/84 (21,4%) informaram ter recebido ou pago por sexo; 3/84 (3,6%) informaram contato homossexual; 2/26 mulheres (7,6%) tinham história pregressa de múltiplos abortos ilegais e 3/84 (3,6%) tinham sido transfundidos. Quatro de 62 doadores (6,5%) tinham fator anti-nuclear (Hep2) positivo, com títulos de até 1:640. Exame parasitológico revelou a presença de ovos de S. mansoni nas fezes de 4/62 (6,4%) e outros parasitas em 8/62 (12,9%). Cinco indivíduos (5,9%) apresentaram franca conversão para HIV-1/2 no WB; 43/84 (51,2%) tinham resultados negativos na última visita, enquanto que 36/84 (42,9%) permaneceram com o WB indeterminado. Concluímos que, embora pudéssemos encontrar algumas condições associadas ao resultado indeterminado para HIV-1/2 no WB e muitos doadores com história pregressa de comportamento de risco, o significado da maioria dos resultados ainda necessita elucidação.
ACKNOWLEDGMENTS
We would like to thank the Social Service of Fundação Hemominas for referring the blood donors and Drs. Suzane P.F. Neves and Laiz E.B. Marzano for performing the Hep2 tests.
Supported by: Fundação Hemominas, FAPEMIG, CNPq, Capes
Correspondence to: Dr. A.B. F.Carneiro-Proietti, Alameda Ezequiel Dias 321, 30130-110 Belo Horizonte, Minas Gerais, Brazil. Phone:55312733377 Fax:5531-2262002. e-mail: arraia@gold.com.br
Received: 19 August 1998
Accepted: 29 March 1999
- 1. CELUM, C.L.; COOMBS, R.W.; LAFFERTY, W. et al. - Indeterminate human immunodeficiency virus type 1 Western blots: seroconversion risk, specificity of supplemental tests, and an algorithm for evaluation. J. infect. Dis., 164: 656-664, 1991.
- 2. CELUM, C.L.; COOMBS, R.W.; JONES, M. et al. - Risk factors for repeatedly reactive HIV-1 EIA and indeterminate Western blots. A population-based case-control study. Arch. intern. Med., 154: 1129-1137, 1994.
- 3. CONSTANTINE, N.T. - Serologic tests for the retroviruses: approaching a decade of evolution. AIDS, 7: 1-13, 1993.
- 4. DOCK, N.L.; KLEINMAN, S.H.; RAYFIELD, M.A. et al. - Human immunodeficiency virus infection and indeterminate Western blot patterns: prospective studies in a low prevalence population. Arch. intern. Med., 151: 525-530, 1991.
- 5. DRABICK, J.J. & BAKER, J.R. - HLA antigen contamination of commercial Western blots strips for detecting human immunodeficiency virus. J. infect. Dis., 159: 357-358., 1989.
- 6. GOLDFARB, M.F. - Effect of heat inactivation on results of HIV antibody detection by Western blot assay. Clin. Chem., 34: 1661-1662, 1988.
- 7. GONDA, M.A.; BRAUN, M.J.; CARTER, S.G. et al. - Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. Nature (Lond.), 330: 388-391, 1987.
- 8. JACKSON, J.B.; MACDONALD, K.L.; CADWEL, J. et al. - Absence of HIV infection in blood donors with indeterminate Western blot tests for antibody to HIV-1. New Engl. J. Med., 322: 217-222, 1990.
- 9. JACKSON, J.B.; HANSON, M.R.; JOHNSON, G.M. et al. - Long-term follow-up of blood donors with indeterminate human immunodeficiency virus type 1 results on Western blot. Transfusion, 35: 98-102, 1995.
- 10. JACOBS, R.M.; SMITH, H.E.; GREGORY, B.; VALLI, V.E.O. & WHETSTONE, C.A. - Detection of multiple retroviral infections in cattle and cross-reaction of bovine immunodeficiency virus and human immunodeficiency virus type 1 proteins using bovine and human sera in a Western blot assay. Canad. J. vet. Res., 56: 353-359, 1992.
- 11. KARPAS, A.; HILL, F.; TENANT-FLOWER, M. et al. - Use of Karpas HIV cell test to detect antibodies to HIV-2. Lancet, 2 (8551): 132-133, 1987.
- 12. KASHALA, O.; MARLINK, R.; ILUNGA, M. et al. - Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J. infect. Dis., 169: 296-304, 1994.
- 13. KLEINMANN, S.; FITZPATRICK, L.; SECORD, K. & WILKE, D. - Follow-up testing and notification of anti-HIV Western blot atypical (indeterminant) donors. Transfusion, 28: 280-282, 1988.
- 14. MIDTHUN, K.; GARRISON, L.; CLEMENTS, M.L. et al. - Frequency of indeterminate Western blot tests in healthy adults at low risk for human immunodeficiency virus infection. The NIAID AIDS vaccine clinical trails network. J. infect. Dis., 162: 1379-1382, 1990.
- 15. NUSBACHER, J. & NAIMAN, R. - Longitudinal follow-up of blood donors found to be reactive for antibody to human immunodeficiency virus (anti-HIV) by enzyme-linked immunoassay (EIA+) but negative by Western blot (WB). Transfusion, 4: 365-367, 1989.
- 16. PERRIN, L.H.; YERLY, S.; ADAMI, N. et al. - Human immunodeficiency virus DNA amplification and serology in blood donors. Blood, 76: 641-645, 1990.
- 17. PROGRAMA NACIONAL DE DOENÇAS SEXUALMENTE TRANSMISSÍVEIS/AIDS. AIDS Bol. Epidem., 10: 3, 1997.
- 18. SCHINDZIELORZ, A.H.; BELSHE, R.B. & MUFSON, M.A. - Occurrence, characteristics, and patterns of HIV-1 and HIV-2 Western blot indeterminate sera in low risk populations in West Virginia and pre-AIDS Africa. Amer. J. trop. Med. Hyg., 42: 460-464, 1990.
- 19. WHETSTONE, C.A.; SAYRE, K.R.; DOCK, N.L. et al. - Examination of whether persistently indeterminate human immunodeficiency virus type 1 Western immunoblot reactions are due to serological reactivity with bovine immunodeficiency-like virus. J. clin. Microbiol., 30: 764-770, 1992.
Publication Dates
-
Publication in this collection
14 Oct 1999 -
Date of issue
May 1999
History
-
Received
19 Aug 1998 -
Accepted
29 Mar 1999